Fundamental Biomaterial Considerations in the Development of a 3D Model Representative of Primary Open Angle Glaucoma
Abstract
1. Introduction
2. TM and eSC Biological Properties
3. Current Biomaterial Approaches to Modelling the TM/eSC
3.1. Biomaterial Composition
3.2. Topography
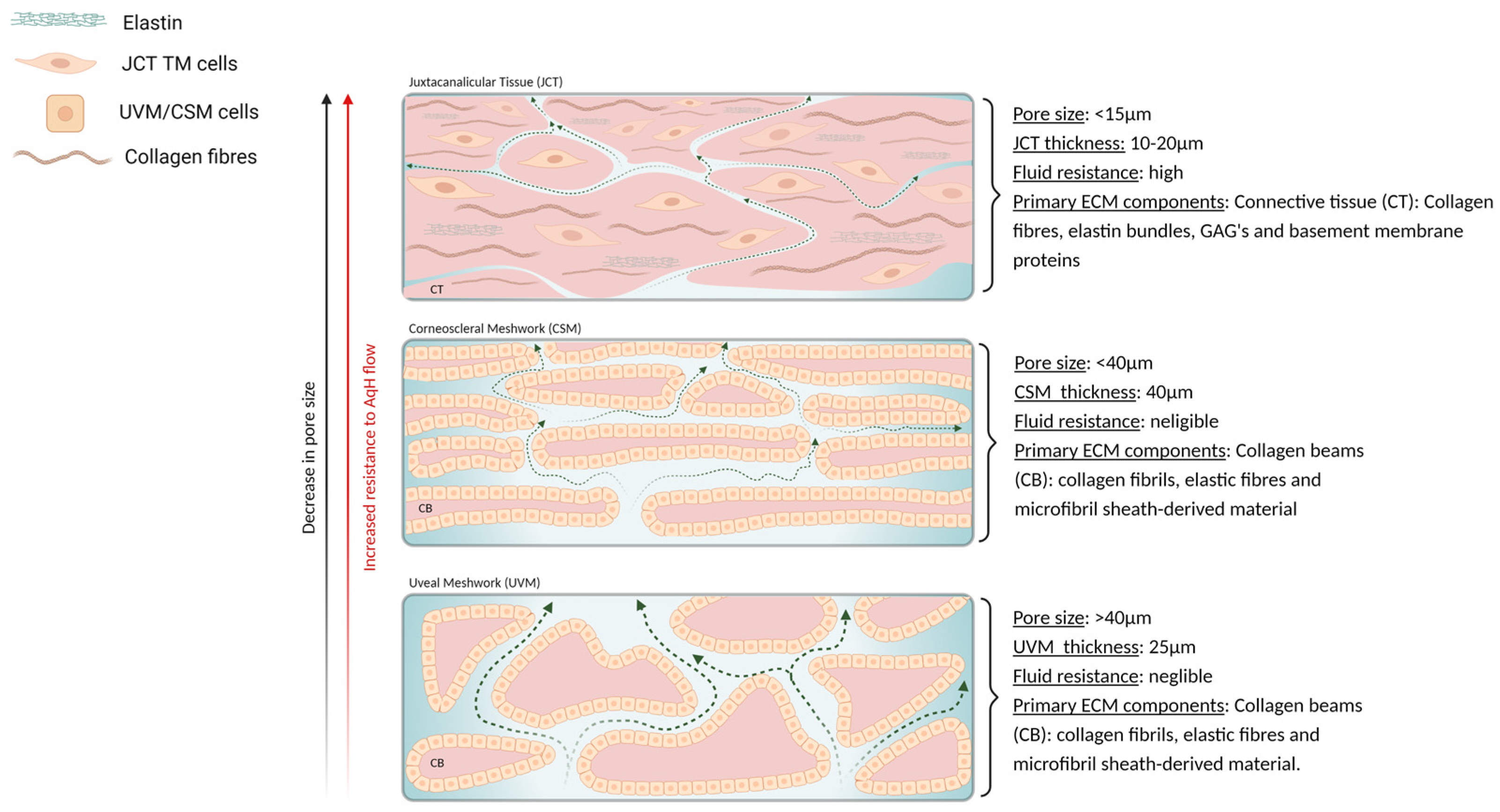
3.3. Porosity
3.4. Mechanical Properties
| Materials | Material Classification | Cells | In Vitro Model | Outputs Measured | Pros (P) and Cons (C) | Author(s) |
|---|---|---|---|---|---|---|
| MAX8B | Shear thinning peptide hydrogel | Human TM cells | 3D TM model drug testing platform | Cytoskeletal and ECM protein expression, cell viability, biomaterial stiffness and permeability | P: Fluid flow system, tuneable material properties, 3D culture, dynamic response C: Lack of ECM remodelling capacity | [18] |
| Type I Collagen + Hyaluronan + elastin like polypeptides | Protein/GAG/peptide--based hydrogel composite | Human TM cells | 3D TM/glaucomatous model drug testing platform | Cell proliferation, viability, hydrogel contraction analysis, scaffold microstructure, actin cytoskeleton formation, gene expression, fibronectin protein expression, elastic moduli | P: ECM remodelling capacity, dynamic response, tuneable material properties, 3D cell culture C: static fluid system | [20] |
| Poly acrylamide (PA) (Type I collagen coated) | Synthetic polymer | Human TM cells /human eSC | Matrix stiffness | Genetic expression, actin stress fibre formation, cell spreading, focal adhesion size, cellular contractility, subcortical stiffness, cellular biomechanics | P: Controlled cellular characteristics, controlled material properties C: 2D culture, lack of ECM remodelling capacity | [25,55,125,126] |
| SU-8 epoxy photoresist + 1% Gelatin | Epoxy-based polymer (negative photoresist) + protein -based hydrogel | Human TM cells/human eSC | TM outflow system/ glaucomatous TM outflow system | Cell viability, TM marker expression, ECM protein expression, actin cytoskeleton formation, phagocytosis assay, gene expression, outflow facility, material pore size | P: fluid flow system, topographical cues, co-culture C: Fixed material parameters, 2D culture | [31,103,104] |
| Type I collagen + chondroitin sulphate | Protein/GAG--based hydrogel composite | Porcine TM cells | TM model | Elastic moduli, material pore size, GAG quantification, gene expression, cell viability and proliferation, fibronectin gene expression, fibronectin protein expression | P: tuneable material properties, ECM remodelling capacity C: static fluid system, decreased biomaterial retention, 2D culture | [32] |
| Matrigel® | Basement membrane-derived extract | Human TM cells | 3D TM model drug testing platform/Gene manipulation | Gene expression; TM markers, stem cell markers, inflammatory cytokine markers, apoptotic markers. Actin cytoskeleton formation, cell viability, reactive oxygen species production | P: 3D culture, topographical cues, controlled cellular characteristics, fluid flow system C: Lack of ECM remodelling capacity, dynamic response and tuneable materials properties | [33,53,91,92] |
| Type I collagen + HA/HA+CS | Protein/GAG--based hydrogel composite | Human TM cells | TM model | Scaffold architecture, material pore size, GAG quantification, cell viability and proliferation, fibronectin gene expression, fibronectin protein expression | P: ECM remodelling capacity, tuneable material properties, topographical cues C: static fluid system, 2D culture, decreased biomaterial retention | [67] |
| Type I Collagen | Protein-based hydrogel | Human TM cells | Collagen contraction assays | Collagen contraction analysis, gene expression, protein expression, actin cytoskeleton formation, cell motility | P: ECM remodelling capacity, dynamic response, tuneable biomaterial properties C: static fluid system, 2D culture | [67,68,69,70,72,73] |
