Nanohydroxyapatite Electrodeposition onto Electrospun Nanofibers: Technique Overview and Tissue Engineering Applications
Abstract
:1. Introduction
2. Rationale for Using nHAp as a Coating in Tissue-Regenerative Scaffolds
3. nHAp Electrodeposition Method
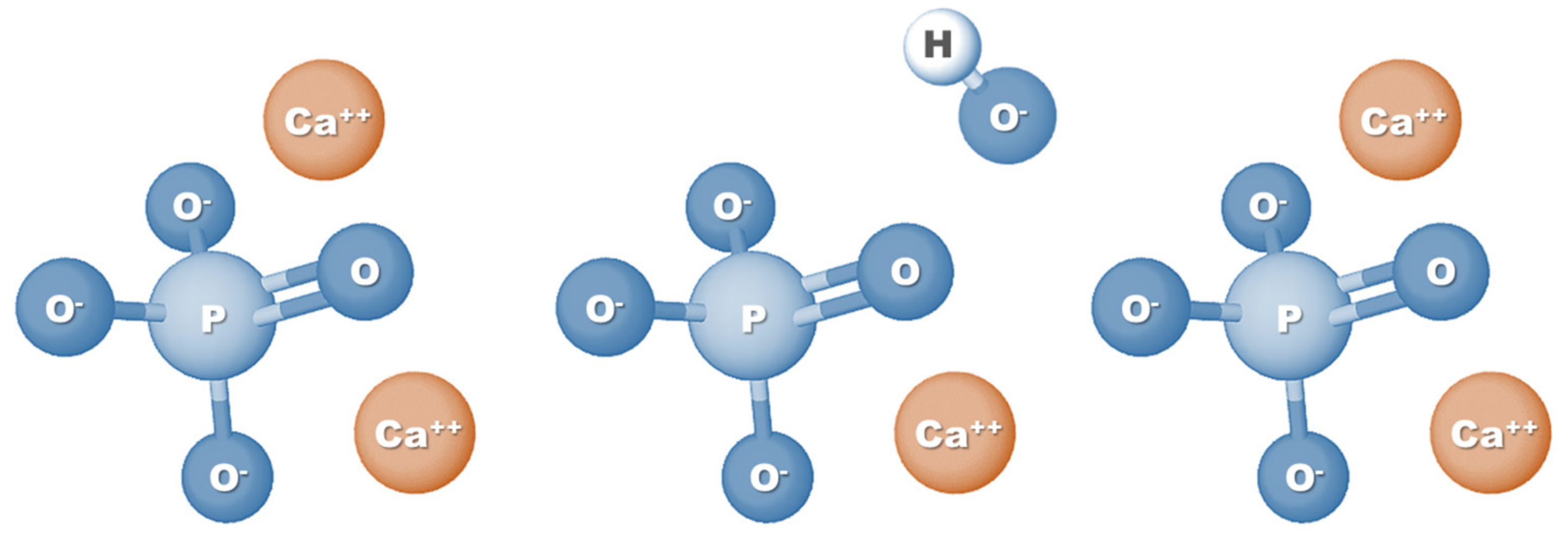
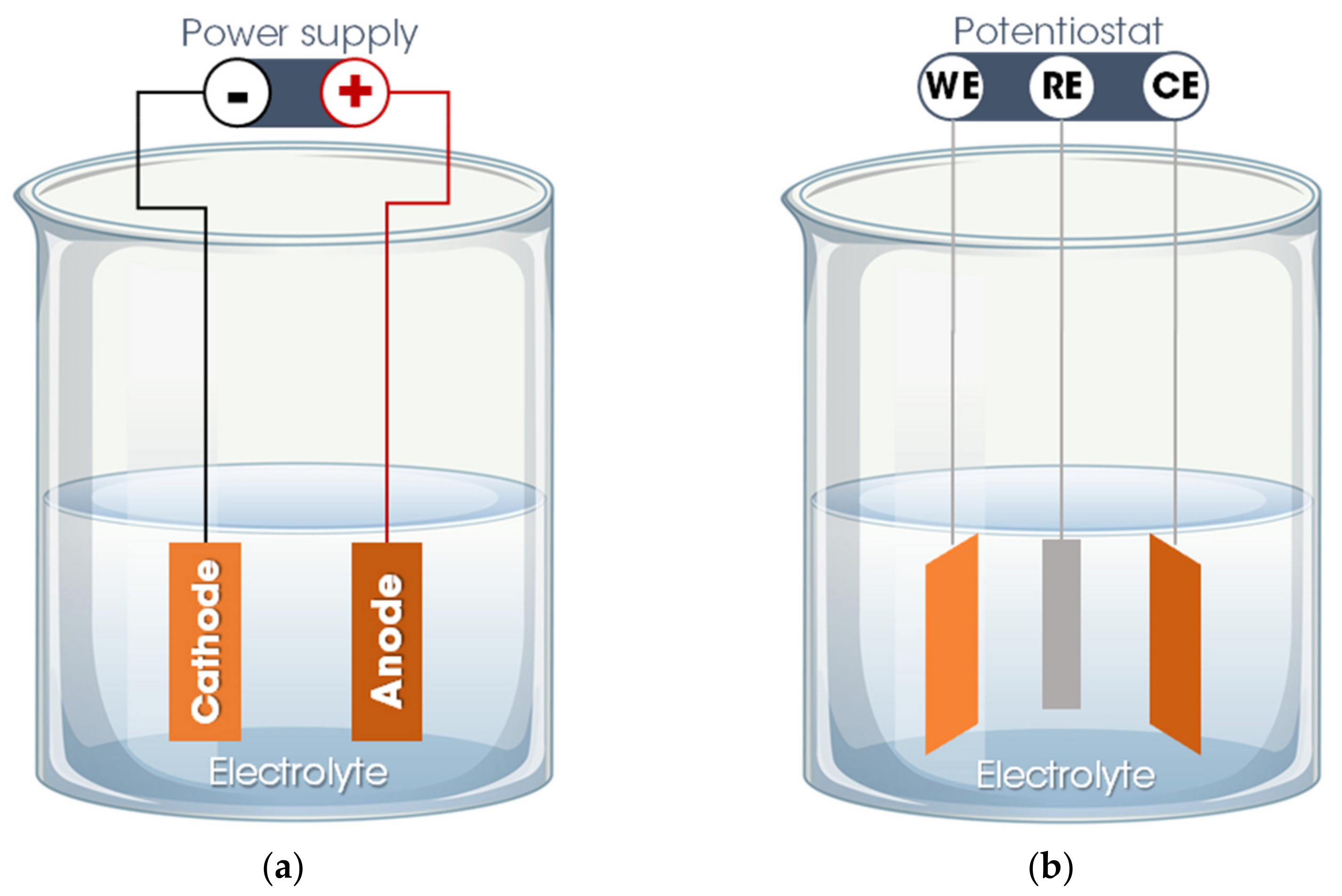
3.1. Method Overview
3.2. nHAp Electrodeposition onto Polymeric Nanofibers
4. Applications of Nanofibrous Scaffolds Coated with Electrodeposited nHAp
5. Final Considerations and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [Green Version]
- Rey, F.; Barzaghini, B.; Nardini, A.; Bordoni, M.; Zuccotti, G.V.; Cereda, C.; Raimondi, M.T.; Carelli, S. Advances in tissue engineering and innovative fabrication techniques for 3-d-structures: Translational applications in neurodegenerative diseases. Cells 2020, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Danie Kingsley, J.; Ranjan, S.; Dasgupta, N.; Saha, P. Nanotechnology for tissue engineering: Need, techniques and applications. J. Pharm. Res. 2013, 7, 200–204. [Google Scholar] [CrossRef]
- Stocco, T.D.; Bassous, N.J.; Zhao, S.; Granato, A.E.C.; Webster, T.J.; Lobo, A.O. Nanofibrous scaffolds for biomedical applications. Nanoscale 2018, 10, 12228–12255. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef]
- Lowe, B.; Hardy, J.G.; Walsh, L.J. Optimizing nanohydroxyapatite nanocomposites for bone tissue engineering. ACS Omega 2020, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Kim, S.-K. Nano-Hydroxyapatite composite biomaterials for bone tissue engineering—A review. J. Biomed. Nanotechnol. 2014, 10, 3124–3140. [Google Scholar] [CrossRef]
- Seethalakshmi, K.; Kaviya, M.; Venkatachalapathy, B.; Mubeena, S.; Punnoose, A.M.; Sridhar, T.M. Nanohydroxyapatite-doped polycaprolactone-based nanoscaffolds as a viable drug delivery agent in bone tissue engineering. J. Mater. Res. 2021, 36, 420–430. [Google Scholar] [CrossRef]
- Stastna, E.; Castkova, K.; Rahel, J. Influence of hydroxyapatite nanoparticles and surface plasma treatment on bioactivity of polycaprolactone nanofibers. Polymers 2020, 12, 1877. [Google Scholar] [CrossRef]
- Ao, C.; Niu, Y.; Zhang, X.; He, X.; Zhang, W.; Lu, C. Fabrication and characterization of electrospun cellulose/nano-hydroxyapatite nanofibers for bone tissue engineering. Int. J. Biol. Macromol. 2017, 97, 568–573. [Google Scholar] [CrossRef]
