From Bench to Bedside: Clinical and Biomedical Investigations on Hepatitis C Virus (HCV) Genotypes and Risk Factors for Albuminuria
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Subjects
2.1.1. Demographics
2.1.2. Dietary Habits
2.1.3. Physical Health
2.1.4. Biochemistry
2.1.5. Questionnaire
2.1.6. Restricted Access and Patient Data Collection
2.2. Study Tools
2.3. Operational Definition of Study Variables
2.3.1. Data Processing and Variable Selection
2.3.2. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.1.1. Gender
3.1.2. Age
3.1.3. Race
3.1.4. Education Level
3.1.5. Smoking
3.1.6. Drug Use
3.1.7. Diabetes Mellitus
3.1.8. Hypertension
3.1.9. Hepatitis B
3.1.10. HIV
3.1.11. Alcohol Use
3.1.12. BMI
3.1.13. Liver Function Tests and Lipid Profiles
3.1.14. Urine ACR
3.2. Distribution of HCV Genotypes
3.3. HCV Genotypes and Urine ACR
3.4. Generalized Linear Equation of the Relationship between HCV Genotype and Urine ACR
4. Discussion
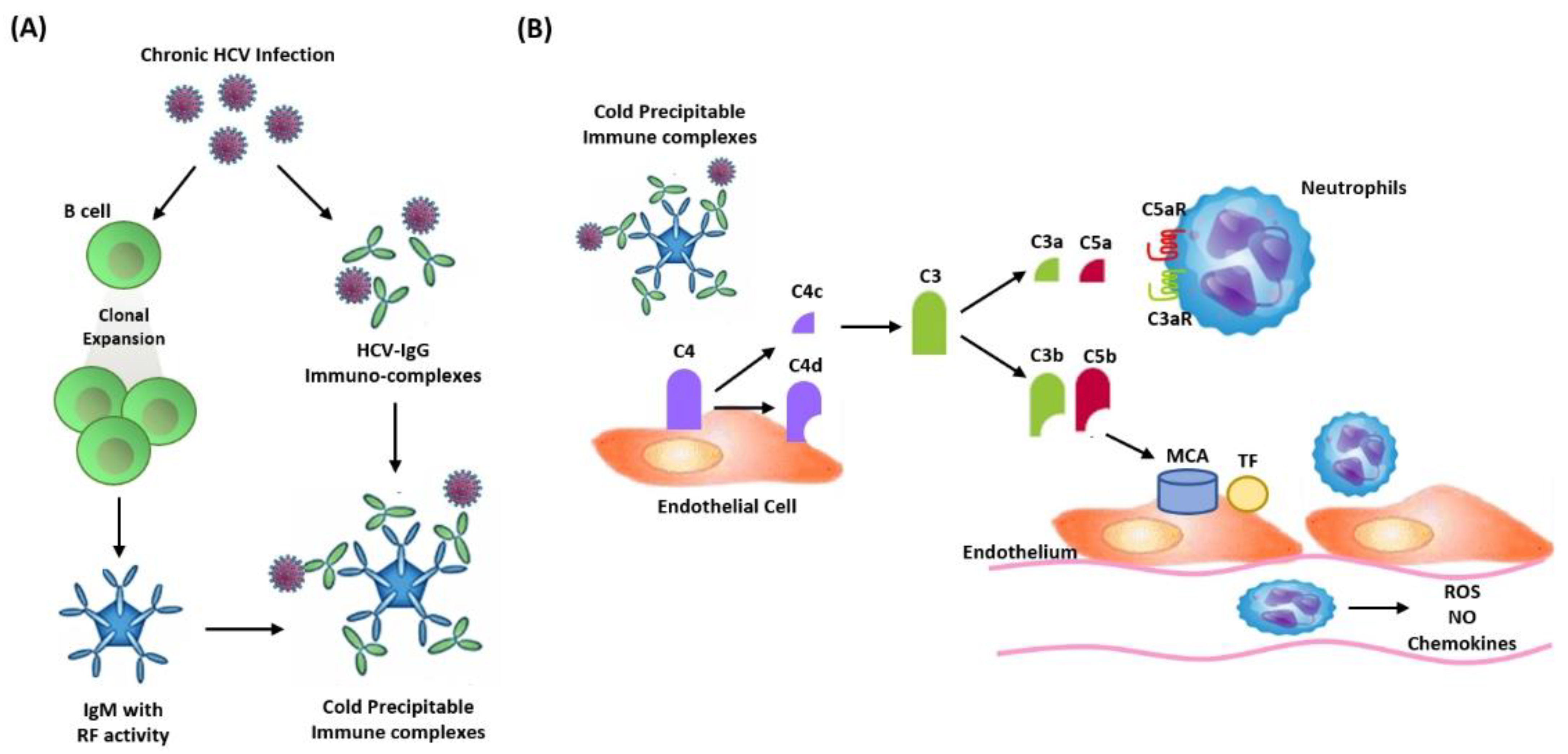
4.1. HCV-Associated Nephropathies
4.2. Management of HCV-Associated Nephropathies
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824–7840. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis C Key Fact. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 3 July 2021).
