Modulation of Macrophage Activity by Pulsed Electromagnetic Fields in the Context of Fracture Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Material
2.2. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
2.3. ELF-PEMF Device and Exposure
2.4. Western Blot
2.5. DCFH-DA Assay
2.6. Human Cytokine Array C5 with Media Conditioned by PBMC Exposed to ELF-PEMFs
2.7. Culture and Differentiation of SCP-1 Cells
2.8. Cell Migration Assay
2.9. Sulforhodamine B (SRB) Staining
2.10. RNA Isolation and RT-PCR
2.11. Statistical Analysis
3. Results
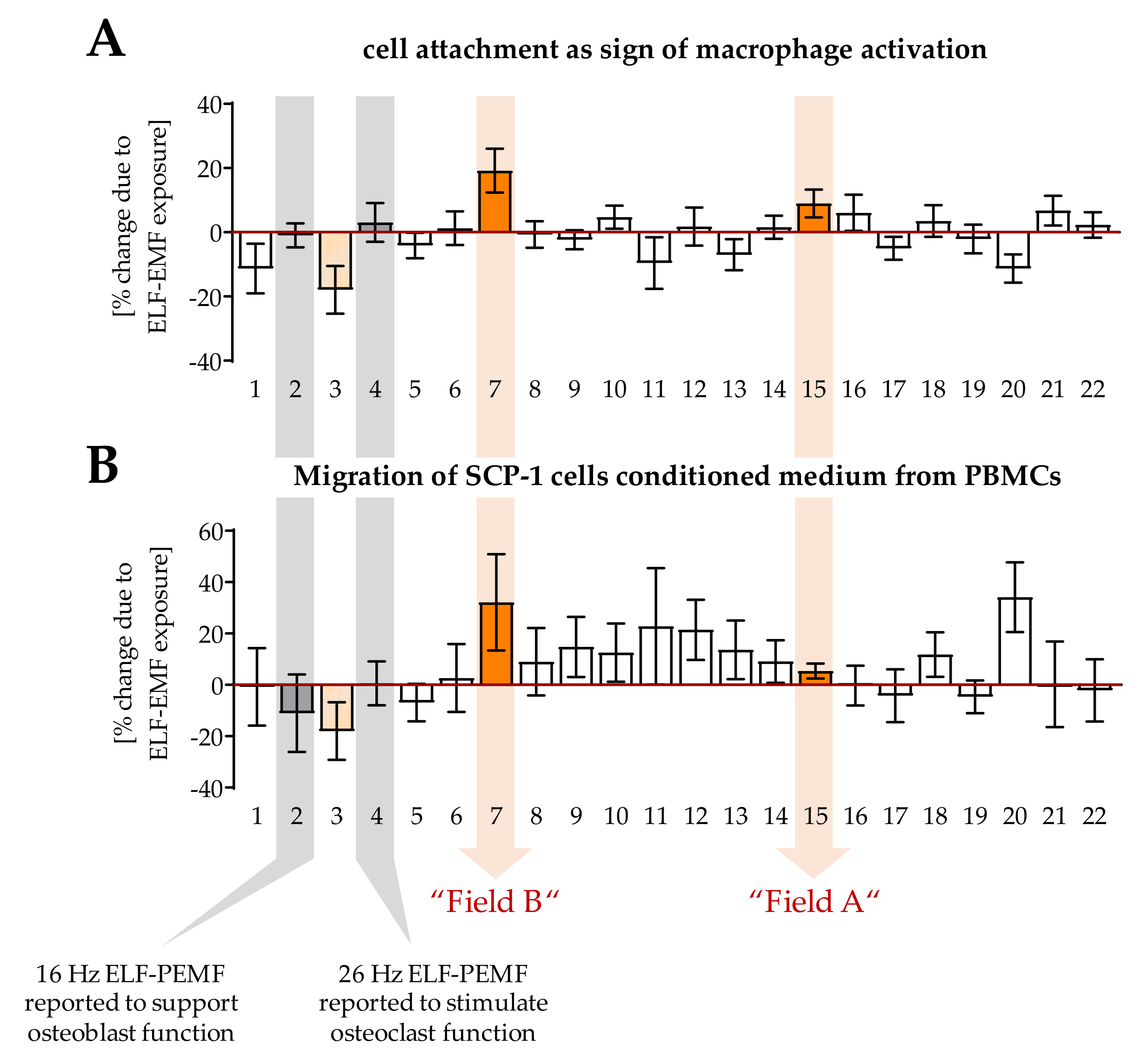
3.1. ELF-PEMFs Exposure Modulates Macrophage Differentiation
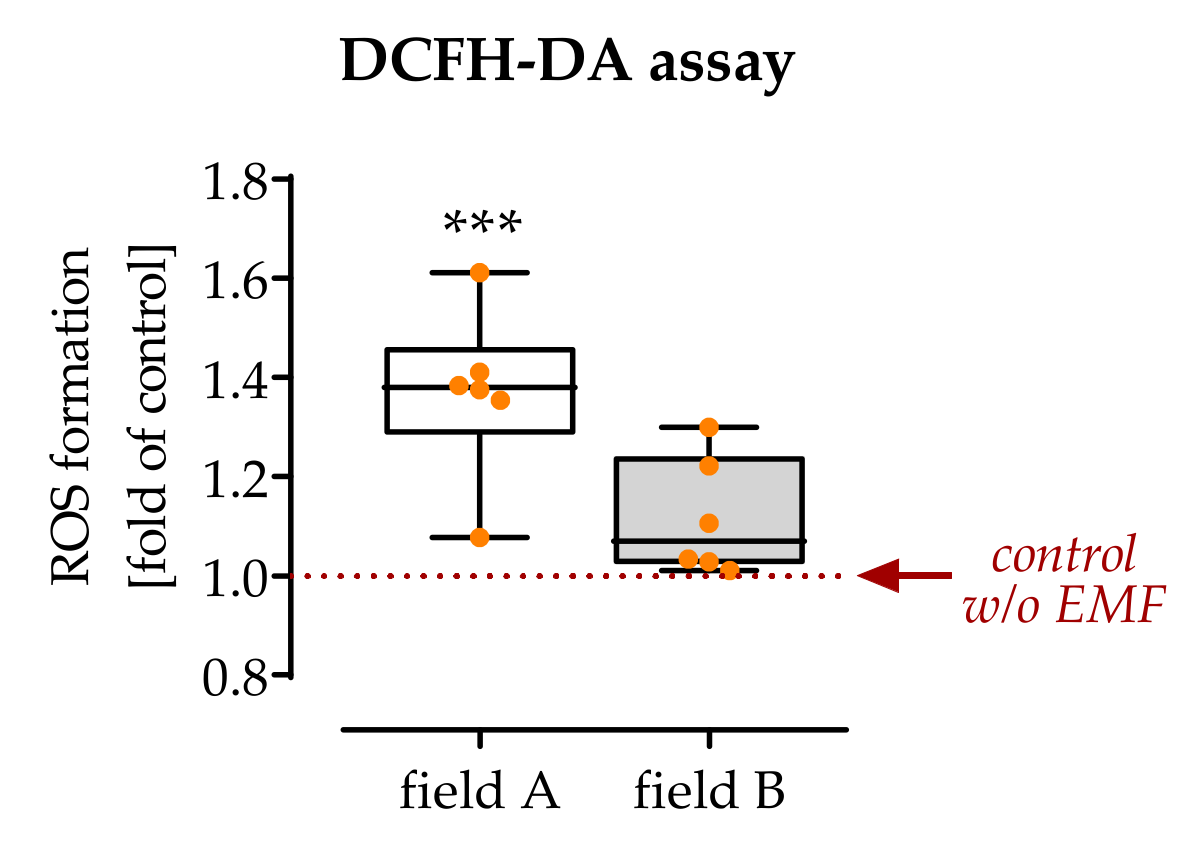
3.2. Exposure to Field A Leads to the Formation of Intracellular Reactive Oxygen Species (ROS)
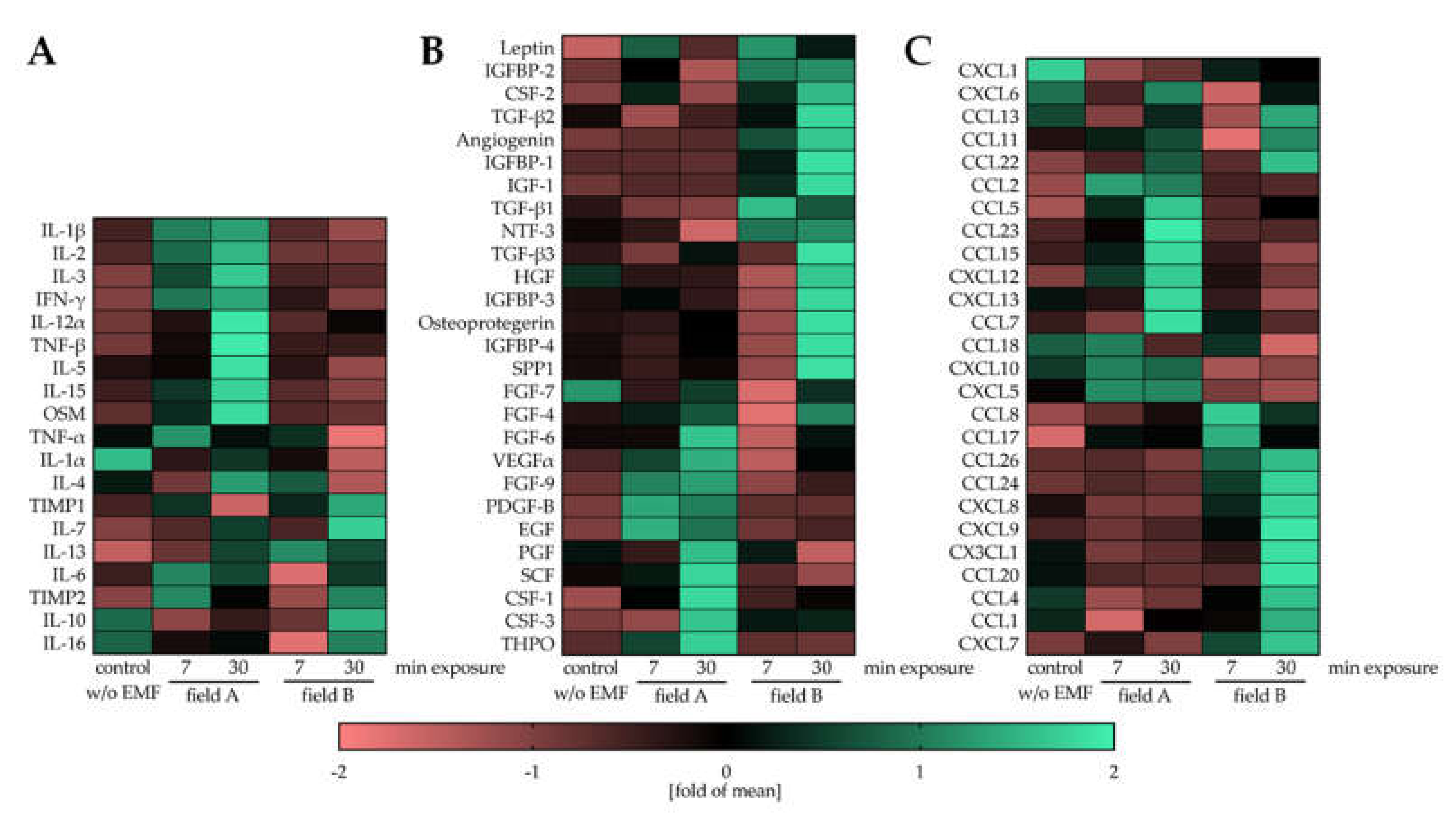
3.3. Field A and Field B Diversely Regulate the Secretion of Cytokines, Growth Factors, and Chemokines by Macrophages
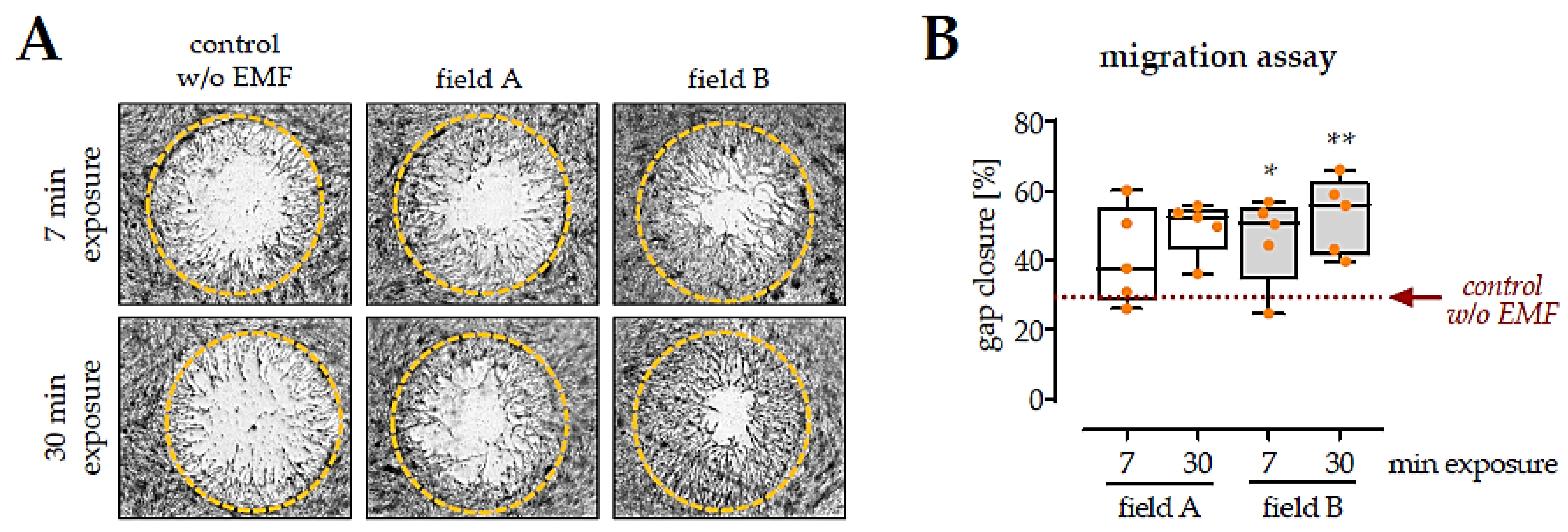
3.4. Factors Secreted by Macrophages Exposed to the Two ELF-PEMF Stimulated Migration of SCP-1 Cells
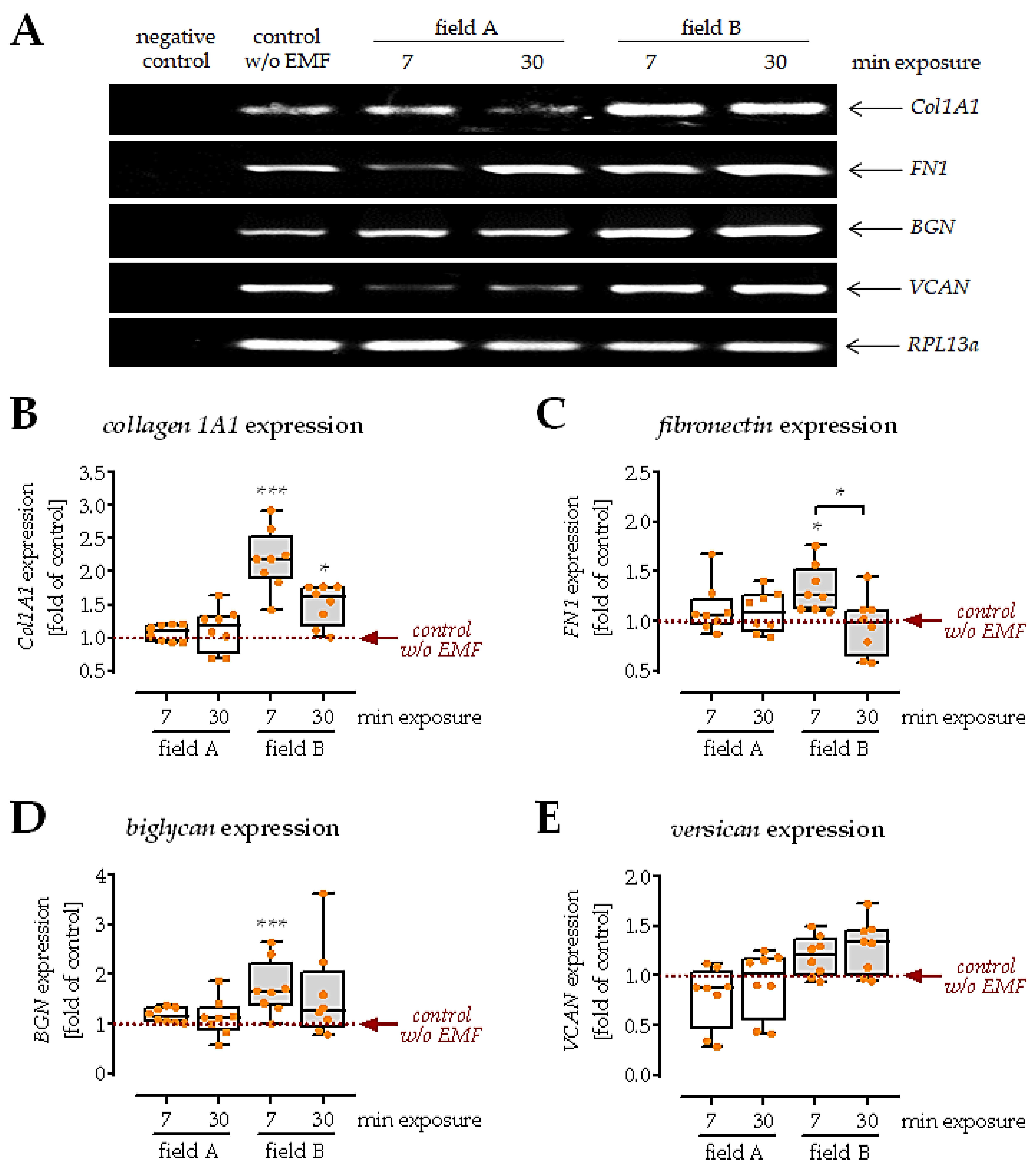
3.5. Conditioned Medium from Macrophages Exposed to Field B Induced Extracellular Matrix Formation in SCP-1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, D. Non-union bone fracture: A quicker fix. Nature 2017, 550, S193. [Google Scholar] [CrossRef]
- Castronuovo, E.; Pezzotti, P.; Franzo, A.; Di Lallo, D.; Guasticchi, G. Early and late mortality in elderly patients after hip fracture: A cohort study using administrative health databases in the Lazio region, Italy. Bmc. Geriatr. 2011, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bucher, C.H.; Lei, H.; Duda, G.N.; Volk, H.D.; Schmidt-Bleek, K. The Role of Immune Reactivity in Bone Regeneration; IntecOpen Limited: London, UK, 2016; pp. 169–194. [Google Scholar]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef]