| SU-8 epoxy photoresist + Extracel™ | Epoxy-based polymer (negative photoresist) + protein/GAG -based hydrogel (Thiol-modified HA and gelatin) | Human eSC | eSC outflow system | Cell viability, cellular characteristics, genetic expression, ECM protein expression, material pore size | P: fluid flow system, controlled cellular characteristics C: Fixed material parameters | [88] |
| Poly (etherurethane) | polymeric elastomer | Human TM cells | Topographical cues/Gene manipulation | Expression of characteristic TM protein expression | P: tuneable material properties, topographical cues, controlled cellular characteristics C: 2D cultures, Lack of ECM remodelling capacity, dynamic response | [95,96] |
| Poly L-lactic + Polycaprolactone | Thermoplastic polyester + thermoplastic polyester | Human TM cells | Gene manipulation | Gene expression, cellular protein expression, scaffold microstructure, fibre diameter, | P: tuneable material properties, topographical cues, controlled cellular characteristics C: Lack of ECM remodelling capacity, dynamic response | [97] |
| Polydimethylsiloxane (PDMS) (Type I Collagen or Laminin) | Silicone-based organic polymer/elastomer | Human/bovine TM cells | Dynamic or static cyclic stress/strain | Genetic expression of cellular stress proteins, ECM proteins and ECM remodelling proteins | P:Controlled material properties C: lack ofECM remodelling capacity, 2D culture | [127,128,129,130,131,132,133,134,135] |
4. Future Perspectives and Potential Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leber, T. Studien über den flüssigkeitswechsel im auge. Graefes Arh. Für Ophthalmol. 1873, 19, 87–185. [Google Scholar] [CrossRef]
- Leber, T.; Pilzecker, A. Neue untersuchungen über den flüssigkeitswechsel des. Auges. Albrecht Von Graefes Arch. Ophthalmol. 1906, 64, 1–127. [Google Scholar] [CrossRef]
- Nathan, J. Hippocrates to Duke-Elder: An overview of the history of glaucoma. Clin. Exp. Optom. 2000, 83, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Connor, L. Remarks on the causes of glaucoma. J. Am. Med. Assoc. 1896, 27, 1037–1040. [Google Scholar] [CrossRef][Green Version]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Tamm, E.R. The trabecular meshwork outflow pathways: Structural and functional aspects. Exp. Eye Res. 2009, 88, 648–655. [Google Scholar] [CrossRef]
- Bill, A. The drainage of aqueous humor. Investig. Ophthalmol. Vis. Sci. 1975, 14, 1–3. [Google Scholar]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal models of glaucoma. J. Biomed. Biotechnol. 2012, 2012, 692609. [Google Scholar] [CrossRef]
- Evangelho, K.; Mastronardi, C.A.; de-la-Torre, A. Experimental models of glaucoma: A powerful translational tool for the future development of new therapies for glaucoma in humans—A review of the literature. Medicina 2019, 55, 280. [Google Scholar] [CrossRef]
- Bradley, J.; Vranka, J.; Colvis, C.M.; Conger, D.M.; Alexander, J.P.; Fisk, A.S.; Samples, J.R.; Acott, T.S. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2649–2658. [Google Scholar]
- Langley, G.R.; Adcock, I.M.; Busquet, F.; Crofton, K.M.; Csernok, E.; Giese, C.; Heinonen, T.; Herrmann, K.; Hofmann-Apitius, M.; Landesmann, B.; et al. Towards a 21st-century roadmap for biomedical research and drug discovery: Consensus report and recommendations. Drug Discov. Today 2017, 22, 327–339. [Google Scholar] [CrossRef]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H. Species similarities and differences in pharmacokinetics. Drug Metab. Dispos. 1995, 23, 1008–1021. [Google Scholar]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Waduthanthri, K.D.; He, Y.; Montemagno, C.; Cetinel, S. An injectable peptide hydrogel for reconstruction of the human trabecular meshwork. Acta Biomater. 2019, 100, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Ryskamp, D.A.; Frye, A.M.; Phuong, T.T.T.; Yarishkin, O.; Jo, A.O.; Xu, Y.; Lakk, M.; Iuso, A.; Redmon, S.N.; Ambati, B.; et al. TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci. Rep. 2016, 6, 30583. [Google Scholar] [CrossRef]