- Santana-Melo, G.F.; Rodrigues, B.V.M.; da Silva, E.; Ricci, R.; Marciano, F.R.; Webster, T.J.; Vasconcellos, L.M.R.; Lobo, A.O. Electrospun ultrathin PBAT/nHAp fibers influenced the in vitro and in vivo osteogenesis and improved the mechanical properties of neoformed bone. Colloids Surf. B 2017, 155, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Sun, T.; Sultana, N. Fabrication of nanohydroxyapatite/poly(caprolactone) composite microfibers using electrospinning technique for tissue engineering applications. J. Nanomater. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lobo, A.O.; Corat, M.A.F.; Ramos, S.C.; Matsushima, J.T.; Granato, A.E.C.; Pacheco-Soares, C.; Corat, E.J. Fast preparation of hydroxyapatite/superhydrophilic vertically aligned multiwalled carbon nanotube composites for bioactive application. Langmuir 2010, 26, 18308–18314. [Google Scholar] [CrossRef] [PubMed]
- Zanin, H.; Saito, E.; Marciano, F.R.; Ceragioli, H.J.; Campos Granato, A.E.; Porcionatto, M.; Lobo, A.O. Fast preparation of nano-hydroxyapatite/superhydrophilic reduced graphene oxide composites for bioactive applications. J. Mater. Chem. B 2013, 1, 4947. [Google Scholar] [CrossRef] [PubMed]
- Idaszek, J.; Kijeńska, E.; Łojkowski, M.; Swieszkowski, W. How important are scaffolds and their surface properties in regenerative medicine. Appl. Surf. Sci. 2016, 388, 762–774. [Google Scholar] [CrossRef]
- He, C.; Jin, X.; Ma, P.X. Calcium phosphate deposition rate, structure and osteoconductivity on electrospun poly(l-lactic acid) matrix using electrodeposition or simulated body fluid incubation. Acta Biomater. 2014, 10, 419–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.; Chang, J. Structure and properties of hydroxyapatite for biomedical applications. In Hydroxyapatite (Hap) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–19. [Google Scholar]
- Sossa, P.A.F.; Giraldo, B.S.; Garcia, B.C.G.; Parra, E.R.; Arango, P.J.A. Comparative study between natural and synthetic Hydroxyapatite: Structural, morphological and bioactivity properties. Matéria 2018, 23. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.S.; Waghmare, U.V.; Ramamurty, U. First-principles study of structure, vibrational, and elastic properties of stoichiometric and calcium-deficient hydroxyapatite. Cryst. Growth Des. 2014, 14, 3131–3141. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, present, and future in bone regeneration. Bone Tissue Regen. Insights 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.J.; Lee, E.C.; Kwon, G.J.; Seo, Y.K. The effect of coated nano-hydroxyapatite concentration on scaffolds for osteogenesis. J. Biomater. Appl. 2020, 34, 827–839. [Google Scholar] [CrossRef]