- Park, J.E.; Barbul, A. Understanding the role of immune regulation in wound healing. Am. J. Surg. 2004, 187, 11–16. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, Y.; Medzhitov, R. Tissue biology perspective on macrophages. Nat. Immunol. 2016, 17, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Behm, B.; Babilas, P.; Landthaler, M.; Schreml, S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, M.; Ren, R.; Gao, C.; Cheng, H.; Li, Y.; Gao, B.; Wei, Y.; Fu, J.; Sun, J.; et al. Improved Immunoregulation of Ultra-Low-Dose Silver Nanoparticle-Loaded TiO2 Nanotubes via M2 Macrophage Polarization by Regulating GLUT1 and Autophagy. Int. J. Nanomed. 2020, 15, 2011–2026. [Google Scholar] [CrossRef] [Green Version]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef] [PubMed]
- George, M.D.; Baker, J.F.; Leonard, C.E.; Mehta, S.; Miano, T.A.; Hennessy, S. Risk of Nonunion with Nonselective NSAIDs, COX-2 Inhibitors, and Opioids. J. Bone Jt. Surg. Am. 2020, 102, 1230–1238. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Akhter, M.P.; Gao, X.; Wang, X.Y.; Wang, X.B.; Zhao, G.; Wei, X.; Wu, H.J.; Chen, H.; Wang, D.; et al. Glucocorticoid-induced delayed fracture healing and impaired bone biomechanical properties in mice. Clin. Interv. Aging 2018, 13, 1465–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehnert, S.; Schroter, S.; Aspera-Werz, R.H.; Eisler, W.; Falldorf, K.; Ronniger, M.; Nussler, A.K. Translational Insights into Extremely Low Frequency Pulsed Electromagnetic Fields (ELF-PEMFs) for Bone Regeneration after Trauma and Orthopedic Surgery. J. Clin. Med. 2019, 8, 2802. [Google Scholar] [CrossRef] [Green Version]