- Li, H.; Bagué, T.; Kirschner, A.; Strat, A.N.; Roberts, H.; Weisenthal, R.W.; Patteson, A.; Annabi, N.; Stamer, W.D.; Ganapathy, P.S.; et al. A tissue-engineered human trabecular meshwork hydrogel for advanced glaucoma disease modeling. Exp. Eye Res. 2021, 205, 1–18. [Google Scholar] [CrossRef]
- Lu, R.; Soden, P.A.; Lee, E. Tissue-engineered models for glaucoma research. Micromachines 2020, 11, 612. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef]
- Keller, K.E.; Acott, T.S. The juxtacanalicular region of ocular trabecular meshwork: A tissue with a unique extracellular matrix and specialized function. J. Ocul. Biol. 2013, 1, 3–18. [Google Scholar]
- Overby, D.R.; Stamer, W.D.; Johnson, M. The changing paradigm of outflow resistance generation: Towards synergistic models of the JCT and inner wall endothelium. Exp. Eye Res. 2009, 88, 656–670. [Google Scholar] [CrossRef]
- Overby, D.R.; Zhou, E.H.; Vargas-Pinto, R.; Pedrigi, R.M.; Fuchshofer, R.; Braakman, S.T.; Gupta, R.; Perkumas, K.M.; Sherwood, J.M.; Vahabikashi, A.; et al. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 13876–13881. [Google Scholar] [CrossRef]
- Acott, T.S.; Kelley, M.J. Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 2008, 86, 543–561. [Google Scholar] [CrossRef]
- Stamer, W.D.; Acott, T.S. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 2012, 23, 135–143. [Google Scholar] [CrossRef]
- Stamer, W.D.; Clark, A.F. The many faces of the trabecular meshwork cell. Exp. Eye Res. 2017, 158, 112–123. [Google Scholar] [CrossRef] [PubMed]
- WuDunn, D. Mechanobiology of trabecular meshwork cells. Exp. Eye Res. 2009, 88, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Acott, T.S.; Kelley, M.J.; Keller, K.E.; Vranka, J.A.; Abu-Hassan, D.W.; Li, X.; Aga, M.; Bradley, J.M. Intraocular pressure homeostasis: Maintaining balance in a high-pressure environment. J. Ocul. Pharmacol. Ther. 2014, 30, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Torrejon, K.Y.; Pu, D.; Bergkvist, M.; Danias, J.; Sharfstein, S.T.; Xie, Y. Recreating a human trabecular meshwork outflow system on microfabricated porous structures. Biotechnol. Bioeng. 2013, 110, 3205–3218. [Google Scholar] [CrossRef]
- Osmond, M.; Bernier, S.M.; Pantcheva, M.B.; Krebs, M.D. Collagen and collagen-chondroitin sulfate scaffolds with uniaxially aligned pores for the biomimetic, three dimensional culture of trabecular meshwork cells. Biotechnol. Bioeng. 2017, 114, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Tirendi, S.; Scarfì, S.; Passalacqua, M.; Oddone, F.; Traverso, C.E.; Vernazza, S.; Bassi, A.M. An advanced in vitro model to assess glaucoma onset. Altex 2020, 37, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Aga, M.; Bradley, J.M.; Kelley, M.J.; Acott, T.S. Extracellular matrix turnover and outflow resistance. Exp. Eye Res. 2009, 88, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Knepper, P.A.; Goossens, W.; Hvizd, M.; Palmberg, P.F. Glycosaminoglycans of the human trabecular meshwork in primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1360–1367. [Google Scholar]
- Knepper, P.A.; Goossens, W.; Palmberg, P.F. Glycosaminoglycan stratification of the juxtacanalicular tissue in normal and primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2414–2425. [Google Scholar]
- Keller, K.E.; Sun, Y.Y.; Yang, Y.-F.; Bradley, J.M.; Acott, T.S. Perturbation of hyaluronan synthesis in the trabecular meshwork and the effects on outflow facility. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4616–4625. [Google Scholar] [CrossRef]
- Keller, K.E.; Bradley, J.M.; Vranka, J.A.; Acott, T.S. Segmental versican expression in the trabecular meshwork and involvement in outflow facility. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5049–5057. [Google Scholar] [CrossRef]
- Abu-Hassan, D.W.; Acott, T.S.; Kelley, M.J. The trabecular meshwork: A basic review of form and function. J. Ocul. Biol. 2014, 2. Available online: http://fulltextarticles.avensonline.org/JOCB-2334–838-02-0017.html (accessed on 23 July 2021).
