Human Peripheral Nerve-Derived Pluripotent Cells Can Be Stimulated by In Vitro Bone Morphogenetic Protein-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Peripheral Nerve Tissue Treatment
2.2. Cell Isolation and Culture
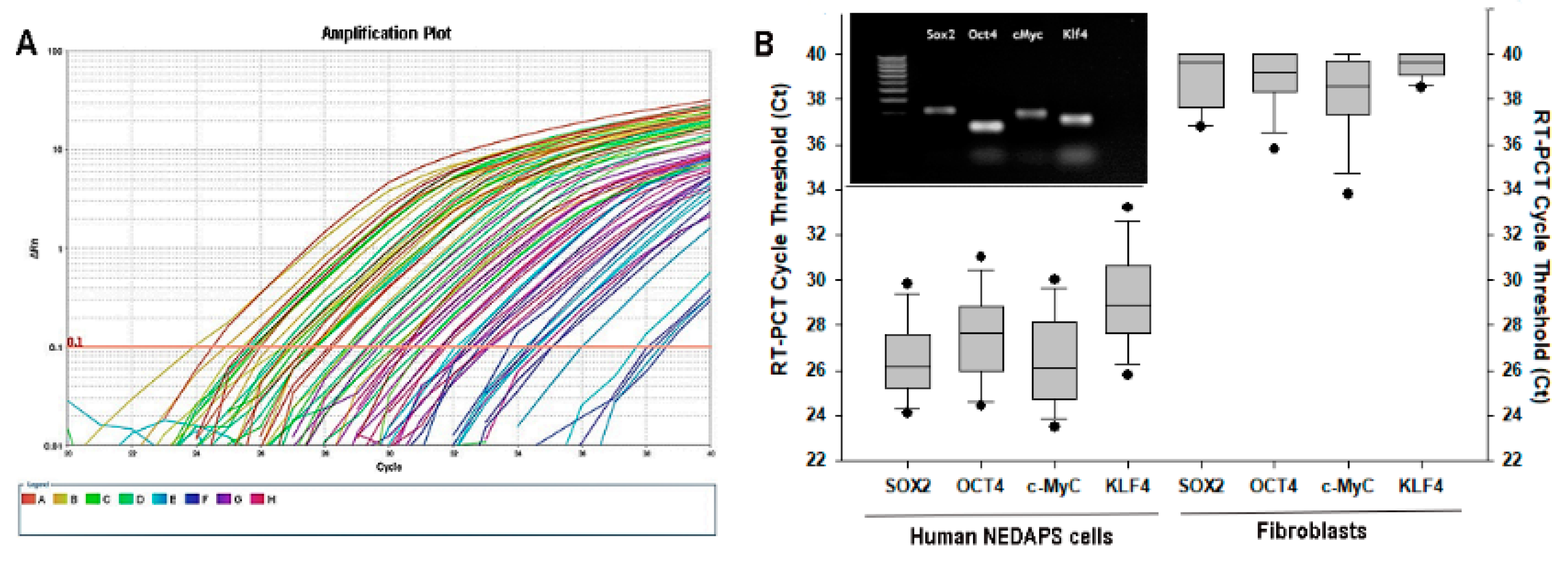
2.3. Real-Time PCR (RT-PCR) Test
2.4. Immunocytochemistry Staining and Image Acquisition
2.5. Statistical Analysis
3. Results
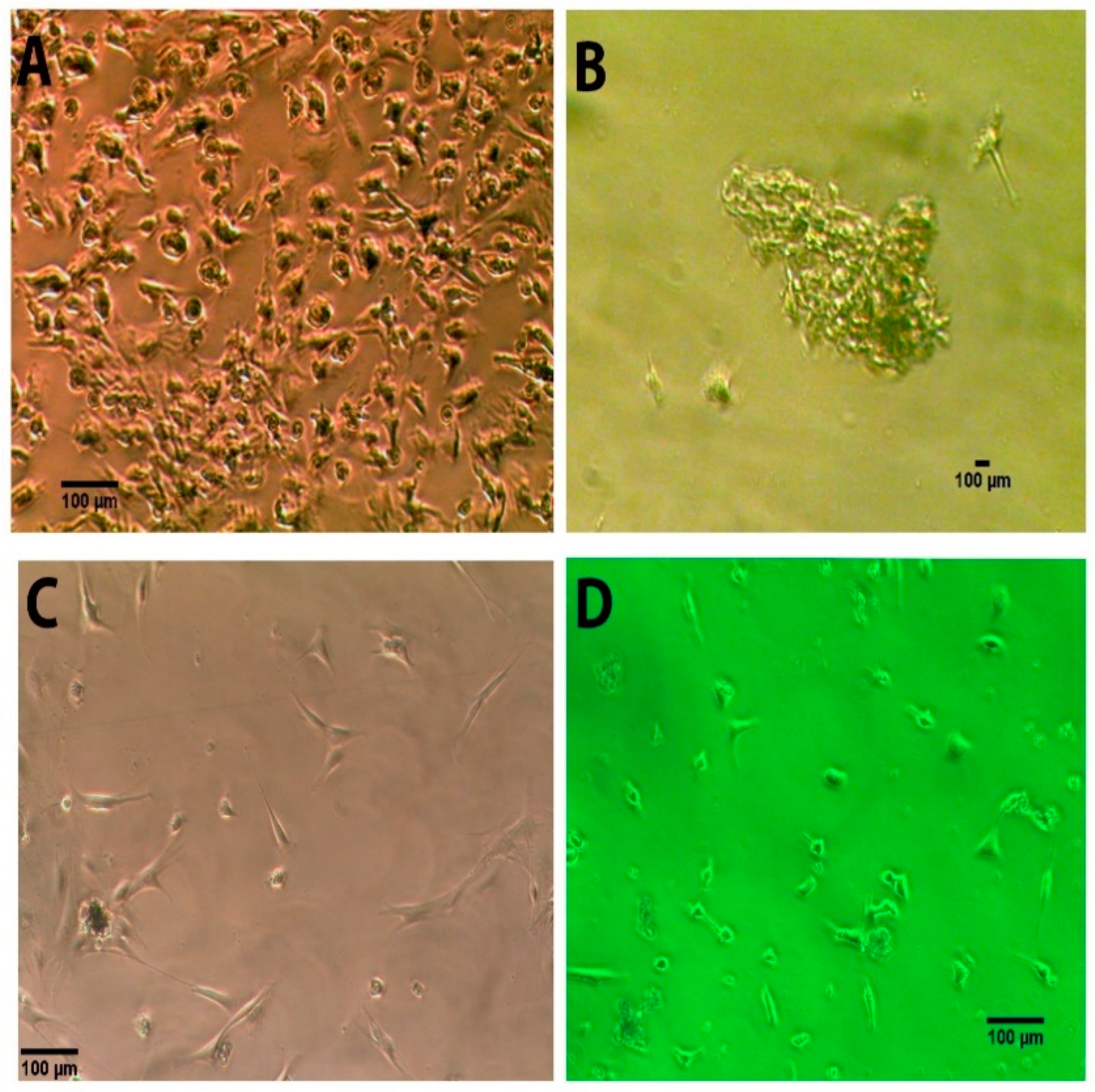
3.1. Morphology of the Human NEDAPS Cells
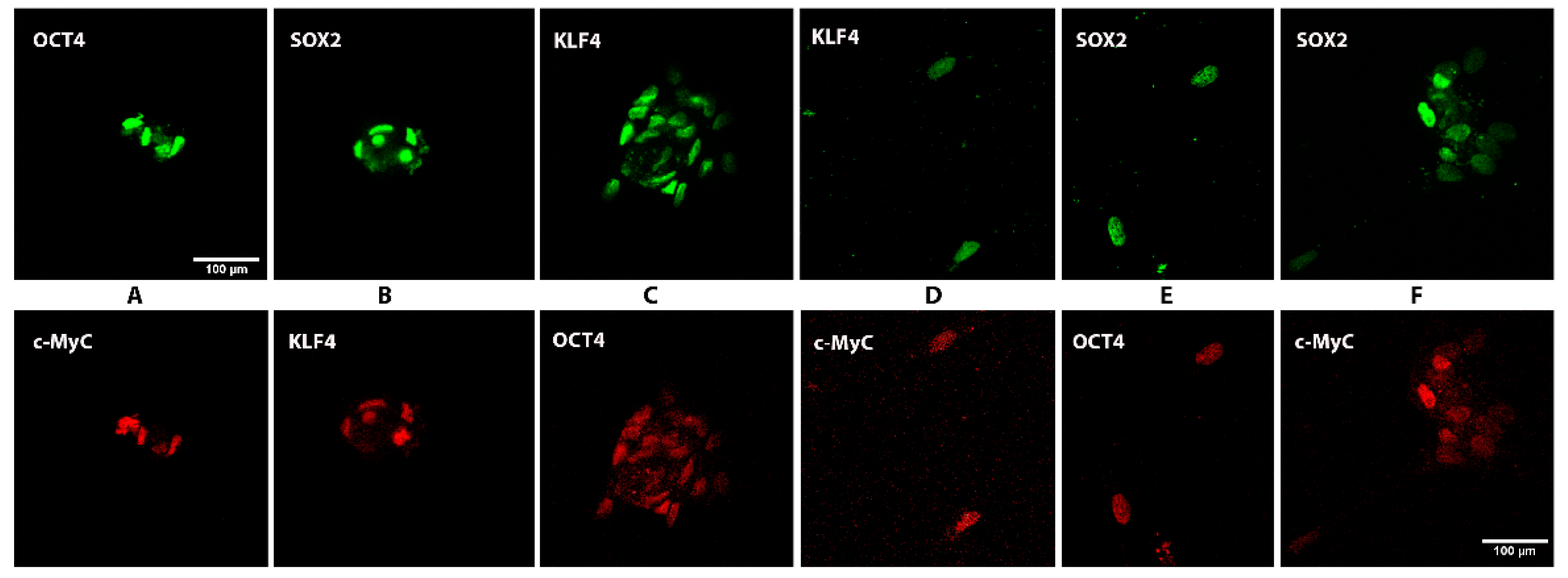
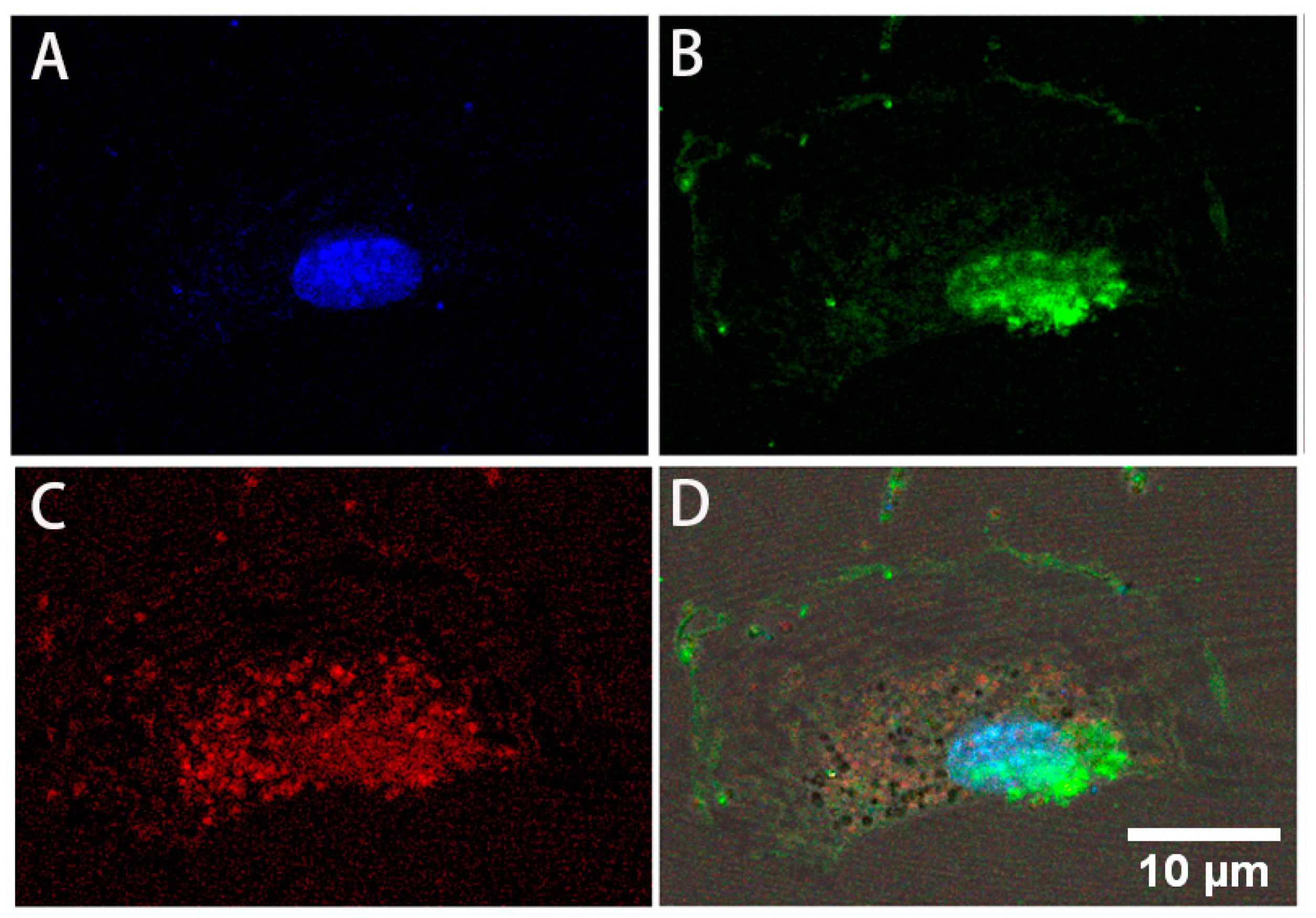
3.2. Expression of Stem Cell Markers
3.3. Differentiation to Fibroblasts
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, M.Z.; Marycz, K.; Poniewierska-Baran, A.; Fiedorowicz, K.; Zbucka-Kretowska, M.; Moniuszko, M. Very small embryonic-like stem cells as a novel developmental concept and the hierarchy of the stem cell compartment. Adv. Med. Sci. 2014, 59, 273–280. [Google Scholar] [CrossRef]