- Ehnert, S.; Falldorf, K.; Fentz, A.K.; Ziegler, P.; Schroter, S.; Freude, T.; Ochs, B.G.; Stacke, C.; Ronniger, M.; Sachtleben, J.; et al. Primary human osteoblasts with reduced alkaline phosphatase and matrix mineralization baseline capacity are responsive to extremely low frequency pulsed electromagnetic field exposure—Clinical implication possible. Bone Rep. 2015, 3, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Ehnert, S.; van Griensven, M.; Unger, M.; Scheffler, H.; Falldorf, K.; Fentz, A.K.; Seeliger, C.; Schroter, S.; Nussler, A.K.; Balmayor, E.R. Co-Culture with Human Osteoblasts and Exposure to Extremely Low Frequency Pulsed Electromagnetic Fields Improve Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasa, K.; Reddi, A.H. Pulsed Electromagnetic Fields and Tissue Engineering of the Joints. Tissue Eng. Part B Rev. 2018, 24, 144–154. [Google Scholar] [CrossRef]
- Miller, S.L.; Coughlin, D.G.; Waldorff, E.I.; Ryaby, J.T.; Lotz, J.C. Pulsed electromagnetic field (PEMF) treatment reduces expression of genes associated with disc degeneration in human intervertebral disc cells. Spine J. 2016, 16, 770–776. [Google Scholar] [CrossRef]
- Simko, M.; Mattsson, M.O. Extremely low frequency electromagnetic fields as effectors of cellular responses in vitro: Possible immune cell activation. J. Cell Biochem. 2004, 93, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Rollwitz, J.; Lupke, M.; Simko, M. Fifty-hertz magnetic fields induce free radical formation in mouse bone marrow-derived promonocytes and macrophages. Biochim. Biophys Acta 2004, 1674, 231–238. [Google Scholar] [CrossRef]