- Gonzalez, J.M.; Ko, M.K.; Pouw, A.; Tan, J.C.H. Tissue-based multiphoton analysis of actomyosin and structural responses in human trabecular meshwork. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- King, B.J.; Burns, S.A.; Sapoznik, K.A.; Luo, T.; Gast, T.J. High-Resolution, Adaptive Optics Imaging of the Human Trabecular Meshwork In Vivo. Transl. Vis. Sci. Technol. 2019, 8, 1–12. [Google Scholar] [CrossRef]
- Grant, W.M. Experimental aqueous perfusion in enucleated human eyes. Arch. Ophthalmol. 1963, 69, 783–801. [Google Scholar] [CrossRef]
- McEwen, W. Application of Poiseuille’s law to aqueous outflow. AMA Arch. Ophthalmol. 1958, 60, 290–294. [Google Scholar] [CrossRef]
- Friedman, N.J.; Kraiser, P.K.; Trattler, W.B. Review of Ophthalmology, 3rd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018; pp. 432–433. [Google Scholar]
- Mäepea, O.; Bill, A. Pressures in the juxtacanalicular tissue and Schlemm’s canal in monkeys. Exp. Eye Res. 1992, 54, 879–883. [Google Scholar] [CrossRef]
- Mäepea, O.; Bill, A. The pressures in the episcleral veins, Schlemm’s canal and the trabecular meshwork in monkeys: Effects of changes in intraocular pressure. Exp. Eye Res. 1989, 49, 645–663. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sagara, T.; Seki, K.; Hirano, S.; Nishida, T. Permissive effect of fibronectin on collagen gel contraction mediated by bovine trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4331–4336. [Google Scholar] [CrossRef]
- Marshall, G.E.; Konstas, A.G.; Lee, W.R. Immunogold localization of type IV collagen and laminin in the aging human outflow system. Exp. Eye Res. 1990, 51, 691–699. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Welge-Lüssen, U.; Lütjen-Drecoll, E.; Birke, M. Biochemical and morphological analysis of basement membrane component expression in corneoscleral and cribriform human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 794–801. [Google Scholar] [CrossRef]
- Ueda, J.; Wentz-Hunter, K.; Yue, B.Y.J.T. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1068–1076. [Google Scholar]
- Zhu, Q.; Zhang, Y.; Tighe, S.; Liu, Y.; Zhu, Y.; Hu, M. Human trabecular meshwork progenitors. Int. J. Med. Sci. 2019, 16, 704–710. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, S.; Tseng, S.C.G.; Zhu, Y.-T. Isolation and Expansion of Multipotent Progenitors from Human Trabecular Meshwork. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fautsch, M.P.; Howell, K.G.; Vrabel, A.M.; Charlesworth, M.C.; Muddiman, D.C.; Johnson, D.H. Primary trabecular meshwork cells incubated in human aqueous humor differ from cells incubated in serum supplements. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2848–2856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlunck, G.; Han, H.; Wecker, T.; Kampik, D.; Meyer-ter-Vehn, T.; Grehn, F. Substrate rigidity modulates cell–matrix interactions and protein expression in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Braakman, S.T.; Zhou, E.H.; Ethier, C.R.; Fredberg, J.J.; Overby, D.R.; Johnson, M. Biomechanics of Schlemm’s canal endothelium and intraocular pressure reduction. Prog. Retin. Eye Res. 2015, 44, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Chan, D.; Read, A.T.; Christensen, C.; Sit, A.; Ethier, C.R. The Pore Density in the Inner Wall Endothelium of Schlemm’s Canal of Glaucomatous Eyes. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2950–2955. [Google Scholar]
- Johnson, M.; Shapiro, A.; Ethier, C.R.; Kamm, R.D. Modulation of outflow resistance by the pores of the inner wall endothelium. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1670–1675. [Google Scholar]
- Tripathi, R.C. Mechanism of the aqueous outflow across the trabecular wall of Schlemm’s canal. Exp. Eye Res. 1971, 11, 116–121. [Google Scholar] [CrossRef]
- Pedrigi, R.M.; Simon, D.; Reed, A.; Stamer, W.D.; Overby, D.R. A model of giant vacuole dynamics in human Schlemm’s canal endothelial cells. Exp. Eye Res. 2011, 92, 57–66. [Google Scholar] [CrossRef]
- Ethier, C. The inner wall of Schlemm’s canal. Exp. Eye Res. 2002, 74, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Allingham, R.R.; de Kater, A.W.; Ethier, C.R.; Anderson, P.J.; Hertzmark, E.; Epstein, D.L. The relationship between pore density and outflow facility in human eyes. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1661–1669. [Google Scholar]
- Grinnell, F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003, 13, 264–269. [Google Scholar] [CrossRef]
- Amorim, S.; Reis, C.A.; Reis, R.L.; Pires, R.A. Extracellular Matrix Mimics Using Hyaluronan-Based Biomaterials. Trends Biotechnol. 2021, 39, 90–104. [Google Scholar] [CrossRef]
