Quantification of the Rupture Potential of Patient-Specific Intracranial Aneurysms under Contact Constraints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Clinical Information
2.2. Governing Model Equations
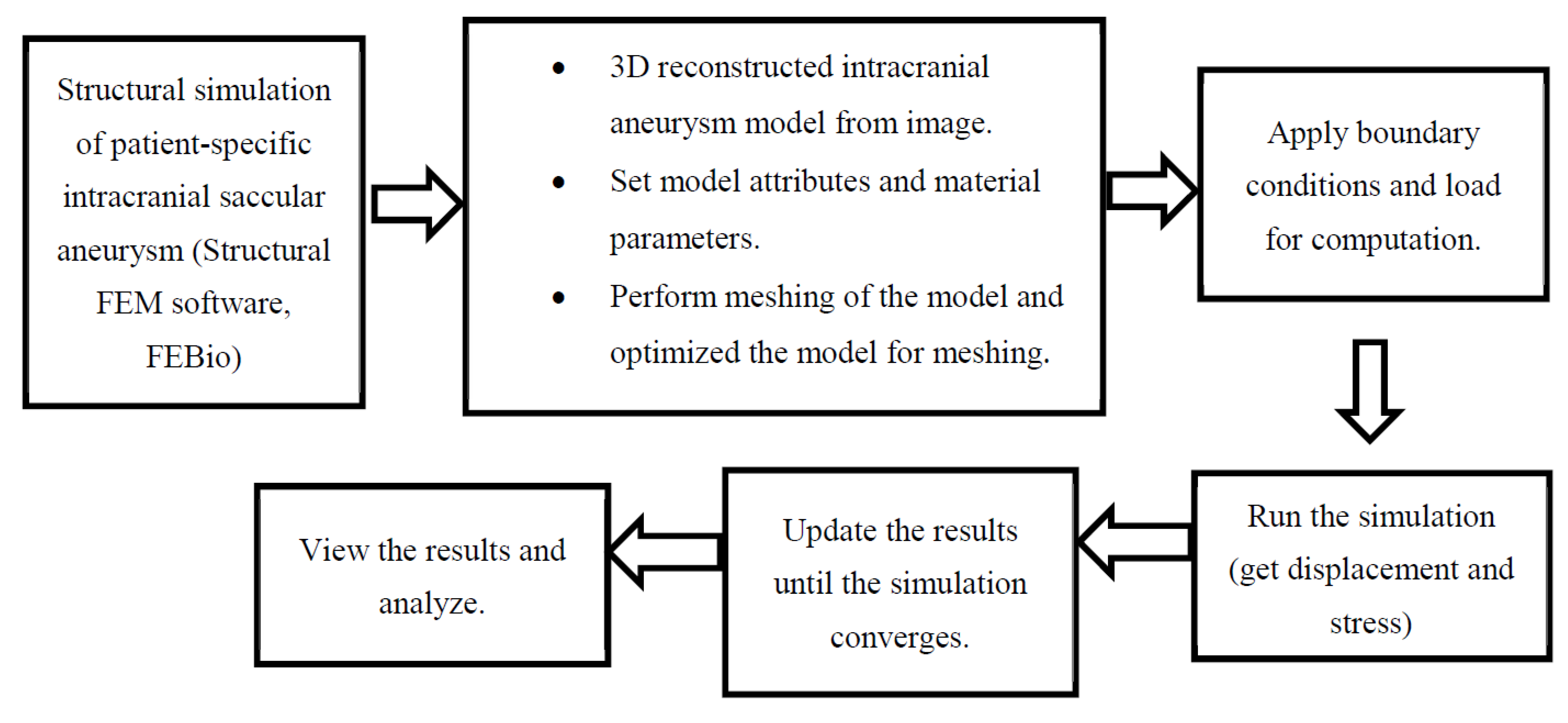
2.3. Computational Simulation
2.4. Boundary Conditions and Loads
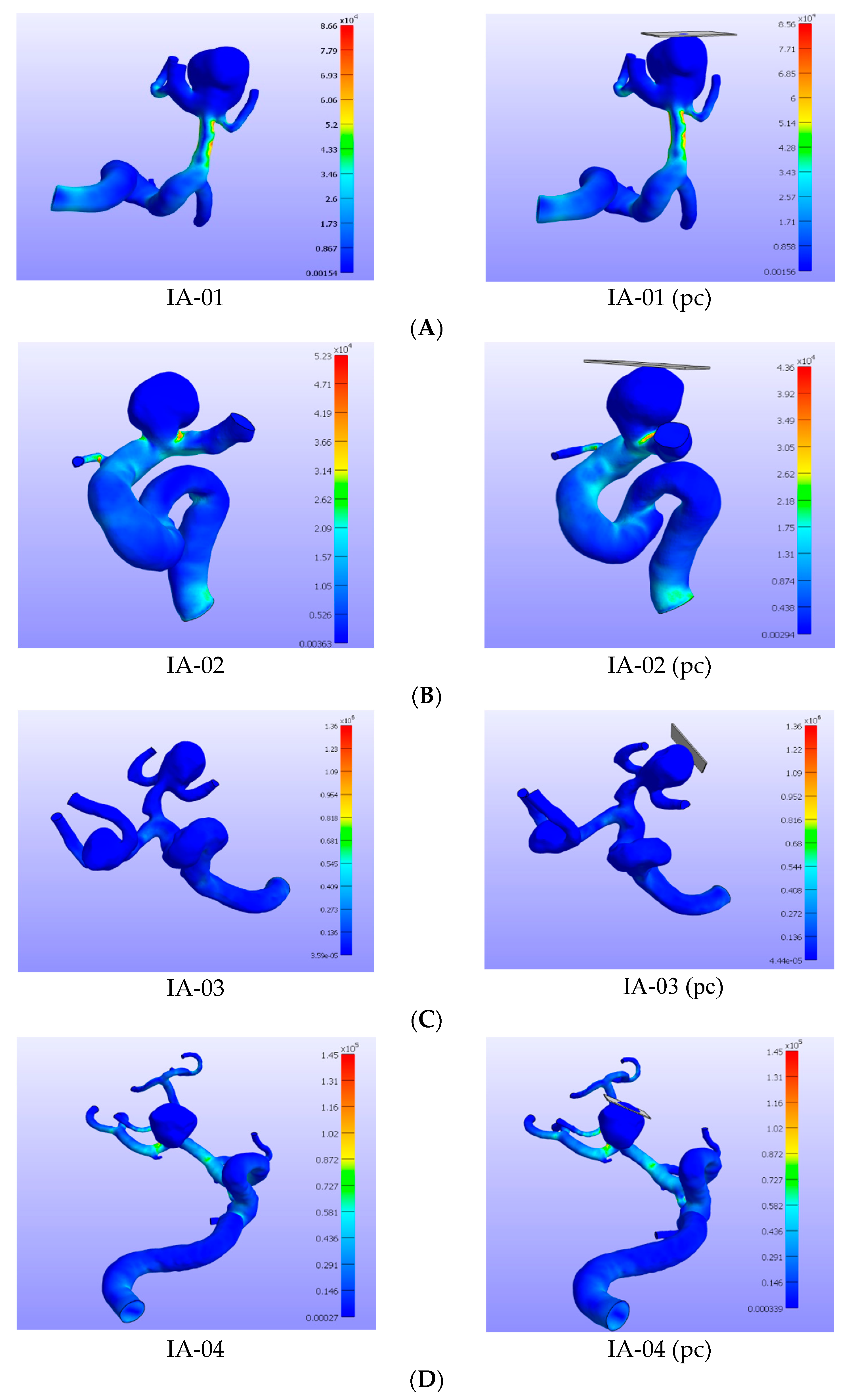
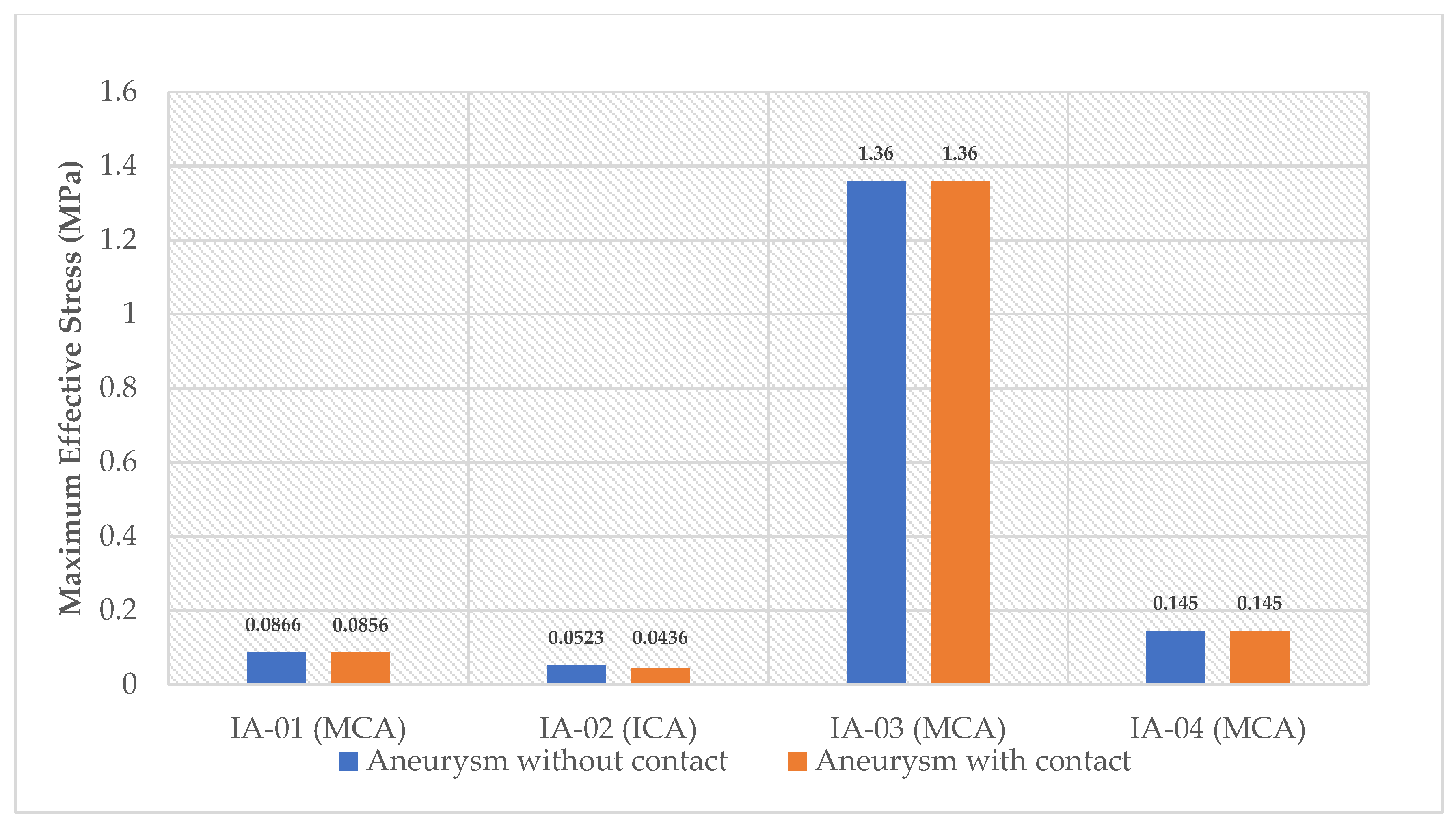
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stehbens, W.E. Pathology of the Cerebral Blood Vessels; C.V. Mosby: St. Louis, MO, USA, 1972; pp. 1–661. [Google Scholar]
- Cebral, J.R.; Castro, M.A.; Burgess, J.E.; Pergolizzi, R.S.; Sheridan, M.J.; Putman, C.M. Characterization of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. AJNR Am. J. Neuroradiol. 2005, 26, 2550–2559. [Google Scholar]
- Alberico, R.A.; Patel, M.; Casey, S.; Jacobs, B.; Maguire, W.; Decker, R. Evaluation of the circle of Willis with three-dimensional CT angiography in patients with suspected intracranial aneurysms. AJNR Am. J. Neuroradiol. 1995, 16, 1571–1578. [Google Scholar]
- Kayembe, K.N.; Sasahara, M.; Hazama, F. Cerebral aneurysms and variations in the circle of Willis. Stroke 1984, 15, 846–850. [Google Scholar] [CrossRef] [Green Version]
- Chien, A.; Castro, M.A.; Tateshima, S.; Sayre, J.; Cebral, J.; Vinuela, F. Quantitative Hemodynamic Analysis of Brain Aneurysms at Different Locations. Am. J. Neuroradiol. 2009, 30, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Juvela, S.; Porras, M.; Poussa, K. Natural history of unruptured intracranial aneurysms: Probability of and risk factors for aneurysm rupture. J. Neurosurg. 2000, 93, 379–387. [Google Scholar] [CrossRef]