- Simko, M.; Droste, S.; Kriehuber, R.; Weiss, D.G. Stimulation of phagocytosis and free radical production in murine macrophages by 50 Hz electromagnetic fields. Eur. J. Cell. Biol. 2001, 80, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Frahm, J.; Lantow, M.; Lupke, M.; Weiss, D.G.; Simko, M. Alteration in cellular functions in mouse macrophages after exposure to 50 Hz magnetic fields. J. Cell Biochem. 2006, 99, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Costantini, E.; Ferrone, A.; Pesce, M.; Diomede, F.; Trubiani, O.; Reale, M. Short ELF-EMF Exposure Targets SIRT1/Nrf2/HO-1 Signaling in THP-1 Cells. Int. J. Mol. Sci. 2020, 21, 7284. [Google Scholar] [CrossRef] [PubMed]
- Akan, Z.; Aksu, B.; Tulunay, A.; Bilsel, S.; Inhan-Garip, A. Extremely low-frequency electromagnetic fields affect the immune response of monocyte-derived macrophages to pathogens. Bioelectromagnetics 2010, 31, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Aspera-Werz, R.H.; Menger, M.M.; Falldorf, K.; Ronniger, M.; Stacke, C.; Histing, T.; Nussler, A.K.; Ehnert, S. Exposure to 16 Hz Pulsed Electromagnetic Fields Protect the Structural Integrity of Primary Cilia and Associated TGF-beta Signaling in Osteoprogenitor Cells Harmed by Cigarette Smoke. Int. J. Mol. Sci. 2021, 22, 7036. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.Q.; Ge, B.F.; Ma, X.N.; Ma, H.P.; Xian, C.J.; Chen, K.M. Different electromagnetic field waveforms have different effects on proliferation, differentiation and mineralization of osteoblasts in vitro. Bioelectromagnetics 2014, 35, 30–38. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Qu, X.; Wen, J. Effects of different extremely low-frequency electromagnetic fields on osteoblasts. Electromagn. Biol. Med. 2007, 26, 167–177. [Google Scholar] [CrossRef]