- Yousefi, A.-M.; Oudadesse, H.; Akbarzadeh, R.; Wers, E.; Lucas-Girot, A. Physical and biological characteristics of nanohydroxyapatite and bioactive glasses used for bone tissue engineering. Nanotechnol. Rev. 2014, 3. [Google Scholar] [CrossRef]
- Galindo, T.G.P.; Chai, Y.; Tagaya, M. Hydroxyapatite nanoparticle coating on polymer for constructing effective biointeractive interfaces. J. Nanomater. 2019, 2019, 6495239. [Google Scholar] [CrossRef] [Green Version]
- Padilla, S.; Benito-Garzón, L.; Enciso Sanz, S.; Garzón-Gutiérrez, A.; García Carrodeguas, R.; Rodríguez, M.A.; Garcia de Castro, A.; Canillas, M. Novel osteoinductive and osteogenic scaffolds of monetite, amorphous calcium phosphate, hydroxyapatite, and silica gel: Influence of the hydroxyapatite/monetite ratio on their in vivo behavior and on their physical and chemical properties. ACS Biomater. Sci. Eng. 2020, 6, 3440–3453. [Google Scholar] [CrossRef]
- Ripamonti, U.; Richter, P.W.; Nilen, R.W.N.; Renton, L. The induction of bone formation by smart biphasic hydroxyapatite tricalcium phosphate biomimetic matrices in the non-human primate Papio ursinus. J. Cell. Mol. Med. 2008, 12, 2609–2621. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Hydroxyapatite coatings for metallic implants. In Hydroxyapatite (Hap) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 143–157. [Google Scholar]
- Arcos, D.; Vallet-Regí, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Malmberg, P.; Bigdeli, N.; Jensen, J.; Nygren, H. Formation of hydroxyapatite on titanium implants in vivo precedes bone-formation during healing. Biointerphases 2017, 12, 041002. [Google Scholar] [CrossRef] [Green Version]
- Darimont, G.L.; Cloots, R.; Heinen, E.; Seidel, L.; Legrand, R. In vivo behaviour of hydroxyapatite coatings on titanium implants: A quantitative study in the rabbit. Biomaterials 2002, 23, 2569–2575. [Google Scholar] [CrossRef]