- Morita, A.; Fujiwara, S.; Hashi, K.; Ohtsu, H.; Kirino, T. Risk of rupture associated with intact cerebral aneurysms in the Japanese population: A systematic review of the literature from Japan. J. Neurosurg. 2005, 102, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Stein, J.; Shafqat, S.; Eskey, C.; Doherty, D.; Chang, Y.; Kurina, A.; Furie, K.L. Functional recovery after rehabilitation for cerebellar stroke. Stroke 2001, 32, 530–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ropper, A.H.; Zervas, N.T. Outcome 1 year after SAH from cerebral aneurysm. Management morbidity, mortality, and functional status in 112 consecutive good-risk patients. J. Neurosurg. 1984, 60, 909–915. [Google Scholar] [CrossRef] [Green Version]
- The International Study of Unruptured Intracranial Aneurysms Investigator, Unruptured intracranial aneurysms-risk of rupture and risks of surgical intervention. N. Engl. J. Med. 1998, 339, 1725–1733. [CrossRef] [PubMed]
- Wanderer, S.; Andereggen, L.; Mrosek, J.; Kashefiolasl, S.; Marbacher, S.; Konczalla, J. The Role of Losartan as a Potential Neuroregenerative Pharmacological Agent after Aneurysmal Subarachnoid Haemorrhage. Int. J. Mol. Sci. 2020, 21, 6496. [Google Scholar] [CrossRef]
- Wanderer, S.; Andereggen, L.; Mrosek, J.; Kashefiolasl, S.; Schubert, G.A.; Schubert, M.; Konczalla, J. Levosimendan as a therapeutic strategy to prevent neuroinflammation after aneurysmal subarachnoid hemorrhage? J. NeuroInterventional Surg. 2021. [Google Scholar] [CrossRef]
- Broderick, J.P.; Brown, R.D.; Sauerbeck, L.; Hornung, R.; Huston, J.; Woo, D.; Anderson, C.; Rouleau, G.; Kleindorfer, D.; Flaherty, M.L.; et al. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke 2009, 40, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.; Eddleman, C.; Bendok, B.; Batjer, H. Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: Sifting through the sands of data. Neurosurg 2009, 26, e2. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.K. Intracranial Aneurysms and Subarachnoid Hemorrhage: An Overview; Wilkins, R.H., Rengachary, S.S., Eds.; Neurosurgery McGraw-Hill: New York, NY, USA, 1985; pp. 1308–1329. [Google Scholar]
- Cebral, J.R.; Raschi, M. Suggested connections between risk factors of intracranial aneurysms: A review. Ann. Biomed. Eng. 2013, 41, 1366–1383. [Google Scholar] [CrossRef] [PubMed]
- Seshaiyer, P.; Humphrey, J.D. On the potentially protective role of contact constraints on saccular aneurysms. J. Biomech. 2001, 34, 607–612. [Google Scholar] [CrossRef]
- Alam, M.; Seshaiyer, P. Quantification of the Rupture Potential of Intracranial Saccular Aneurysms under Contact Constraints. In Proceedings of the APS March Meeting, Los Angeles, CA, USA, 5–9 March 2018. Abstract: V46.00005. [Google Scholar]
- Alam, M.; Seshaiyer, P. Impact of Contact Constraints on the Dynamics of Idealized Intracranial Saccular Aneurysms. Bioengineering 2019, 6, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, A.M.; Duan, X.; Aziz, K.M.; Hill, M.R.; Watkins, S.C.; Cebral, J.R. Diversity in the Strength and Structure of Unruptured Cerebral Aneurysms. Ann. Biomed. Eng. 2015, 43, 1502–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, S.A.; Ellis, B.J.; Ateshian, G.A.; Weiss, J.A. FEBio: Finite elements for biomechanics. J. Biomech. Eng. 2012, 134. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Canham, P.B. Structure, mechanical properties, and mechanics of intracranial saccular aneurysms. J. Elast. 2000, 61, 49–81. [Google Scholar] [CrossRef]