- Martínez, I.; Ryan, P.; Valencia, J.; Resino, S. The Challenging Road to Hepatitis C Virus Eradication. J. Clin. Med. 2021, 10, 611. [Google Scholar] [CrossRef]
- Juanbeltz, R.; Martínez-Baz, I.; Miguel, R.S.; Goñi-Esarte, S.; Cabasés, J.M.; Castilla, J. Impact of successful treatment with direct-acting antiviral agents on health-related quality of life in chronic hepatitis C patients. PLoS ONE 2018, 13, e0205277. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Feld, J.J. What Are the Benefits of a Sustained Virologic Response to Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection? Gastroenterology 2019, 156, 446–460.e2. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, S.; Riggio, O.; Rosati, D.; Gioia, S.; Farcomeni, A.; Ridola, L. Hepatitis C virus eradication with directly acting antivirals improves health-related quality of life and psychological symptoms. World J. Gastroenterol. 2019, 25, 6928–6938. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Cacoub, P. Renal involvement in HCV-related vasculitis. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Martin, P.; Dixit, V.; Messa, P. Hepatitis C virus infection and kidney disease: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2012, 7, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Liangpunsakul, S.; Chalasani, N. Relationship between hepatitis C and microalbuminuria: Results from the NHANES III. Kidney Int. 2005, 67, 285–290. [Google Scholar] [CrossRef][Green Version]
- Lai, T.S.; Lee, M.H.; Yang, H.I.; You, S.L.; Lu, S.N.; Wang, L.Y.; Yuan, Y.; L’Italien, G.; Chien, K.L.; Chen, C.J. High hepatitis C viral load and genotype 2 are strong predictors of chronic kidney disease. Kidney Int. 2017, 92, 703–709. [Google Scholar] [CrossRef]
- Lai, T.S.; Lee, M.H.; Yang, H.I.; You, S.L.; Lu, S.N.; Wang, L.Y.; Yuan, Y.; L’Italien, G.; Chien, K.L.; Chen, C.J. REVEAL-HCV Study Group, Hepatitis C viral load, genotype, and increased risk of developing end-stage renal disease: Reveal-HCV study. Hepatology 2017, 66, 784–793. [Google Scholar] [CrossRef]
- Hall, E.W.; Rosenberg, E.S.; Sullivan, P.S. Estimates of state-level chronic hepatitis C virus infection, stratified by race and sex, United States, 2010. BMC Infect. Dis. 2018, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC); National Centre for Health Statistics (NCHS). National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 15 September 2017).
- Edlin, B.R.; Eckhardt, B.J.; Shu, M.A.; Holmberg, S.D.; Swan, T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015, 62, 1353–1363. [Google Scholar] [CrossRef]
- Chen, Y.M.; Lin, M.X. The 23rd International Conference on Research Methods Organized by Statistics Canada-Population and Health Measurement Research Methods. pdf. 2007.