- Pan, J.; Prabakaran, S.; Rajan, M. In-vivo assessment of minerals substituted hydroxyapatite/poly sorbitol sebacate glutamate (PSSG) composite coating on titanium metal implant for orthopedic implantation. Biomed. Pharmacother. 2019, 119, 109404. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Yazdani, J.; Ahmadian, E.; Sharifi, S.; Shahi, S.; Maleki Dizaj, S. A short view on nanohydroxyapatite as coating of dental implants. Biomed. Pharmacother. 2018, 105, 553–557. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Guo, B.; Wang, X.; Fan, H.; Zhang, X. Fabrication and cellular biocompatibility of porous carbonated biphasic calcium phosphate ceramics with a nanostructure. Acta Biomater. 2009, 5, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, M.; Chen, F.; Wei, Y.; Chen, X.; Zhou, Y.; Yang, X.; Zhu, X.; Tu, C.; Zhang, X. Nano-hydroxyapatite coating promotes porous calcium phosphate ceramic-induced osteogenesis via BMP/smad signaling pathway. Int. J. Nanomed. 2019, 14, 7987–8000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Lin, K.; Xia, L.; Gan, J.; Zhang, Z.; Chen, H.; Jiang, X.; Chang, J. Tailoring the nanostructured surfaces of hydroxyapatite bioceramics to promote protein adsorption, osteoblast growth, and osteogenic differentiation. ACS Appl. Mater. Interfaces 2013, 5, 8008–8017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Herten, M.; Rothamel, D.; Schwarz, F.; Friesen, K.; Koegler, G.; Becker, J. Surface- and nonsurface-dependent in vitro effects of bone substitutes on cell viability. Clin. Oral Investig. 2009, 13, 149–155. [Google Scholar] [CrossRef]
- Webster, T. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, Y.; Huang, L.; Liu, J.; Lu, H. Effect of nano-hydroxyapatite coating on the osteoinductivity of porous biphasic calcium phosphate ceramics. BMC Musculoskelet. Disord. 2014, 15, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants. Surf. Coatings Technol. 2011, 205, 2785–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahi, M.; Nadari, M.; Sahmani, M.; Seyedjafari, E.; Ahmadbeigi, N.; Peymani, A. Osteoconduction of unrestricted somatic stem cells on an electrospun polylactic-co-glycolic acid scaffold coated with nanohydroxyapatite. Cells Tissues Organs 2018, 205, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, J.; Fortunato, G.; Woźniak, B.; Chodara, A.; Domaschke, S.; Męczyńska-Wielgosz, S.; Kruszewski, M.; Dommann, A.; Łojkowski, W. Polymer membranes sonocoated and electrosprayed with nano-hydroxyapatite for periodontal tissues regeneration. Nanomaterials 2019, 9, 1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grinet, M.A.V.M.; Zanin, H.; Campos Granato, A.E.; Porcionatto, M.; Marciano, F.R.; Lobo, A.O. Fast preparation of free-standing nanohydroxyapatite–vertically aligned carbon nanotube scaffolds. J. Mater. Chem. B 2014, 2, 1196. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.L.; Perez, X.I.; Ciprano, J.; Freeman, C.O.U.; Goldstein, A.; Freeman, J. Three-dimensional porous trabecular scaffold exhibits osteoconductive behaviors in vitro. Regen. Eng. Transl. Med. 2020, 6, 241–250. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited hydroxyapatite-based biocoatings: Recent progress and future challenges. Coatings 2021, 11, 110. [Google Scholar] [CrossRef]
- Gurrappa, I.; Binder, L. Electrodeposition of nanostructured coatings and their characterization—A review. Sci. Technol. Adv. Mater. 2008, 9, 043001. [Google Scholar] [CrossRef]
- Li, T.-T.; Ling, L.; Lin, M.-C.; Peng, H.-K.; Ren, H.-T.; Lou, C.-W.; Lin, J.-H. Recent advances in multifunctional hydroxyapatite coating by electrochemical deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Sobha Jayakrishnan, D. Electrodeposition: The versatile technique for nanomaterials. In Corrosion Protection and Control Using Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2012; pp. 86–125. [Google Scholar]
- Li, T.-T.; Ling, L.; Lin, M.-C.; Jiang, Q.; Lin, Q.; Lin, J.-H.; Lou, C.-W. Properties and mechanism of hydroxyapatite coating prepared by electrodeposition on a braid for biodegradable bone scaffolds. Nanomaterials 2019, 9, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotrut, C.M.; Vladescu, A.; Dinu, M.; Vranceanu, D.M. Influence of deposition temperature on the properties of hydroxyapatite obtained by electrochemical assisted deposition. Ceram. Int. 2018, 44, 669–677. [Google Scholar] [CrossRef]
- Vladescu, A.; Vranceanu, D.M.; Kulesza, S.; Ivanov, A.N.; Bramowicz, M.; Fedonnikov, A.S.; Braic, M.; Norkin, I.A.; Koptyug, A.; Kurtukova, M.O.; et al. Influence of the electrolytes pH on the properties of electrochemically deposited hydroxyapatite coating on additively manufactured Ti64 alloy. Sci. Rep. 2017, 7, 16819. [Google Scholar] [CrossRef]
- Fornell, J.; Feng, Y.P.; Pellicer, E.; Suriñach, S.; Baró, M.D.; Sort, J. Mechanical behaviour of brushite and hydroxyapatite coatings electrodeposited on newly developed FeMnSiPd alloys. J. Alloys Compd. 2017, 729, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhu, S.; Wang, L.; Feng, Y.; Ma, X.; Guan, S. Formation mechanism of Ca-deficient hydroxyapatite coating on Mg–Zn–Ca alloy for orthopaedic implant. Appl. Surf. Sci. 2014, 307, 92–100. [Google Scholar] [CrossRef]