- Linnemann, C.; Savini, L.; Rollmann, M.F.; Histing, T.; Nussler, A.K.; Ehnert, S. Altered Secretome of Diabetic Monocytes Could Negatively Influence Fracture Healing-An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 9212. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Fentz, A.K.; Schreiner, A.; Birk, J.; Wilbrand, B.; Ziegler, P.; Reumann, M.K.; Wang, H.; Falldorf, K.; Nussler, A.K. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of *O2(-) and H2O2. Sci. Rep. 2017, 7, 14544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocker, W.; Yin, Z.; Drosse, I.; Haasters, F.; Rossmann, O.; Wierer, M.; Popov, C.; Locher, M.; Mutschler, W.; Docheva, D.; et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J. Cell Mol. Med. 2008, 12, 1347–1359. [Google Scholar] [CrossRef] [Green Version]
- Bedi, B.; Li, J.Y.; Grassi, F.; Tawfeek, H.; Weitzmann, M.N.; Pacifici, R. Inhibition of antigen presentation and T cell costimulation blocks PTH-induced bone loss. Ann. N. Y. Acad. Sci. 2010, 1192, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Parihar, A.; Eubank, T.D.; Doseff, A.I. Monocytes and Macrophages Regulate Immunity through Dynamic Networks of Survival and Cell Death. J. Innate Immun. 2010, 2, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Funk, R.H. Coupling of pulsed electromagnetic fields (PEMF) therapy to molecular grounds of the cell. Am. J. Transl. Res. 2018, 10, 1260–1272. [Google Scholar] [PubMed]
- Ziegler, P.; Nussler, A.K.; Wilbrand, B.; Falldorf, K.; Springer, F.; Fentz, A.K.; Eschenburg, G.; Ziegler, A.; Stockle, U.; Maurer, E.; et al. Pulsed Electromagnetic Field Therapy Improves Osseous Consolidation after High Tibial Osteotomy in Elderly Patients-A Randomized, Placebo-Controlled, Double-Blind Trial. J. Clin. Med. 2019, 8, 2008. [Google Scholar] [CrossRef] [Green Version]