- Sambataro, G.; Giuffrè, M.; Sambataro, D.; Palermo, A.; Vignigni, G.; Cesareo, R.; Crimi, N.; Torrisi, S.E.; Vancheri, C.; Malatino, L.; et al. The Model for Early COvid-19 Recognition (MECOR) Score: A Proof-of-Concept for a Simple and Low-Cost Tool to Recognize a Possible Viral Etiology in Community-Acquired Pneumonia Patients during COVID-19 Outbreak. Diagnostics 2020, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Price, C.P.; Newall, R.G.; Boyd, J.C. Use of Protein: Creatinine Ratio Measurements on Random Urine Samples for Prediction of Significant Proteinuria: A Systematic Review. Clin. Chem. 2005, 51, 1577–1586. [Google Scholar] [CrossRef]
- Hong, D.S.C.; Oh, I.H.; Park, J.-S.; Lee, C.H.; Kang, C.M.; Kim, G.-H. Evaluation of Urinary Indices for Albuminuria and Proteinuria in Patients with Chronic Kidney Disease. Kidney Blood Press. Res. 2016, 41, 258–266. [Google Scholar] [CrossRef] [PubMed]
- De Jong, P.E.; Curhan, G.C. Screening, monitoring, and treatment of albuminuria: Public health perspectives. J. Am. Soc. Nephrol. 2006, 17, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Lamb, E.J.; McTaggart, M.P.; Stevens, P.E. Why albumin to creatinine ratio should replace protein to creatinine ratio: It is not just about nephrologists. Ann. Clin. Biochem. 2013, 50, 301–305. [Google Scholar] [CrossRef]
- Wu, C.C.; Liao, M.T.; Hsiao, P.J.; Lu, C.L.; Hsu, Y.J.; Lu, K.C.; Chu, P. Antiproteinuria Effect of Calcitriol in Patients with Chronic Kidney Disease and Vitamin D Deficiency: A Randomized Controlled Study. J. Ren. Nutr. 2020, 30, 200–207. [Google Scholar] [CrossRef]
- Hsiao, P.J.; Lin, H.C.; Chang, S.T.; Hsu, J.T.; Lin, W.S.; Chung, C.M.; Chang, J.J.; Hung, K.C.; Shih, Y.W.; Chen, F.C.; et al. Albuminuria and neck circumference are determinate factors of successful accurate estimation of glomerular filtration rate in high cardiovascular risk patients. PLoS ONE 2018, 13, e0185693. [Google Scholar] [CrossRef] [PubMed]
- MacIsaac, R.J.; Jerums, G.; Cooper, M.E. New insights into the significance of microalbuminuria. Curr. Opin. Nephrol. Hypertens. 2004, 13, 83–91. [Google Scholar] [CrossRef]
- Matsushita, K.; Van Der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; De Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [PubMed]
- Kdigo Clinical Practice Guideline on Glomerular Disease. Available online: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-GN-GL-Public-Review-Draft_1-June-2020.pdf (accessed on 1 June 2020).
