Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels
Abstract
1. Introduction
2. Collagen Alone with No Crosslinkers
3. Collagen with Crosslinkers
4. Collagen–GAG Hydrogels
4.1. Collagen–HA Hydrogels
4.2. Collagen–CS Hydrogels
4.3. Collagen-Heparin Gels
4.4. Collagen-Alginate Gels
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gorgieva, S.; Kokol, V. Collagen- vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. Biomater. Appl. Nanomed. 2011. [Google Scholar] [CrossRef]
- Lovell, C.R.; Smolenski, K.A.; Duance, V.C.; Light, N.D.; Young, S.; Dyson, M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987, 117, 419–428. [Google Scholar] [CrossRef]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef]
- Boskey, A.L.; Robey, P.G. The Composition of Bone. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; American Society for Bone and Mineral Research: Washington, DC, USA, 2018; pp. 84–92. [Google Scholar]
- Meek, K.M. Corneal collagen-its role in maintaining corneal shape and transparency. Biophys. Rev. 2009, 1, 83–93. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Simons, M. The extracellular matrix and blood vessel formation: Not just a scaffold. J. Cell Mol. Med. 2007, 11, 176–205. [Google Scholar] [CrossRef]
- Komuro, T. The lattice arrangement of the collagen fibres in the submucosa of the rat small intestine: Scanning electron microscopy. Cell Tissue Res. 1988, 251, 117–121. [Google Scholar] [CrossRef]
- Sharabi, M.; Wade, K.; Haj-Ali, R. The Mechanical Role of Collagen Fibers in the Intervertebral Disc. In Biomechanics of the Spine; Galbusera, F., Wilke, H.-J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 105–123. [Google Scholar]
- Ramachandran, G.N.; Kartha, G. Structure of collagen. Nature 1954, 174, 269–270. [Google Scholar] [CrossRef]
- Rich, A.; Crick, F.H. The structure of collagen. Nature 1955, 176, 915–916. [Google Scholar] [CrossRef]
- Cowan, P.M.; McGavin, S.; North, A.C. The polypeptide chain configuration of collagen. Nature 1955, 176, 1062–1064. [Google Scholar] [CrossRef]
- Pinnell, S.R.; Martin, G.R. The cross-linking of collagen and elastin: Enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc. Natl. Acad. Sci. USA 1968, 61, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R.; Paz, M.A.; Gallop, P.M. Cross-linking in collagen and elastin. Annu. Rev. Biochem. 1984, 53, 717–748. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef]
- Meek, K.M.; Chapman, J.A.; Hardcastle, R.A. The staining pattern of collagen fibrils. Improved correlation with sequence data. J. Biol. Chem. 1979, 254, 10710–10714. [Google Scholar]
- Fietzek, P.P.; Kuhn, K. Information contained in the amino acid sequence of the alpha1(I)-chain of collagen and its consequences upon the formation of the triple helix, of fibrils and crosslinks. Mol. Cell Biochem. 1975, 8, 141–157. [Google Scholar] [CrossRef]
- Berg, R.A.; Prockop, D.J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem. Biophys. Res. Commun. 1973, 52, 115–120. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Knight, C.G.; Morton, L.F.; Peachey, A.R.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J. Biol. Chem. 2000, 275, 35–40. [Google Scholar] [CrossRef]
- Legate, K.R.; Wickstrom, S.A.; Fassler, R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009, 23, 397–418. [Google Scholar] [CrossRef]
- Guan, J.L. Role of focal adhesion kinase in integrin signaling. Int. J. Biochem. Cell Biol. 1997, 29, 1085–1096. [Google Scholar] [CrossRef]
- Moraes, J.A.; Frony, A.C.; Dias, A.M.; Renovato-Martins, M.; Rodrigues, G.; Marcinkiewicz, C.; Assreuy, J.; Barja-Fidalgo, C. Alpha1beta1 and integrin-linked kinase interact and modulate angiotensin II effects in vascular smooth muscle cells. Atherosclerosis 2015, 243, 477–485. [Google Scholar] [CrossRef]