- Barros, M.H.M.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage Polarisation: An Immunohistochemical Approach for Identifying M1 and M2 Macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, H.M.; Chen, C.L.; Yang, C.M.; Wu, P.H.; Tsou, C.J.; Chiang, K.W.; Wu, W.B. The carotenoid lutein enhances matrix metalloproteinase-9 production and phagocytosis through intracellular ROS generation and ERK1/2, p38 MAPK, and RARbeta activation in murine macrophages. J. Leukoc Biol. 2013, 93, 723–735. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Brumell, J.H. NADPH oxidases contribute to autophagy regulation. Autophagy 2009, 5, 887–889. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef]
- Brune, B.; Dehne, N.; Grossmann, N.; Jung, M.; Namgaladze, D.; Schmid, T.; von Knethen, A.; Weigert, A. Redox control of inflammation in macrophages. Antioxid. Redox Signal. 2013, 19, 595–637. [Google Scholar] [CrossRef] [Green Version]
- Herb, M.; Gluschko, A.; Wiegmann, K.; Farid, A.; Wolf, A.; Utermohlen, O.; Krut, O.; Kronke, M.; Schramm, M. Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of NEMO. Sci. Signal. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Dresner-Pollak, R.; Gelb, N.; Rachmilewitz, D.; Karmeli, F.; Weinreb, M. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology 2004, 127, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Henle, P.; Zimmermann, G.; Weiss, S. Matrix metalloproteinases and failed fracture healing. Bone 2005, 37, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C.; Thompson, Z.; Miclau, T.; Werb, Z.; Helms, J.A. Altered fracture repair in the absence of MMP9. Development 2003, 130, 4123–4133. [Google Scholar] [CrossRef] [Green Version]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys 2008, 473, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.; Xu, Z. Three decades of research on angiogenin: A review and perspective. Acta Biochim. Biophys Sin. 2016, 48, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, S.; Zimmermann, G.; Pufe, T.; Varoga, D.; Henle, P. The systemic angiogenic response during bone healing. Arch. Orthop. Trauma Surg. 2009, 129, 989–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, G.; Henle, P.; Kusswetter, M.; Moghaddam, A.; Wentzensen, A.; Richter, W.; Weiss, S. TGF-beta1 as a marker of delayed fracture healing. Bone 2005, 36, 779–785. [Google Scholar] [CrossRef]
- Ehnert, S.; Sreekumar, V.; Aspera-Werz, R.H.; Sajadian, S.O.; Wintermeyer, E.; Sandmann, G.H.; Bahrs, C.; Hengstler, J.G.; Godoy, P.; Nussler, A.K. TGF-beta(1) impairs mechanosensation of human osteoblasts via HDAC6-mediated shortening and distortion of primary cilia. J. Mol. Med. 2017, 95, 653–663. [Google Scholar] [CrossRef]
- Moghaddam, A.; Weiss, S.; Wolfl, C.G.; Schmeckenbecher, K.; Wentzensen, A.; Grutzner, P.A.; Zimmermann, G. Cigarette smoking decreases TGF-b1 serum concentrations after long bone fracture. Injury 2010, 41, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Muszynska, M.; Ambrozewicz, E.; Gegotek, A.; Grynkiewicz, G.; Skrzydlewska, E. Protective Effects of Vitamin K Compounds on the Proteomic Profile of Osteoblasts under Oxidative Stress Conditions. Molecules 2020, 25, 1990. [Google Scholar] [CrossRef]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef] [Green Version]

| Target | Gene Bank Accession Number | Sequence Forward Primer | Sequence Reverse Primer | Ta [°C] | # of Cycles | Amplicon Size [bp] |
|---|---|---|---|---|---|---|
| RPL13a | NM_012423.3 | AAGTACCAGG CAGTGACAG | CCTGTTTCCGT AGCCTCATG | 56 | 30 | 100 |