- Mehta, S.H.; Brancati, F.L.; Strathdee, S.A.; Pankow, J.S.; Netski, D.; Coresh, J.; Szklo, M.; Thomas, D.L. Hepatitis C virus infection and incident type 2 diabetes. Hepatology 2003, 38, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.I.; Vittinghoff, E.; Shlipak, M.G.; O’Hare, A.M. Relationship between hepatitis C and chronic kidney disease: Results from the third national health and nutrition examination survey. J. Am. Soc. Nephrol. 2006, 17, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Wang, H.W.; Huang, Y.T.; Jiang, M.Y. Association of hepatitis C virus infection status and genotype with kidney disease risk: A population-based cross-sectional study. PLoS ONE 2022, 17, e0271197. [Google Scholar] [CrossRef]
- Angeletti, A.; Cantarelli, C.; Cravedi, P. HCV-associated nephropathies in the era of direct acting antiviral agents. Front. Med. 2019, 6, 20. [Google Scholar] [CrossRef]
- Pawlotsky, J.M.; Tsakiris, L.; Roudot-Thoraval, F.; Pellet, C.; Stuyver, L.; Duval, J.; Dhumeaux, D. Relationship between hepatitis C virus genotypes and sources of infection in patients with chronic hepatitis C. J. Infect. Dis. 1995, 171, 1607–1610. [Google Scholar] [CrossRef]
- Morozov, V.A.; Lagaye, S. Hepatitis C virus: Morphogenesis, infection and therapy. World J. Hepatol. 2018, 10, 186–212. [Google Scholar] [CrossRef]
- Sise, M.E.; Backman, E.; Ortiz, G.A.; Hundemer, G.L.; Ufere, N.N.; Chute, D.F.; Brancale, J.; Xu, D.; Wisocky, J.; Lin, M.V.; et al. Effect of Sofosbuvir-Based Hepatitis C Virus Therapy on Kidney Function in Patients with CKD. Clin. J. Am. Soc. Nephrol. CJASN 2017, 12, 1615–1623. [Google Scholar] [CrossRef]
- Aby, E.S.; Dong, T.S.; Kawamoto, J.; Pisegna, J.R.; Benhammou, J.N. Impact of sustained virologic response on chronic kidney disease progression in hepatitis C. World J. Hepatol. 2017, 9, 1352–1360. [Google Scholar] [CrossRef]
- Ogawa, E.; Furusyo, N.; Azuma, K.; Nakamuta, M.; Nomura, H.; Dohmen, K.; Satoh, T.; Kawano, A.; Koyanagi, T.; Ooho, A.; et al. Kyushu University Liver Disease Study (KULDS) Group. Elbasvir plus grazoprevir for patients with chronic hepatitis C genotype 1: A multicenter, real-world cohort study focusing on chronic kidney disease. Antivir. Res. 2018, 159, 143–152. [Google Scholar] [CrossRef]