- Heino, J. Cellular Signaling by Collagen-Binding Integrins. In I Domain Integrins; Gullberg, D., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 143–155. [Google Scholar] [CrossRef]
- Wu, D.; Witt, R.L.; Harrington, D.A.; Farach-Carson, M.C. Dynamic Assembly of Human Salivary Stem/Progenitor Microstructures Requires Coordinated alpha1beta1 Integrin-Mediated Motility. Front. Cell Dev. Biol. 2019, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E. Affinity of integrins for damaged extracellular matrix: Alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem. Biophys. Res. Commun. 1992, 182, 1025–1031. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed]
- Sapudom, J.; Rubner, S.; Martin, S.; Kurth, T.; Riedel, S.; Mierke, C.T.; Pompe, T. The phenotype of cancer cell invasion controlled by fibril diameter and pore size of 3D collagen networks. Biomaterials 2015, 52, 367–375. [Google Scholar] [CrossRef]
- Roeder, B.A.; Kokini, K.; Voytik-Harbin, S.L. Fibril microstructure affects strain transmission within collagen extracellular matrices. J. Biomech. Eng. 2009, 131, 031004. [Google Scholar] [CrossRef]
- Corin, K.A.; Gibson, L.J. Cell contraction forces in scaffolds with varying pore size and cell density. Biomaterials 2010, 31, 4835–4845. [Google Scholar] [CrossRef]
- Ray, A.; Morford, R.K.; Ghaderi, N.; Odde, D.J.; Provenzano, P.P. Dynamics of 3D carcinoma cell invasion into aligned collagen. Integr. Biol. 2018, 10, 100–112. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Cliche, S.; Gariépy, C.; Germain, L.; Berthod, F. Comparative study of bovine, porcine and avian collagens for the production of a tissue engineered dermis. Acta Biomater. 2011, 7, 3757–3765. [Google Scholar] [CrossRef]
- Kreger, S.T.; Bell, B.J.; Bailey, J.; Stites, E.; Kuske, J.; Waisner, B.; Voytik-Harbin, S.L. Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers 2010, 93, 690–707. [Google Scholar] [CrossRef]
- Bailey, J.L.; Critser, P.J.; Whittington, C.; Kuske, J.L.; Yoder, M.C.; Voytik-Harbin, S.L. Collagen oligomers modulate physical and biological properties of three-dimensional self-assembled matrices. Biopolymers 2011, 95, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Ramanujan, S.; Pluen, A.; McKee, T.D.; Brown, E.B.; Boucher, Y.; Jain, R.K. Diffusion and convection in collagen gels: Implications for transport in the tumor interstitium. Biophys. J. 2002, 83, 1650–1660. [Google Scholar] [CrossRef]
- Erikson, A.; Andersen, H.N.; Naess, S.N.; Sikorski, P.; Davies Cde, L. Physical and chemical modifications of collagen gels: Impact on diffusion. Biopolymers 2008, 89, 135–143. [Google Scholar] [CrossRef] [PubMed]
- McPherson, J.M.; Wallace, D.G.; Sawamura, S.J.; Conti, A.; Condell, R.A.; Wade, S.; Piez, K.A. Collagen fibrillogenesis in vitro: A characterization of fibril quality as a function of assembly conditions. Coll Relat. Res. 1985, 5, 119–135. [Google Scholar] [CrossRef]
- Raub, C.B.; Suresh, V.; Krasieva, T.; Lyubovitsky, J.; Mih, J.D.; Putnam, A.J.; Tromberg, B.J.; George, S.C. Noninvasive Assessment of Collagen Gel Microstructure and Mechanics Using Multiphoton Microscopy. Biophys. J. 2007, 92, 2212–2222. [Google Scholar] [CrossRef]
- Gobeaux, F.; Mosser, G.; Anglo, A.; Panine, P.; Davidson, P.; Giraud-Guille, M.M.; Belamie, E. Fibrillogenesis in Dense Collagen Solutions: A Physicochemical Study. J. Mol. Biol. 2008, 376, 1509–1522. [Google Scholar] [CrossRef]
- Naciri, M.; Kuystermans, D.; Al-Rubeai, M. Monitoring pH and dissolved oxygen in mammalian cell culture using optical sensors. Cytotechnology 2008, 57, 245–250. [Google Scholar] [CrossRef]
- Millerot-Serrurot, E.; Guilbert, M.; Fourre, N.; Witkowski, W.; Said, G.; Van Gulick, L.; Terryn, C.; Zahm, J.M.; Garnotel, R.; Jeannesson, P. 3D collagen type I matrix inhibits the antimigratory effect of doxorubicin. Cancer Cell Int. 2010, 10, 26. [Google Scholar] [CrossRef]