- Lamba, D.A.; McUsic, A.; Hirata, R.K.; Wang, P.R.; Russell, D.; Reh, T.A. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS ONE 2010, 5, e8763. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Narita, M.; Yokura, M.; Ichisaka, T.; Yamanaka, S. Human induced pluripotent stem cells on autologous feeders. PLoS ONE 2009, 4, e8067. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, P.; Takahashi, K.; Saito, M.; Yoshida, Y.; Okita, K.; Watanabe, A.; Inoue, H.; Yamashita, J.K.; Todani, M.; Nakagawa, M.; et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol. Rev. 2019, 99, 79–114. [Google Scholar] [CrossRef]
- Worringer, K.A.; Rand, T.A.; Hayashi, Y.; Sami, S.; Takahashi, K.; Tanabe, K.; Narita, M.; Srivastava, D.; Yamanaka, S. The let-7/LIN-41 pathway regulates reprogramming to human induced pluripotent stem cells by controlling expression of prodifferentiation genes. Cell Stem Cell 2014, 14, 40–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Xu, Y. Challenges to the clinical application of pluripotent stem cells: Towards genomic and functional stability. Genome Med. 2012, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Serra, M.; Brito, C.; Correia, C.; Alves, P.M. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 2012, 30, 350–359. [Google Scholar] [CrossRef]
- Hu, B.Y.; Weick, J.P.; Yu, J.; Ma, L.X.; Zhang, X.Q.; Thomson, J.A.; Zhang, S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 2010, 107, 4335–4340. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.Y.; Strong, N.; Gong, X.; Heggeness, M.H. Differentiation of nerve-derived adult pluripotent stem cells into osteoblastic and endothelial cells. Spine J. 2017, 17, 277–281. [Google Scholar] [CrossRef]
- Heggeness, M.H.; Strong, N.; Wooley, P.H.; Yang, S.Y. Quiescent pluripotent stem cells reside within murine peripheral nerves that can be stimulated to proliferate by recombinant human bone morphogenic protein 2 or by nerve trauma. Spine J. 2017, 17, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Brockes, J.P. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012, 35, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.S.; Sacchi, N. Single step method of RNA isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [Green Version]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Daniel, G.; Schultz, M. FDA Public Health Notification: Life-Threatening Complications Associated with Recombinant Human Bone Morphogenetic Protein in Cervical Spine Fusion. 2008. Available online: http://www.tccortho.com/pdf/FDAPublic%20Health%20Note.pdf (accessed on 5 December 2018).