- Alric, L.; Ollivier-Hourmand, I.; Bérard, E.; Hillaire, S.; Guillaume, M.; Vallet-Pichard, A.; Bernard-Chabert, B.; Loustaud-Ratti, V.; Bourlière, M.; de Ledinghen, V.; et al. Grazoprevir plus elbasvir in HCV genotype-1 or -4 infected patients with stage 4/5 severe chronic kidney disease is safe and effective. Kidney Int. 2018, 94, 206–213. [Google Scholar] [CrossRef]
- Tsai, M.C.; Lin, C.Y.; Hung, C.H.; Lu, S.N.; Tung, S.Y.; Chien, R.N.; Lin, C.L.; Wang, J.H.; Chien-Hung, C.; Chang, K.C.; et al. Evolution of renal function under direct-acting antivirals treatment for chronic hepatitis C: A real-world experience. J. Viral Hepat. 2019, 26, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.M.; Tsai, M.C.; Lin, C.Y.; Chen, C.H.; Lu, S.N.; Hung, C.H.; Sheen, I.S.; Chien, R.N.; Lin, C.L.; Hu, T.H.; et al. Serial changes of renal function after directly acting antivirals treatment for chronic hepatitis C: A 1-year follow-up study after treatment. PLoS ONE 2020, 15, e0231102. [Google Scholar] [CrossRef]
- Sise, M.E.; Chute, D.F.; Oppong, Y.; Davis, M.I.; Long, J.D.; Silva, S.T.; Rusibamayila, N.; Jean-Francois, D.; Raji, S.; Zhao, S.; et al. Direct-acting antiviral therapy slows kidney function decline in patients with Hepatitis C virus infection and chronic kidney disease. Kidney Int. 2020, 97, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Lin, J.W.; Liu, C.J.; Su, T.H.; Wu, J.H.; Tseng, T.C.; Chen, P.J.; Kao, J.H. Long-term Evolution of Estimated Glomerular Filtration Rate in Patients with Antiviral Treatment for Hepatitis C Virus Infection. Clin. Gastroenterol. Hepatol. 2022, in press. [CrossRef]
- Smith, D.B.; Bukh, J.; Kuiken, C.; Muerhoff, A.S.; Rice, C.M.; Stapleton, J.T.; Simmonds, P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2014, 59, 318–327. [Google Scholar] [CrossRef]
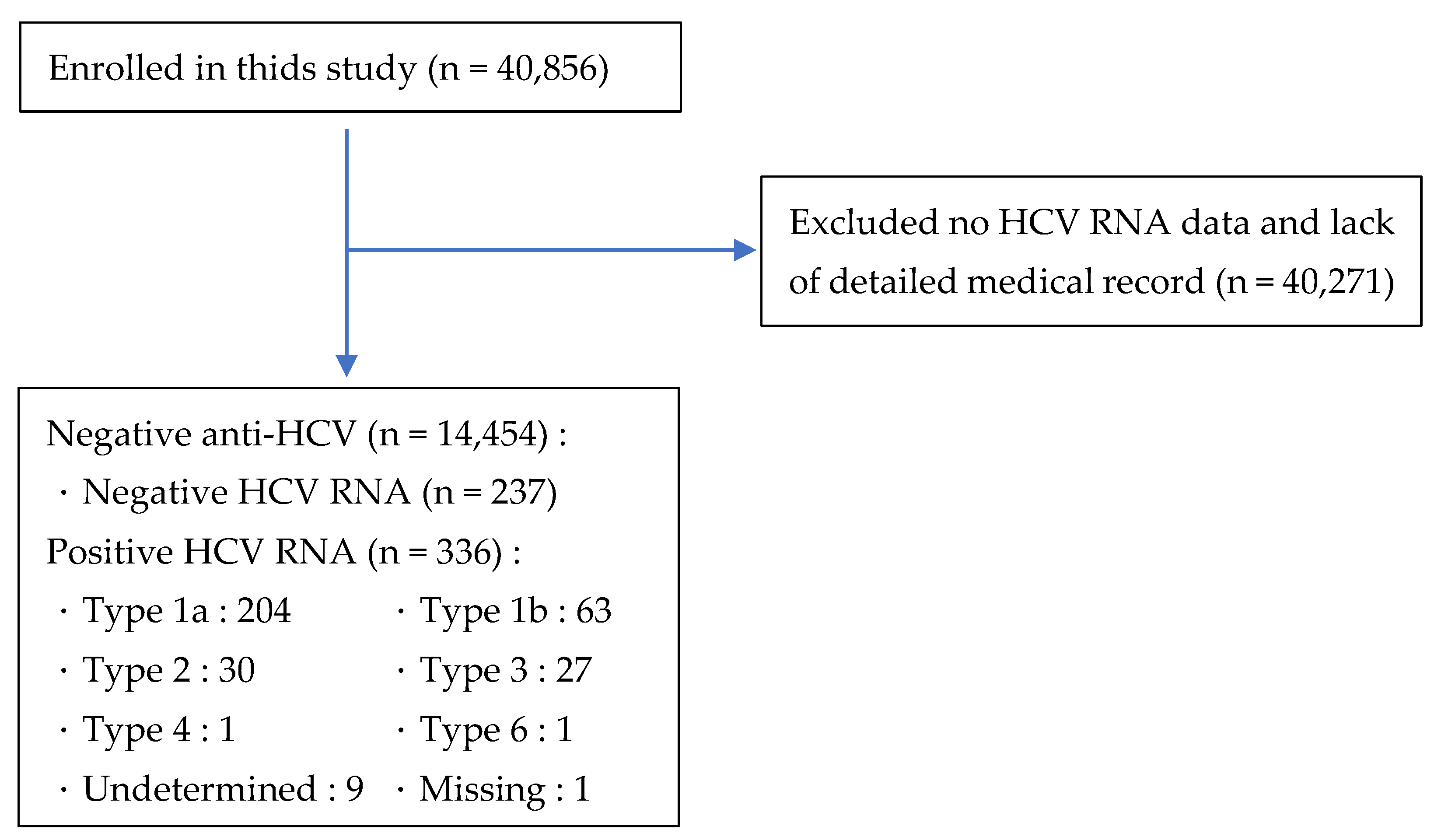
| All = 573 | Positive (%) | Negative (%) | p-Value | Genotype 1a | Genotype 1b | Genotype 2 | Genotype 3 | p-Value |
|---|---|---|---|---|---|---|---|---|
| n = 336 | n = 237 | n = 204 | n = 63 | n = 30 | n = 27 | |||
| Gender | ||||||||
| Male | 222 (66.1) | 131 (55.3) | <0.009 ** | 141 (69.1) | 36 (57.1) | 20 (66.7) | 14 (51.9) | 0.15 |
| Female | 114 (33.9) | 106 (44.7) | 63 (30.9) | 27 (42.9) | 10 (33.3) | 13 (48.1) | ||
| Age (years) | ||||||||
| <20 | 0 | 19 (8) | <0.001 *** | - | - | - | - | 0.072 |
| 20–39 | 32 (9.5) | 46 (19.4) | 23 (11.3) | 1 (1.6) | 4 (13.3) | 3 (11.1) | ||