- Kumar, N.; Rao, V.V. Hyper-elastic Mooney-Rivlin Model: Determination and Physical Interpretation of Material Constants. MIT Int. J. Mech. Eng. 2016, 6, 43–46. [Google Scholar]
- William, M.L. Structural analysis of viscoelastic materials. J. Am. Inst. Aero. Astro. 1964, 5, 785–808. [Google Scholar] [CrossRef]
- Mooney, M. A theory of large elastic deformation. J. Appl. Phys. 1940, 11, 582–592. [Google Scholar] [CrossRef]
- Simo, J.C.; Taylor, R.L. Quasi-incompressible finite elasticity in principal stretches: Continuum basis and numerical algorithms. Comput. Methods Appl. Mech. Eng. 1991, 85, 273–310. [Google Scholar] [CrossRef]
- Humphrey, J.D. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs; Springer: Verlag New York Inc.: New York, NY, USA, 2013; pp. 1–300. [Google Scholar]
- Voyiadjis, G.Z.; Kattan, P.I. Advance in Damage Mechanics: Metals and Metal Matrix Composites; Elsevier: London, UK, 1999; pp. 1–700. [Google Scholar]
- Ming-Shaung, J.U.; Chou-Ching, K.L.; Chang, C.T. Researches on biomechanical properties and models of peripheral nerves-a review. J. Biomech. Sc. Eng. 2017, 12, 16-00678. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhang, Q.; Jin, Y.; Gao, Z. Stress and strain analysis on the anastomosis site sutured with either epineurial or perineurial sutures after simulation of sciatic nerve injury. Neural Regen. Res. 2012, 7, 2299–2304. [Google Scholar] [PubMed]
- Beckmann, F.; Heise, K.; Kolsch, B.; Bonse, U.; Rajewsky, M.F.; Bartscher, M.; Biermann, T. Three-Dimensional Imaging of Nerve Tissue by X-Ray Phase-Contrast Microtomography. Biophys. J. 1999, 76, 98–102. [Google Scholar] [CrossRef]
| Aneurysm | Location of IA | Gender | Age | SAH | Family History of IA | Number of IA | Cigarette Smoker (Packs/Week) | Hypertension | Diabetic |