- Gagen, D.; Faralli, J.A.; Filla, M.S.; Peters, D.M. The role of integrins in the trabecular meshwork. J. Ocul. Pharmacol. Ther. 2014, 30, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Evanko, S.P.; Potter-Perigo, S.; Petty, L.J.; Workman, G.A.; Wight, T.N. Hyaluronan controls the deposition of fibronectin and collagen and modulates TGF-β1 induction of lung myofibroblasts. Matrix Biol. 2015, 42, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.J.; Krebs, M.D.; Pantcheva, M.B. Human trabecular meshwork cell behavior is influenced by collagen scaffold pore architecture and glycosaminoglycan composition. Biotechnol. Bioeng. 2020, 117, 3150–3159. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Hirano, S.; Suzuki, K.; Seki, K.; Sagara, T.; Nishida, T. Signaling Mechanism of TGF-β1–induced collagen contraction mediated by bovine trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3465–3472. [Google Scholar]
- Zhao, Y.; Zhu, H.; Yang, Y.; Ye, Y.; Yao, Y.; Huang, X.; Zhang, Y.; Shu, X.; Chen, X.; Yang, Y.; et al. AQP1 suppression by ATF4 triggers trabecular meshwork tissue remodelling in ET-1-induced POAG. J. Cell Mol. Med. 2020, 24, 3469–3480. [Google Scholar] [CrossRef]
- Dismuke, W.M.; Liang, J.; Overby, D.R.; Stamer, W.D. Concentration-related effects of nitric oxide and endothelin-1 on human trabecular meshwork cell contractility. Exp. Eye Res. 2014, 120, 28–35. [Google Scholar] [CrossRef]
- Stumpff, F.; Wiederholt, M. Regulation of trabecular meshwork contractility. Ophthalmologica 2000, 214, 33–53. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.; Huang, J.; Qiu, J.; Wu, J.; Yuan, F.; Epstein, D.L.; Gonzalez, P. Regulation of trabecular meshwork cell contraction and intraocular pressure by miR-200c. PLoS ONE 2012, 7, e51688. [Google Scholar] [CrossRef]
- Koga, T.; Koga, T.; Awai, M.; Tsutsui, J.-I.; Yue, B.Y.J.T.; Tanihara, H. Rho-associated protein kinase inhibitor, Y-27632, induces alterations in adhesion, contraction and motility in cultured human trabecular meshwork cells. Exp. Eye Res. 2006, 82, 362–370. [Google Scholar] [CrossRef]
- Knepper, P.A.; Farbman, A.; Telser, A. Aqueous outflow pathway glycosaminoglycans. Exp. Eye Res. 1981, 32, 265–277. [Google Scholar] [CrossRef]
- Binninger, E.A.; Schachtschabel, D.O.; Rohen, J.W. Exogenous glycosaminoglycans stimulate hyaluronic acid synthesis by cultured human trabecular-meshwork cells. Exp. Eye Res. 1987, 45, 169–177. [Google Scholar] [CrossRef]
- Berggren, L.; Vrabec, F. Demonstration of a coating substance in the trabecular meshwork of the eye: And its decrease after perfusion experiments with different kinds of hyaluronidase. Am. J. Ophthalmol. 1957, 44, 200–208. [Google Scholar] [CrossRef]
- Wolf, J. Inner surface of regions in the anterior chamber taking part in the regulation of the intraocular tension, including the demonstration of the covering viscous substance. Doc. Ophthalmol. 1968, 25, 113–149. [Google Scholar] [CrossRef]
- Lerner, L.E.; Polansky, J.R.; Howes, E.L.; Stern, R. Hyaluronan in the human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1222–1228. [Google Scholar]
- Knepper, P.A.; Collins, J.A.; Frederick, R. Effects of dexamethasone, progesterone, and testosterone on IOP and GAGs in the rabbit eye. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1093–1100. [Google Scholar]
- Ethier, C.; Kamm, R.; Palaszewski, B.; Johnson, M.; Richardson, T. Calculations of flow resistance in the juxtacanalicular meshwork. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1741–1750. [Google Scholar]
- Filla, M.S.; Dimeo, K.D.; Tong, T.; Peters, D.M. Disruption of fibronectin matrix affects type IV collagen, fibrillin and laminin deposition into extracellular matrix of human trabecular meshwork (HTM) cells. Exp. Eye Res. 2017, 165, 7–19. [Google Scholar] [CrossRef]
- Stamer, W.D.; Roberts, B.C.; Howell, D.N.; Epstein, D.L. Isolation, culture, and characterization of endothelial cells from Schlemm’s canal. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1804–1812. [Google Scholar]
- Jumper, M.D.; Stern, R.; Lui, G.M.; Formby, B.; Schwartz, D.M. Expression of CD44 isoforms in cultured human trabecular Meshwork Cells. Ophthalmic Res. 1998, 30, 314–320. [Google Scholar] [CrossRef]
- Acott, T.S.; Westcott, M.; Passo, M.S.; Van Buskirk, E.M. Trabecular meshwork glycosaminoglycans in human and cynomolgus monkey eye. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1320–1329. [Google Scholar]
- Mitra, A.K.; Arnott, S.; Sheehan, J.K. Hyaluronic acid: Molecular conformation and interactions in the tetragonal form of the potassium salt containing extended chains. J. Mol. Biol. 1983, 169, 813–827. [Google Scholar] [CrossRef]