- Mao, C.; Kisaalita, W.S. Characterization of 3-D collagen hydrogels for functional cell-based biosensing. Biosens. Bioelectron. 2004, 19, 1075–1088. [Google Scholar] [CrossRef]
- Buchanan, C.F.; Voigt, E.E.; Szot, C.S.; Freeman, J.W.; Vlachos, P.P.; Rylander, M.N. Three-dimensional microfluidic collagen hydrogels for investigating flow-mediated tumor-endothelial signaling and vascular organization. Tissue Eng. Part C Methods 2014, 20, 64–75. [Google Scholar] [CrossRef]
- Vazquez-Portalati, N.N.; Kilmer, C.E.; Panitch, A.; Liu, J.C. Characterization of Collagen Type I and II Blended Hydrogels for Articular Cartilage Tissue Engineering. Biomacromolecules 2016, 17, 3145–3152. [Google Scholar] [CrossRef] [PubMed]
- Kilmer, C.; Battistoni, C.M.; Cox, A.; Breur, G.J.; Panitch, A.; Liu, J.C. Collagen Type I and II Blend Hydrogel with Autologous Mesenchymal Stem Cells as a Scaffold for Articular Cartilage Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 3464–3476. [Google Scholar] [CrossRef]
- Ren, X.; Wang, F.; Chen, C.; Gong, X.; Yin, L.; Yang, L. Engineering zonal cartilage through bioprinting collagen type II hydrogel constructs with biomimetic chondrocyte density gradient. BMC Musculoskelet. Disord. 2016, 17, 301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yuan, T.; Guo, L.; Zhang, X. An in vitro study of collagen hydrogel to induce the chondrogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. A 2012, 100, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.; Hefferan, T.E.; Tarara, J.E.; Haasper, C.; Meller, R.; Krettek, C.; Lu, L.; Yaszemski, M.J. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J. Biomed. Mater. Res. A 2010, 94, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Lee, H.Y.; Lee, J.H.; Kim, T.H.; Jang, J.H.; Kim, H.W.; Wall, I. Collagen three-dimensional hydrogel matrix carrying basic fibroblast growth factor for the cultivation of mesenchymal stem cells and osteogenic differentiation. Tissue Eng. Part A 2012, 18, 1087–1100. [Google Scholar] [CrossRef]
- Noth, U.; Schupp, K.; Heymer, A.; Kall, S.; Jakob, F.; Schutze, N.; Baumann, B.; Barthel, T.; Eulert, J.; Hendrich, C. Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy 2005, 7, 447–455. [Google Scholar] [CrossRef]
- Szot, C.S.; Buchanan, C.F.; Freeman, J.W.; Rylander, M.N. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials 2011, 32, 7905–7912. [Google Scholar] [CrossRef]
- Responte, D.J.; Natoli, R.M.; Athanasiou, K.A. Collagens of articular cartilage: Structure, function, and importance in tissue engineering. Crit. Rev. Biomed. Eng. 2007, 35, 363–411. [Google Scholar] [CrossRef]
- Hassell, J.R.; Birk, D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar] [CrossRef]
- Cheng, X.; Gurkan, U.A.; Dehen, C.J.; Tate, M.P.; Hillhouse, H.W.; Simpson, G.J.; Akkus, O. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials 2008, 29, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Bullock, W.; Sun, X.; Van Dyke, W.S.; Akkus, O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials 2012, 33, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Panitch, A.; Neu, C.P. Incorporation of an aggrecan mimic prevents proteolytic degradation of anisotropic cartilage analogs. Acta Biomater. 2013, 9, 4618–4625. [Google Scholar] [CrossRef] [PubMed]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, A.; Ruggiero, F.; Hulmes, D.J. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 2007, 28, 4268–4276. [Google Scholar] [CrossRef]
- Builles, N.; Janin-Manificat, H.; Malbouyres, M.; Justin, V.; Rovere, M.R.; Pellegrini, G.; Torbet, J.; Hulmes, D.J.; Burillon, C.; Damour, O.; et al. Use of magnetically oriented orthogonal collagen scaffolds for hemi-corneal reconstruction and regeneration. Biomaterials 2010, 31, 8313–8322. [Google Scholar] [CrossRef]