|---|---|---|---|---|---|---|---|---|---|
| IA-01 | MCA | F | 61 | No | Yes | 1 | Yes (10) | Yes | No |
| IA-02 | ICA | M | 63 | No | No | 2 | No | Yes | Yes |
| IA-03 | MCA | F | 69 | No | No | 2 | Yes (1) | No | No |
| IA-04 | MCA | F | 66 | No | No | 1 | Yes (7) | Yes | No |
| Aneurysm Domes | Ultimate Stress (MPa) | Ultimate Stretch | Young’s Modulus, E (MPa) | Bulk Modulus, K (MPa) | Poisson’s Ratio (ϑ) | ||
|---|---|---|---|---|---|---|---|
| IA-01 | 1.50 | 1.27 | 1.18 | 5.47 | 1.10 | 1.967 | 0.3 |
| IA-02 | 0.625 | 1.24 | 0.50 | 3.76 | 0.54 | 0.834 | 0.3 |
| IA-03 | 0.73 | 1.05 | 0.70 | 338.13 | 0.54 | 1.166 | 0.3 |
| IA-04 | 1.34 | 1.33 | 1.008 | 1.45 | 8.38 | 1.679 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.; Mut, F.; Cebral, J.R.; Seshaiyer, P. Quantification of the Rupture Potential of Patient-Specific Intracranial Aneurysms under Contact Constraints. Bioengineering 2021, 8, 149. https://doi.org/10.3390/bioengineering8110149
Alam M, Mut F, Cebral JR, Seshaiyer P. Quantification of the Rupture Potential of Patient-Specific Intracranial Aneurysms under Contact Constraints. Bioengineering. 2021; 8(11):149. https://doi.org/10.3390/bioengineering8110149
Chicago/Turabian StyleAlam, Manjurul, Fernando Mut, Juan R. Cebral, and Padmanabhan Seshaiyer. 2021. "Quantification of the Rupture Potential of Patient-Specific Intracranial Aneurysms under Contact Constraints" Bioengineering 8, no. 11: 149. https://doi.org/10.3390/bioengineering8110149
APA StyleAlam, M., Mut, F., Cebral, J. R., & Seshaiyer, P. (2021). Quantification of the Rupture Potential of Patient-Specific Intracranial Aneurysms under Contact Constraints. Bioengineering, 8(11), 149. https://doi.org/10.3390/bioengineering8110149