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef]
- Jacob, M.; Chang, L.; Pure, E. Fibroblast activation protein in remodeling tissues. Curr. Mol. Med. 2012, 12, 1220–1243. [Google Scholar] [CrossRef] [PubMed]
- Strutz, F.; Okada, H.; Lo, C.W.; Danoff, T.; Carone, R.L.; Tomaszewski, J.E.; Neilson, E.G. Identification and characterization of a fibroblast marker: FSP1. J. Cell Biol. 1995, 130, 393–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grillo, H.C. Derivation of Fibroblasts in the Healing Wound. Arch. Surg. 1964, 88, 218–224. [Google Scholar] [CrossRef]
- Hemmati-Brivanlou, A.; Melton, D. Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell 1997, 88, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Bales, J.G.; Meals, R. Peripheral neuropathy of the upper extremity: Medical comorbidity that confounds common orthopedic pathology. Orthopedics 2009, 32, 758. [Google Scholar] [CrossRef]

| Target | Forward Primer | Reverse Primer | Product Size |
|---|---|---|---|
| Sox2 | agaaccccaagatgcacaac | gggcagcgtgtacttatcct | 200 |
| c-Myc | acccgctcaacgacagcagc | ccgtggggaggactcggagg | 104 |
| Klf4 | ctgaacagcagggactgtca | gtgtgggtggctgttctttt | 218 |
| Oct4 | agcgatcaagcagcgactat | agagtggtgacggagacagg | 202 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Jia, T.; Dart, B.; Shrestha, S.; Bretches, M.; Heggeness, M.H.; Yang, S.-Y. Human Peripheral Nerve-Derived Pluripotent Cells Can Be Stimulated by In Vitro Bone Morphogenetic Protein-2. Bioengineering 2021, 8, 132. https://doi.org/10.3390/bioengineering8100132
Sun R, Jia T, Dart B, Shrestha S, Bretches M, Heggeness MH, Yang S-Y. Human Peripheral Nerve-Derived Pluripotent Cells Can Be Stimulated by In Vitro Bone Morphogenetic Protein-2. Bioengineering. 2021; 8(10):132. https://doi.org/10.3390/bioengineering8100132
Chicago/Turabian StyleSun, Renyi, Tanghong Jia, Bradley Dart, Sunaina Shrestha, Morgan Bretches, Michael H. Heggeness, and Shang-You Yang. 2021. "Human Peripheral Nerve-Derived Pluripotent Cells Can Be Stimulated by In Vitro Bone Morphogenetic Protein-2" Bioengineering 8, no. 10: 132. https://doi.org/10.3390/bioengineering8100132
APA StyleSun, R., Jia, T., Dart, B., Shrestha, S., Bretches, M., Heggeness, M. H., & Yang, S.-Y. (2021). Human Peripheral Nerve-Derived Pluripotent Cells Can Be Stimulated by In Vitro Bone Morphogenetic Protein-2. Bioengineering, 8(10), 132. https://doi.org/10.3390/bioengineering8100132