| 40–59 | 191 (56.8) | 93 (39.2) | 116 (56.9) | 33 (52.4) | 15 (50.0) | 19 (70.4) | ||
| ≥60 | 113 (33.6) | 79 (33.3) | 65 (31.9) | 29 (46.0) | 11 (36.7) | 5 (18.5) | ||
| Race | ||||||||
| Mexican-American | 34 (10.1) | 33 (13.9) | <0.001 *** | 17 (8.5) | 7 (11.3) | 3 (10.0) | 6 (23.1) | <0.001 *** |
| Hispanic | 31 (9.2) | 24 (10.1) | 18 (9.0) | 3 (4.8) | 5 (16.7) | 4 (15.4) | ||
| Non-Hispanic white | 124 (36.9) | 116 (48.9) | 75 (37.7) | 13 (21.0) | 19 (63.3) | 11 (42.3) | ||
| Non-Hispanic black | 133 (39.6) | 42 (17.7) | 89 (44.7) | 38 (61.3) | 2 (6.7) | 2 (7.7) | ||
| Other | 14 (4.2) | 22 (9.3) | 5 (2.5) | 2 (3.2) | 1 (3.3) | 4 (14.8) | ||
| Education level | ||||||||
| High school | 228 (68.3) | 117 (53.7) | <0.001 ** | 139 (68.1) | 38 (60.3) | 22 (75.9) | 21 (80.8) | 0.306 |
| College or equivalent | 88 (26.3) | 68 (31.2) | 57 (27.9) | 19 (30.2) | 5 (17.2) | 4 (15.4) | ||
| Post-graduate | 18 (5.4) | 33 (15.1) | 8 (3.9) | 6 (9.5) | 2 (6.9) | 1 (3.8) | ||
| Smoking | ||||||||
| Yes | 116 (49.4%) | 72 (30.4%) | <0.001 *** | 123 (73.2) | 29 (60.4) | 19 (70.4) | 16 (69.6) | 0.402 |
| No | 170(50.6%) | 165 (69.6%) | 45 (26.8) | 19 (39.6) | 8 (29.6) | 7 (30.4) | ||
| Diabetes mellitus | ||||||||
| Yes | 51 (15.2%) | 30 (12.7%) | 0.386 | 29 (14.2) | 13 (20.6) | 1 (3.3) | 6 (22.2) | 0.115 |
| No | 284 (84.8%) | 207 (87.3%) | 175 (85.8) | 50 (79.4) | 29 (96.7) | 21 (77.8) | ||
| Hypertension | ||||||||
| Yes | 151 (85.3%) | 74 (76.3%) | 0.062 | 96 (88.9) | 33 (84.6) | 7 (50.0) | 9 (90.0) | 0.002 * |
| No | 26 (14.7%) | 23 (23.7%) | 12 (11.1) | 6(15.4) | 7 (50.0) | 1 (10.0) | ||
| Hepatitis B | ||||||||
| Yes | 4 (1.3%) | 2 (1.1%) | 0.8 | 2 (1.1) | 0 | 0 | 1 (4.3) | 0.336 |
| No | 300 (98.7%) | 187 (98.9%) | 184 (63.4) | 59 (20.3) | 25 (8.6) | 22 (7.6) | ||
| HIV | ||||||||
| Yes | 5 (2.6%) | 4 (2.9%) | 0.865 | 4 (3.3) | 1 (3.7) | 0 | 0 | 0.749 |
| No | 188 (97.4%) | 134 (97.1%) | 119 (96.7) | 26 (96.3) | 17 (100) | 18 (100) | ||
| Alcohol use | ||||||||
| Yes | 272 (86.9) | 162 (77.9) | <0.007 ** 0.729 | 176 (91.7) | 50 (83.3) | 22 (78.6) | 17 (73.9) | 0.019 * 0.911 |
| Week | 97 (49.2) | 55 (46.2) | 63 (49.2) | 19 (54.3) | 6 (35.3) | 6 (50.0) | ||
| Month | 36 (19.8) | 22 (18.5) | 26 (20.3) | 6 (17.1) | 5 (29.4) | 2 (16.7) | ||
| Year | 61 (31) | 42 (35.3) | 39 (30.5) | 10 (28.6) | 6 (35.3) | 4 (33.3) | ||