- Ling, L.; Li, T.-T.; Lin, M.-C.; Jiang, Q.; Ren, H.-T.; Lou, C.-W.; Lin, J.-H. Effect of hydrogen peroxide concentration on the nanostructure of hydroxyapatite coatings via ultrasonic-assisted electrodeposition. Mater. Lett. 2020, 261, 126989. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mohammadi, I.; Sadrnezhaad, S.K. Hydroxyapatite based and anodic Titania nanotube biocomposite coatings: Fabrication, characterization and electrochemical behavior. Surf. Coatings Technol. 2016, 287, 67–75. [Google Scholar] [CrossRef]
- Han, Y.; Fu, T.; Lu, J.; Xu, K. Characterization and stability of hydroxyapatite coatings prepared by an electrodeposition and alkaline-treatment process. J. Biomed. Mater. Res. 2001, 54, 96–101. [Google Scholar] [CrossRef]
- Chakraborty, R.; Sengupta, S.; Saha, P.; Das, K.; Das, S. Synthesis of calcium hydrogen phosphate and hydroxyapatite coating on SS316 substrate through pulsed electrodeposition. Mater. Sci. Eng. C 2016, 69, 875–883. [Google Scholar] [CrossRef]
- Xue, J.; Farris, A.; Wang, Y.; Yeh, W.; Romany, C.; Guest, J.K.; Grayson, W.L.; Hall, A.S.; Weihs, T.P. Electrodeposition of hydroxyapatite on a metallic 3D-woven bioscaffold. Coatings 2020, 10, 715. [Google Scholar] [CrossRef]
- Doustgani, A. Effect of electrospinning process parameters of polycaprolactone and nanohydroxyapatite nanocomposite nanofibers. Text. Res. J. 2015, 85, 1445–1454. [Google Scholar] [CrossRef]
- Fu, S.; Ni, P.; Wang, B.; Chu, B.; Peng, J.; Zheng, L.; Zhao, X.; Luo, F.; Wei, Y.; Qian, Z. In vivo biocompatibility and osteogenesis of electrospun poly(ε-caprolactone)–poly(ethylene glycol)–poly(ε-caprolactone)/nano-hydroxyapatite composite scaffold. Biomaterials 2012, 33, 8363–8371. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Chandra, V.; Bhonde, R.R.; Soni, V.P.; Bellare, J.R. Mineralization of nanohydroxyapatite on electrospun poly(L-lactic acid)/gelatin by an alternate soaking process: A biomimetic scaffold for bone regeneration. J. Bioact. Compat. Polym. 2012, 27, 356–374. [Google Scholar] [CrossRef]
- Tong, H.-W.; Wang, M.; Li, Z.-Y.; Lu, W.W. Electrospinning, characterization and in vitro biological evaluation of nanocomposite fibers containing carbonated hydroxyapatite nanoparticles. Biomed. Mater. 2010, 5, 054111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanin, H.; Rosa, C.M.R.; Eliaz, N.; May, P.W.; Marciano, F.R.; Lobo, A.O. Assisted deposition of nano-hydroxyapatite onto exfoliated carbon nanotube oxide scaffolds. Nanoscale 2015, 7, 10218–10232. [Google Scholar] [CrossRef]