- Barkalow, F.J.; Schwarzbauer, J.E. Interactions between fibronectin and chondroitin sulfate are modulated by molecular context. J. Biol. Chem. 1994, 269, 3957–3962. [Google Scholar] [CrossRef]
- Faralli, J.A.; Schwinn, M.K.; Gonzalez, J.M.; Filla, M.S.; Peters, D.M. Functional properties of fibronectin in the trabecular meshwork. Exp. Eye Res. 2009, 88, 689–693. [Google Scholar] [CrossRef]
- Dautriche, C.N.; Szymanski, D.; Kerr, M.; Torrejon, K.Y.; Bergkvist, M.; Xie, Y.; Danias, J.; Stamer, W.; Sharfstein, S.T. A biomimetic Schlemm’s canal inner wall: A model to study outflow physiology, glaucoma pathology and high-throughput drug screening. Biomaterials 2015, 65, 86–92. [Google Scholar] [CrossRef]
- Last, J.A.; Pan, T.; Ding, Y.; Reilly, C.M.; Keller, K.; Acott, T.S.; Fautsch, M.P.; Murphy, C.J.; Russell, P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2147–2152. [Google Scholar] [CrossRef]
- Johnson, M. What controls aqueous humour outflow resistance? Exp. Eye Res. 2006, 82, 545–557. [Google Scholar] [CrossRef]
- Vernazza, S.; Tirendi, S.; Scarfì, S.; Passalacqua, M.; Oddone, F.; Traverso, C.E.; Rizzato, I.; Bassi, A.M.; Sacca’, S. 2D- and 3D-cultures of human trabecular meshwork cells: A preliminary assessment of an in vitro model for glaucoma study. PLoS ONE 2019, 14, e0221942. [Google Scholar] [CrossRef]
- Bouchemi, M.; Roubeix, C.; Kessal, K.; Riancho, L.; Raveu, A.-L.; Soualmia, H.; Baudouin, C.; Brignole-Baudouin, F. Effect of benzalkonium chloride on trabecular meshwork cells in a new in vitro 3D trabecular meshwork model for glaucoma. Toxicol. In Vitro 2017, 41, 21–29. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Russell, P.; Gasiorowski, J.Z.; Nealy, P.F.; Murphy, C.J. Response of human trabecular meshwork cells to topographic cues on the nanoscale level. Investig. Ophthalmol. Vis. Sci. 2008, 49, 629–635. [Google Scholar] [CrossRef]
- Kim, B.; Roberts, C.J.; Mahmoud, A.M.; Grzybowski, D.M.; Weber, P.A.; Yi, Z. Topographic Effect of Micro/Nanoengineered Polymer Substrates on Cultured Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4666. [Google Scholar]
- Jedari, B.; Rahmani, A.; Naderi, M.; Nadri, S. MicroRNA-7 promotes neural differentiation of trabecular meshwork mesenchymal stem cell on nanofibrous scaffold. J. Cell Biochem. 2019, 121, 2818–2827. [Google Scholar] [CrossRef]
- Tamm, E.R.; Russell, P.; Epstein, D.L.; Johnson, D.H.; Piatigorsky, J. Modulation of Myocilin/TIGR expression in human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2577–2582. [Google Scholar]
- Spencer, W.; Alvarado, J.; Hayes, T. Scanning electron microscopy of human ocular tissues: Trabecular meshwork. Investig. Ophthalmol. 1968, 7, 651–662. [Google Scholar]
- Fisher, O.Z.; Khademhosseini, A.; Langer, R.; Peppas, N.A. Bioinspired materials for controlling stem cell fate. Acc. Chem. Res. 2010, 43, 419–428. [Google Scholar] [CrossRef]
- Kim, E.; Tae, G. Direct reprogramming and biomaterials for controlling cell fate. Biomater. Res. 2016, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Coroneo, M.; Korbmacher, C.; Flügel, C.; Stiemer, B.; Lütjen-Drecoll, E.; Wiederholt, M. Electrical and morphological evidence for heterogeneous populations of cultured bovine trabecular meshwork cells. Exp. Eye Res. 1991, 52, 375–388. [Google Scholar] [CrossRef]
- Torrejon, K.Y.; Papke, E.L.; Halman, J.R.; Stolwijk, J.; Dautriche, C.N.; Bergkvist, M.; Danias, J.; Sharfstein, S.; Xie, Y. Bioengineered glaucomatous 3D human trabecular meshwork as an in vitro disease model. Biotechnol. Bioeng. 2016, 113, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Torrejon, K.Y.; Papke, E.L.; Halman, J.R.; Bergkvist, M.; Danias, J.; Sharfstein, S.T.; Xie, Y. TGFβ2-induced outflow alterations in a bioengineered trabecular meshwork are offset by a rho-associated kinase inhibitor. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Bill, A.; Svedbergh, B. Scanning electron microscopic studies of the trabecular meshwork and the canal of Schlemm--an attempt to localize the main resistance to outflow of aqueous humor in man. Acta Ophthalmol. 1972, 50, 295–320. [Google Scholar] [CrossRef]
- Mauro, A.; Massarotti, N.; Salahudeen, M.; Romano, M.R.; Romano, V.; Nithiarasu, P. A generalised porous medium approach to study thermo-fluid dynamics in human eyes. Med. Biol. Eng. Comput. 2018, 56, 1823–1839. [Google Scholar] [CrossRef]
- Wang, K.; Read, A.T.; Sulchek, T.; Ethier, C.R. Trabecular meshwork stiffness in glaucoma. Exp. Eye Res. 2017, 158, 3–12. [Google Scholar] [CrossRef]