| No | 41 (13.1) | 46 (22.1) | 16 (8.3) | 10 (16.7) | 6 (21.4) | 6 (26.1) | ||
| BMI | ||||||||
| Underweight | 5 (1.5) | 12 (5.1) | <0.039 * | 4 (2.0) | 1 (1.6) | 0 | 0 | 0.856 |
| Normal weight | 81 (24.5) | 60 (25.6) | 47 (23.4) | 12 (19.4) | 9 (31.0) | 7 (25.9) | ||
| Overweight | 245 (74.0) | 162 (69.2) | 150 (74.6) | 49 (79) | 20 (69.0) | 20 (74.1) | ||
| Urine ACR | ||||||||
| <30 mg/g | 260 (79.5) | 192 (82.1) | 0.723 | 158 (79.8) | 45 (72.6) | 23 (82.1) | 24 (88.9) | 0.617 |
| 30–300 mg/g | 56 (17.1) | 36 (15.4) | 32 (16.2) | 15 (24.2) | 4 (14.3) | 3 (11.1) | ||
| >300 mg/g | 11 (3.4) | 6 (2.6) | 8 (4.0) | 2 (3.2) | 1 (3.6) | 0 |
| All = 335 | N (%) |
|---|---|
| Genotype | |
| Genotype 1a | 204 (60.9) |
| Genotype 1b | 63 18.8) |
| Genotype 2 | 30 (9.0) |
| Genotype 3 | 27 (8.1) |
| Genotype 4 | 1 (0.3) |
| Genotype 6 | 1 (0.3) |
| Genotype undetermined | 9 (2.7) |
| Independent Variable | Coefficient | Standard Error | Hypothesis Test | |
|---|---|---|---|---|
| Wald Chi-Square Test | p-Value | |||
| (Intercept) | 599.567 | 553.9132 | 1.172 | 0.279 |
| Genotype 1a | 66.698 | 93.5948 | 0.508 | 0.476 |
| Genotype 1b | −29.669 | 131.0363 | 0.051 | 0.821 |
| Genotype 2 | 635.457 | 181.5780 | 12.247 | <0.001 *** |
| Genotype 3 | −157.011 | 199.4850 | 0.619 | 0.431 |
| HCV RNA negative (ref) | ||||
| Male | −46.886 | 82.3127 | 0.324 | 0.569 |
| Mexican-American | −20.579 | 215.4718 | 0.009 | 0.924 |
| Hispanic | 352.414 | 220.6777 | 2.550 | 0.110 |
| Non-Hispanic white | 69.235 | 190.8292 | 0.132 | 0.717 |
| Non-Hispanic black | 85.560 | 199.1391 | 0.185 | 0.667 |
| Other (ref) | 0 a | |||
| High school | 190.048 | 141.5909 | 1.802 | 0.180 |
| College or equivalent | 149.012 | 149.3898 | 0.995 | 0.319 |
| Post-graduate (ref) | 0 a | |||
| Smoker | −188.896 | 85.1148 | 4.925 | 0.026 * |
| Diabetes mellitus | 592.153 | 114.6121 | 26.694 | <0.001 *** |
| Hepatitis B | 1894.796 | 357.1796 | 28.142 | <0.001 *** |
| Alcohol use | 94.895 | 112.4882 | 0.712 | 0.399 |
| Age | −11.892 | 6.9587 | 2.921 | 0.087 |
| Age 20–39 years | −316.498 | 269.1206 | 1.383 | 0.240 |
| Age 40–59 years | −58.615 | 140.2000 | 0.175 | 0.676 |
| Age ≥ 60 years (ref) | 0 a | |||
| BMI: Underweight | 48.622 | 310.0694 | 0.025 | 0.875 |
| BMI: Overweight | 23.14 | 115.8813 | 0.04 | 0.842 |
| BMI | −6.205 | 7.9142 | 0.615 | 0.433 |
| BMI: Normal (ref) | 0 a | |||