- De Castro, J.G.; Rodrigues, B.V.M.; Ricci, R.; Costa, M.M.; Ribeiro, A.F.C.; Marciano, F.R.; Lobo, A.O. Designing a novel nanocomposite for bone tissue engineering using electrospun conductive PBAT/polypyrrole as a scaffold to direct nanohydroxyapatite electrodeposition. RSC Adv. 2016, 6, 32615–32623. [Google Scholar] [CrossRef]
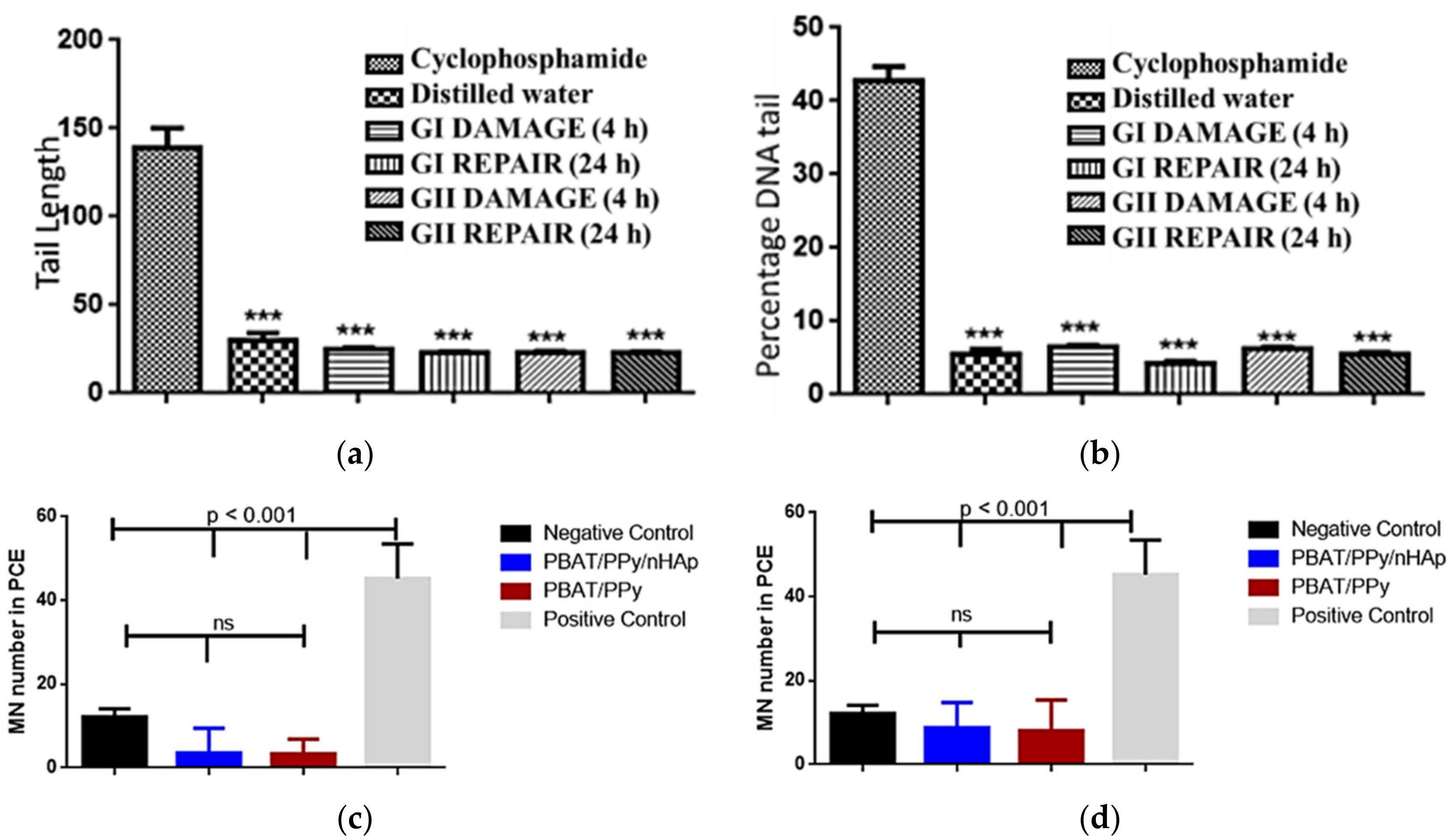
- De Maria Vaz Elias, C.; Maia Filho, A.L.M.; da Silva, L.R.; de Moura do Amaral, F.P.; Webster, T.J.; Marciano, F.R.; Lobo, A.O. In vivo evaluation of the genotoxic effects of poly (butylene adipate-co-terephthalate)/polypyrrole with nanohydroxyapatite scaffolds for bone regeneration. Materials 2019, 12, 1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
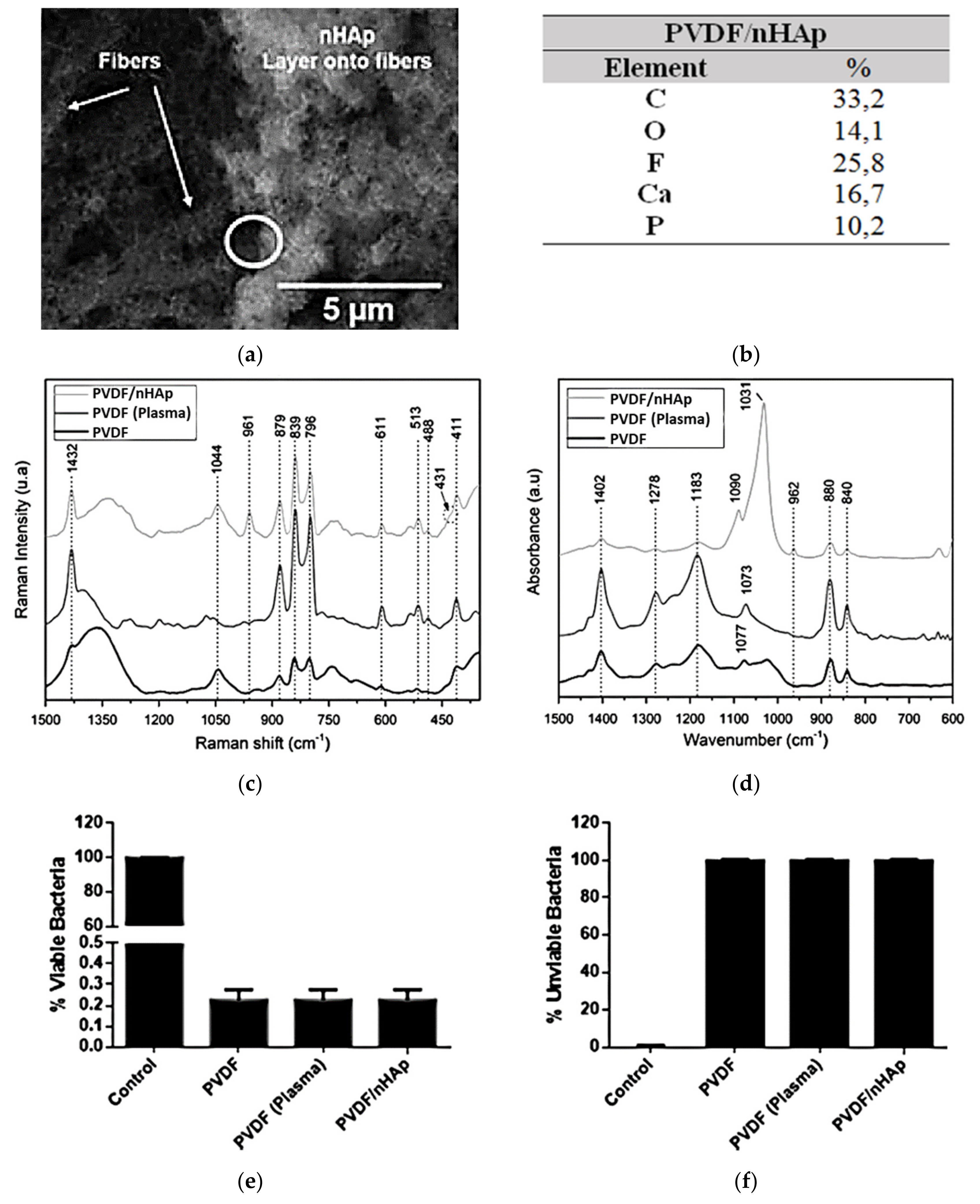
- Rodrigues, P.J.G.; de Elias M.V., C.; Viana, B.C.; de Hollanda, L.M.; Stocco, T.D.; de Vasconcellos, L.M.R.; de Mello C.R., D.; Santos, F.E.P.; Marciano, F.R.; Lobo, A.O. Electrodeposition of bactericidal and bioactive nano-hydroxyapatite onto electrospun piezoelectric polyvinylidene fluoride scaffolds. J. Mater. Res. 2020, 35, 3265–3275. [Google Scholar] [CrossRef]
- Molino, G.; Palmieri, M.C.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C. Biomimetic and mesoporous nano-hydroxyapatite for bone tissue application: A short review. Biomed. Mater. 2020, 15, 022001. [Google Scholar] [CrossRef]
- Xin, Y.; Zhu, J.; Sun, H.; Xu, Y.; Liu, T.; Qian, C. A brief review on piezoelectric PVDF nanofibers prepared by electrospinning. Ferroelectrics 2018, 526, 140–151. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Electrospun polyvinylidene fluoride-based fibrous scaffolds with piezoelectric characteristics for bone and neural tissue engineering. Nanomaterials 2019, 9, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szewczyk, P.K.; Metwally, S.; Karbowniczek, J.E.; Marzec, M.M.; Stodolak-Zych, E.; Gruszczyński, A.; Bernasik, A.; Stachewicz, U. Surface-potential-controlled cell proliferation and collagen mineralization on electrospun polyvinylidene fluoride (PVDF) fiber scaffolds for bone regeneration. ACS Biomater. Sci. Eng. 2019, 5, 582–593. [Google Scholar] [CrossRef]