| Biglycan | NM_001711.5 | CGCCTCGTGT CTCTGCTGGC | GCGGATGCGG TTGTCGTGGA | 64 | 35 | 501 |
| Versican | NM_001164098.1 | AATGCCGTCT GCAGGGTGCC | GGCCGCAAGC GACTGTTCCT | 64 | 35 | 306 |
| Collagen 1A1 | NM_000088.3 | CAGCCGCTTC ACCTACAGC | TTTGTATTCAAT CACTGTCTTGCC | 56 | 35 | 83 |
| Fibronectin | NM_002026.2 | CCCCATTCCAG GACACTTCTG | GCCCACGGTA ACAACCTCTT | 60 | 35 | 203 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Menger, M.M.; Braun, B.J.; Schweizer, S.; Linnemann, C.; Falldorf, K.; Ronniger, M.; Wang, H.; Histing, T.; Nussler, A.K.; et al. Modulation of Macrophage Activity by Pulsed Electromagnetic Fields in the Context of Fracture Healing. Bioengineering 2021, 8, 167. https://doi.org/10.3390/bioengineering8110167
Chen Y, Menger MM, Braun BJ, Schweizer S, Linnemann C, Falldorf K, Ronniger M, Wang H, Histing T, Nussler AK, et al. Modulation of Macrophage Activity by Pulsed Electromagnetic Fields in the Context of Fracture Healing. Bioengineering. 2021; 8(11):167. https://doi.org/10.3390/bioengineering8110167
Chicago/Turabian StyleChen, Yangmengfan, Maximilian M. Menger, Benedikt J. Braun, Sara Schweizer, Caren Linnemann, Karsten Falldorf, Michael Ronniger, Hongbo Wang, Tina Histing, Andreas K. Nussler, and et al. 2021. "Modulation of Macrophage Activity by Pulsed Electromagnetic Fields in the Context of Fracture Healing" Bioengineering 8, no. 11: 167. https://doi.org/10.3390/bioengineering8110167
APA StyleChen, Y., Menger, M. M., Braun, B. J., Schweizer, S., Linnemann, C., Falldorf, K., Ronniger, M., Wang, H., Histing, T., Nussler, A. K., & Ehnert, S. (2021). Modulation of Macrophage Activity by Pulsed Electromagnetic Fields in the Context of Fracture Healing. Bioengineering, 8(11), 167. https://doi.org/10.3390/bioengineering8110167