- Wang, K.; Li, G.; Read, A.T.; Navarro, I.; Mitra, A.K.; Stamer, W.D.; Sulchek, T.; Ethier, C.R. The relationship between outflow resistance and trabecular meshwork stiffness in mice. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Camras, L.J.; Stamer, W.D.; Epstein, D.; Gonzalez, P.; Yuan, F. Differential effects of trabecular meshwork stiffness on outflow facility in normal human and porcine eyes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5242–5250. [Google Scholar] [CrossRef] [PubMed]
- Camras, L.J.; Stamer, W.D.; Epstein, D.; Gonzalez, P.; Yuan, F. Circumferential tensile stiffness of glaucomatous trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2014, 55, 814–823. [Google Scholar] [CrossRef]
- McKee, C.T.; Last, J.A.; Russell, P.; Murphy, C.J. Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue Eng. Part B Rev. 2011, 17, 155–164. [Google Scholar] [CrossRef]
- Chang, J.; Huang, J.; Li, L.; Liu, Z.; Yuan, F. Stiffness characterization of anisotropic trabecular meshwork. J. Biomech. 2017, 61, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Wenger, M.P.; Bozec, L.; Horton, M.A.; Mesquida, P. Mechanical properties of collagen fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Dutov, P.; Antipova, O.; Varma, S.; Orgel, J.P.R.O.; Schieber, J.D. Measurement of elastic modulus of collagen type I single fiber. PLoS ONE 2016, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lin, J. Mechanical roles in formation of oriented collagen fibers. Tissue Eng. Part B Rev. 2020, 26, 116–128. [Google Scholar] [CrossRef]
- Schneider, M.; Pawlak, R.; Weber, G.R.; Dillinger, A.E.; Kuespert, S.; Iozzo, R.V.; Quigley, H.A.; Ohlmann, A.; Tamm, E.R.; Fuchshofer, R. A novel ocular function for decorin in the aqueous humor outflow. Matrix Biol. 2021, 97, 1–19. [Google Scholar] [CrossRef]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef]
- Dai, Y.; Lindsey, J.D.; Duong-Polk, X.; Nguyen, D.; Hofer, A.; Weinreb, R.N. Outflow facility in mice with a targeted type I collagen mutation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5749–5753. [Google Scholar] [CrossRef]
- Aihara, M.; Lindsey, J.D.; Weinreb, R.N. Ocular hypertension in mice with a targeted type I collagen mutation. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1581–1585. [Google Scholar] [CrossRef]
- Gong, H.; Tripathi, R.C.; Tripathi, B.J. Morphology of the aqueous outflow pathway. Microsc. Res. Tech. 1996, 33, 336–367. [Google Scholar] [CrossRef]
- Johnson, M.; Schuman, J.S.; Kagemann, L. Trabecular meshwork stiffness in the living human eye. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3541. [Google Scholar]
- Raghunathan, V.K.; Benoit, J.; Kasetti, R.; Zode, G.; Salemi, M.; Phinney, B.S.; Keller, K.E.; Staverosky, J.A.; Murphy, C.J.; Acott, T.; et al. Glaucomatous cell derived matrices differentially modulate non-glaucomatous trabecular meshwork cellular behavior. Acta Biomater. 2018, 71, 444–459. [Google Scholar] [CrossRef]
- Vranka, J.A.; Staverosky, J.A.; Reddy, A.P.; Wilmarth, P.A.; David, L.L.; Acott, T.S.; Russell, P.; Raghunathan, V.K. Biomechanical rigidity and quantitative proteomics analysis of segmental regions of the trabecular meshwork at physiologic and elevated pressures. Investig. Ophthalmol. Vis. Sci. 2018, 59, 246–259. [Google Scholar] [CrossRef]
- Thomasy, S.M.; Morgan, J.T.; Wood, J.A.; Murphy, C.J.; Russell, P. Substratum stiffness and latrunculin B modulate the gene expression of the mechanotransducers YAP and TAZ in human trabecular meshwork cells. Exp. Eye Res. 2013, 113, 66–73. [Google Scholar] [CrossRef]
- Thomasy, S.M.; Wood, J.A.; Kass, P.H.; Murphy, C.J.; Russell, P. Substratum stiffness and latrunculin B regulate matrix gene and protein expression in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Liton, P.; Liu, X.; Challa, P.; Epstein, D.; Gonzalez, P. Induction of TGF-β1 in the trabecular meshwork under cyclic mechanical stress. J. Cell. Physiol. 2005, 205, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Liton, P.B.; Luna, C.; Bodman, M.; Hong, A.; Epstein, D.L.; Gonzalez, P. Induction of IL-6 expression by mechanical stress in the trabecular meshwork. Biochem. Biophys. Res. Commun. 2005, 337, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Li, G.; Liton, P.B.; Epstein, D.L.; Gonzalez, P. Alterations in gene expression induced by cyclic mechanical stress in trabecular meshwork cells. Mol. Vis. 2009, 15, 534–544. [Google Scholar]
- Luna, C.; Li, G.; Qiu, J.; Epstein, D.L.; Gonzalez, P. MicroRNA-24 regulates the processing of latent TGFβ1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J. Cell. Physiol. 2011, 226, 1407–1414. [Google Scholar] [CrossRef]
- WuDunn, D. The effect of mechanical strain on matrix metalloproteinase production by bovine trabecular meshwork cells. Curr. Eye Res. 2001, 22, 394–397. [Google Scholar] [CrossRef]