- More, N.; Kapusetti, G. Piezoelectric material—A promising approach for bone and cartilage regeneration. Med. Hypotheses 2017, 108, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Pärssinen, J.; Sencadas, V.; Correia, V.; Miettinen, S.; Hytönen, V.P.; Lanceros-Méndez, S. Dynamic piezoelectric stimulation enhances osteogenic differentiation of human adipose stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 2172–2175. [Google Scholar] [CrossRef] [Green Version]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-J.; Kim, H.-W.; Lee, J.-H. Electrospun nanofibers applications in dentistry. J. Nanomater. 2016, 2016, 5931946. [Google Scholar] [CrossRef]
- Berton, F.; Porrelli, D.; Di Lenarda, R.; Turco, G. A critical review on the production of electrospun nanofibres for guided bone regeneration in oral surgery. Nanomaterials 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Electrodeposition Parameters | Values for the Deposition of nHAp in Polymeric Nanofibers |
| Electrodeposition mode | Potentiostatic |
| Calcium precursor/concentration | Ca(NO3)2·4H2O/0.042 mol L–1 |
| Phosphate precursor/concentration | NH4H2PO4/0.025 mol L–1 |
| Ca/P ratio | 1.67 |
| Temperature | ~70 °C |
| pH value | 4.7–5.0 |
| Deposition time | 30 min |
| Potential | −3.8 V |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stocco, T.D.; Rodrigues, P.J.G.; de Almeida Filho, M.A.; Lobo, A.O. Nanohydroxyapatite Electrodeposition onto Electrospun Nanofibers: Technique Overview and Tissue Engineering Applications. Bioengineering 2021, 8, 151. https://doi.org/10.3390/bioengineering8110151
Stocco TD, Rodrigues PJG, de Almeida Filho MA, Lobo AO. Nanohydroxyapatite Electrodeposition onto Electrospun Nanofibers: Technique Overview and Tissue Engineering Applications. Bioengineering. 2021; 8(11):151. https://doi.org/10.3390/bioengineering8110151
Chicago/Turabian StyleStocco, Thiago Domingues, Pedro José Gomes Rodrigues, Mauricio Augusto de Almeida Filho, and Anderson Oliveira Lobo. 2021. "Nanohydroxyapatite Electrodeposition onto Electrospun Nanofibers: Technique Overview and Tissue Engineering Applications" Bioengineering 8, no. 11: 151. https://doi.org/10.3390/bioengineering8110151
APA StyleStocco, T. D., Rodrigues, P. J. G., de Almeida Filho, M. A., & Lobo, A. O. (2021). Nanohydroxyapatite Electrodeposition onto Electrospun Nanofibers: Technique Overview and Tissue Engineering Applications. Bioengineering, 8(11), 151. https://doi.org/10.3390/bioengineering8110151