| Categories | Cryoglobulin Type | Phenotype of GN | Histological Findings |
|---|---|---|---|
| Cryoglobulinemic GN | |||
| Type I: isolated monoclonal IgA, IgM, or IgG Type II: IgG and a monoclonal IgM RF Type III: IgG and a polyclonal IgM RF | Membranoproliferative GN (frequently associated with type II cryoglobulinemia) | IC deposition in:
| |
| Non-cryoglobulinemic GN | |||
| Membranoproliferative GN | Mesangial deposition of IC with viral-like particles, IgG and other complement fractions | ||
| Membranous GN | IC containing HCV proteins deposition in subepithelial glomerular basement membrane | ||
| IgA nephropathy | Impaired IgA clearance and IgA-containing IC | ||
| FSGS | Possible HCV direct injury to the podocytes | ||
| Fibrillary and immunotactoid glomerulopathy | Extracellular deposition of microfibrils within the mesangium and glomerular capillary walls (predominance of IgG4 deposition) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, P.-J.; Hsiao, C.-J.; Tsai, F.-R.; Hou, Y.-L.; Chiu, C.-C.; Chiang, W.-F.; Wu, K.-L.; Li, Y.-K.; Lin, C.; Chan, J.-S.; et al. From Bench to Bedside: Clinical and Biomedical Investigations on Hepatitis C Virus (HCV) Genotypes and Risk Factors for Albuminuria. Bioengineering 2022, 9, 509. https://doi.org/10.3390/bioengineering9100509
Hsiao P-J, Hsiao C-J, Tsai F-R, Hou Y-L, Chiu C-C, Chiang W-F, Wu K-L, Li Y-K, Lin C, Chan J-S, et al. From Bench to Bedside: Clinical and Biomedical Investigations on Hepatitis C Virus (HCV) Genotypes and Risk Factors for Albuminuria. Bioengineering. 2022; 9(10):509. https://doi.org/10.3390/bioengineering9100509
Chicago/Turabian StyleHsiao, Po-Jen, Chia-Jen Hsiao, Fu-Ru Tsai, Yen-Lin Hou, Chih-Chien Chiu, Wen-Fang Chiang, Kun-Lin Wu, Yuan-Kuei Li, Chen Lin, Jenq-Shyong Chan, and et al. 2022. "From Bench to Bedside: Clinical and Biomedical Investigations on Hepatitis C Virus (HCV) Genotypes and Risk Factors for Albuminuria" Bioengineering 9, no. 10: 509. https://doi.org/10.3390/bioengineering9100509
APA StyleHsiao, P.-J., Hsiao, C.-J., Tsai, F.-R., Hou, Y.-L., Chiu, C.-C., Chiang, W.-F., Wu, K.-L., Li, Y.-K., Lin, C., Chan, J.-S., Chang, C.-W., & Chu, C.-M. (2022). From Bench to Bedside: Clinical and Biomedical Investigations on Hepatitis C Virus (HCV) Genotypes and Risk Factors for Albuminuria. Bioengineering, 9(10), 509. https://doi.org/10.3390/bioengineering9100509