- Baetz, N.W.; Hoffman, E.A.; Yool, A.J.; Stamer, W.D. Role of aquaporin-1 in trabecular meshwork cell homeostasis during mechanical strain. Exp. Eye Res. 2009, 89, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Perkumas, K.; Stamer, W.D.; Liu, Y. An in vitro bovine cellular model for human Schlemm’s canal endothelial cells and their response to TGFβ Treatment. Transl. Vis. Sci. Technol. 2020, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tumminia, S.J.; Mitton, K.P.; Arora, J.; Zelenka, P.; Epstein, D.L.; Russell, P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1361–1371. [Google Scholar]
- Porter, K.M.; Jeyabalan, N.; Liton, P.B. MTOR-independent induction of autophagy in trabecular meshwork cells subjected to biaxial stretch. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Soofi, S.S.; Last, J.A.; Liliensiek, S.J.; Nealey, P.F.; Murphy, C.J. The elastic modulus of Matrigel as determined by atomic force microscopy. J. Struct. Biol. 2009, 167, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. BioMedical Eng. OnLine 2020, 19, 1–16. [Google Scholar] [CrossRef]
- Theobald, J.; Ghanem, A.; Wallisch, P.; Banaeiyan, A.A.; Andrade-Navarro, M.A.; Taškova, K.; Haltmeier, M.; Kurtz, A.; Becker, H.; Reuter, S.; et al. Liver-kidney-on-chip to study toxicity of drug metabolites. ACS Biomater. Sci. Eng. 2018, 4, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Lin, R.Z.; Chen, R.J.; Chin, C.K.; Gong, S.E.; Chang, H.Y.; Peng, H.L.; Hsu, L.; Yew, T.R.; Chang, S.F.; et al. Liver-cell patterning lab chip: Mimicking the morphology of liver lobule tissue. Lab A Chip 2013, 13, 3578–3587. [Google Scholar] [CrossRef]
- He, Y.; Mao, T.; Gu, Y.; Yang, Y.; Ding, J. A simplified yet enhanced and versatile microfluidic platform for cyclic cell stretching on an elastic polymer. Biofabrication 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Yahyazadeh Shourabi, A.; Kashaninejad, N.; Saidi, M.S. An integrated microfluidic concentration gradient generator for mechanical stimulation and drug delivery. J. Sci. Adv. Mater. Devices 2021, 6, 280–290. [Google Scholar] [CrossRef]
- Chang, J.Y.; Folz, S.J.; Laryea, S.N.; Overby, D.R. Multi-scale analysis of segmental outflow patterns in human trabecular meshwork with changing intraocular pressure. J. Ocul. Pharmacol. Ther. 2014, 30, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Loke, C.Y.; Ooi, E.H.; Salahudeen, M.S.; Ramli, N.; Samsudin, A. Segmental aqueous humour outflow and eye orientation have strong influence on ocular drug delivery. Appl. Math. Model. 2018, 57, 474–491. [Google Scholar] [CrossRef]
- Montecchi-Palmer, M.; Bermudez, J.Y.; Webber, H.C.; Patel, G.C.; Clark, A.F.; Mao, W. TGFβ2 induces the formation of cross-linked actin networks (CLANs) in human trabecular meshwork cells through the smad and non-smad dependent pathways. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Stamer, D.W.; Roberts, B.C.; Epstein, D.L.; Allingham, R.R. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr. Eye Res. 2000, 20, 347–350. [Google Scholar] [CrossRef]
- Fan, B.J.; Leung, Y.F.; Wang, N.; Lam, S.C.; Liu, Y.; Tam, O.S.; Pang, C.P. Genetic and environmental risk factors for primary open-angle glaucoma. Chin. Med. J. 2004, 117, 706–710. [Google Scholar]
- Kaur, A.; Vanita, V.; Singh, J. Screening of CYP1B1 Arg368His as predominant mutation in North Indian primary open angle glaucoma and juvenile onset glaucoma patients. Mol. Biol. Res. Commun. 2018, 7, 181–186. [Google Scholar]
- Weinshilboum, R.; Wang, L. Pharmacogenomics: Bench to bedside. Nat. Rev. Drug Discov. 2004, 3, 739–748. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamont, H.C.; Masood, I.; Grover, L.M.; El Haj, A.J.; Hill, L.J. Fundamental Biomaterial Considerations in the Development of a 3D Model Representative of Primary Open Angle Glaucoma. Bioengineering 2021, 8, 147. https://doi.org/10.3390/bioengineering8110147
Lamont HC, Masood I, Grover LM, El Haj AJ, Hill LJ. Fundamental Biomaterial Considerations in the Development of a 3D Model Representative of Primary Open Angle Glaucoma. Bioengineering. 2021; 8(11):147. https://doi.org/10.3390/bioengineering8110147
Chicago/Turabian StyleLamont, Hannah C., Imran Masood, Liam M. Grover, Alicia J. El Haj, and Lisa J. Hill. 2021. "Fundamental Biomaterial Considerations in the Development of a 3D Model Representative of Primary Open Angle Glaucoma" Bioengineering 8, no. 11: 147. https://doi.org/10.3390/bioengineering8110147
APA StyleLamont, H. C., Masood, I., Grover, L. M., El Haj, A. J., & Hill, L. J. (2021). Fundamental Biomaterial Considerations in the Development of a 3D Model Representative of Primary Open Angle Glaucoma. Bioengineering, 8(11), 147. https://doi.org/10.3390/bioengineering8110147