- Paten, J.A.; Siadat, S.M.; Susilo, M.E.; Ismail, E.N.; Stoner, J.L.; Rothstein, J.P.; Ruberti, J.W. Flow-Induced Crystallization of Collagen: A Potentially Critical Mechanism in Early Tissue Formation. ACS Nano 2016, 10, 5027–5040. [Google Scholar] [CrossRef]
- Gao, Y.; Li, B.; Kong, W.; Yuan, L.; Guo, L.; Li, C.; Fan, H.; Fan, Y.; Zhang, X. Injectable and self-crosslinkable hydrogels based on collagen type II and activated chondroitin sulfate for cell delivery. Int. J. Biol. Macromol. 2018, 118, 2014–2020. [Google Scholar] [CrossRef]
- Antman-Passig, M.; Levy, S.; Gartenberg, C.; Schori, H.; Shefi, O. Mechanically Oriented 3D Collagen Hydrogel for Directing Neurite Growth. Tissue Eng. Part A 2017, 23, 403–414. [Google Scholar] [CrossRef]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef]
- Helary, C.; Bataille, I.; Abed, A.; Illoul, C.; Anglo, A.; Louedec, L.; Letourneur, D.; Meddahi-Pelle, A.; Giraud-Guille, M.M. Concentrated collagen hydrogels as dermal substitutes. Biomaterials 2010, 31, 481–490. [Google Scholar] [CrossRef]
- Braziulis, E.; Diezi, M.; Biedermann, T.; Pontiggia, L.; Schmucki, M.; Hartmann-Fritsch, F.; Luginbuhl, J.; Schiestl, C.; Meuli, M.; Reichmann, E. Modified plastic compression of collagen hydrogels provides an ideal matrix for clinically applicable skin substitutes. Tissue Eng. Part C Methods 2012, 18, 464–474. [Google Scholar] [CrossRef]
- Sohutskay, D.O.; Buno, K.P.; Tholpady, S.S.; Nier, S.J.; Voytik-Harbin, S.L. Design and biofabrication of dermal regeneration scaffolds: Role of oligomeric collagen fibril density and architecture. Regen. Med. 2020, 15, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Rýglová, Š.; Braun, M.; Suchý, T. Collagen and Its Modifications-Crucial Aspects with Concern to Its Processing and Analysis. Macromol. Mater. Eng. 2017, 302, 1600460. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen-gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Z.W.; Wang, B.H.; Wen, Z.K. Construction and biocompatibility of a thin type I/II collagen composite scaffold. Cell Tissue Bank 2018, 19, 47–59. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Larsen, J.; Krasieva, T.B.; Lyubovitsky, J.G. Effect of genipin crosslinking on the optical spectral properties and structures of collagen hydrogels. ACS Appl. Mater. Interfaces 2011, 3, 2579–2584. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Yang, T.; Zhang, N.; Dong, L.; Ma, S.; Liu, X.; Zhou, M.; Li, B. The effects of different crossing-linking conditions of genipin on type I collagen scaffolds: An in vitro evaluation. Cell Tissue Bank 2014, 15, 531–541. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. Part A 2008, 87, 308–320. [Google Scholar] [CrossRef]
- Zhou, X.; Tao, Y.; Chen, E.; Wang, J.; Fang, W.; Zhao, T.; Liang, C.; Li, F.; Chen, Q. Genipin-cross-linked type II collagen scaffold promotes the differentiation of adipose-derived stem cells into nucleus pulposus-like cells. J. Biomed. Mater. Res. Part A 2018, 106, 1258–1268. [Google Scholar] [CrossRef]
- Selling, G.W.; Woods, K.K.; Sessa, D.; Biswas, A. Electrospun zein fibers using glutaraldehyde as the crosslinking reagent: Effect of time and temperature. Macromol. Chem. Phys. 2008, 209, 1003–1011. [Google Scholar] [CrossRef]
- Goissis, G.; Marcantonio, E., Jr.; Marcantonio, R.A.; Lia, R.C.; Cancian, D.C.; de Carvalho, W.M. Biocompatibility studies of anionic collagen membranes with different degree of glutaraldehyde cross-linking. Biomaterials 1999, 20, 27–34. [Google Scholar] [CrossRef]
- Olde Damink, L.H.H.; Dijkstra, P.J.; Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995, 6, 460–472. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Speer, D.P.; Chvapil, M.; Eskelson, C.D.; Ulreich, J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J. Biomed. Mater. Res. 1980, 14, 753–764. [Google Scholar] [CrossRef]
- Liu, Y.; Shu, X.Z.; Gray, S.D.; Prestwich, G.D. Disulfide-crosslinked hyaluronan-gelatin sponge: Growth of fibrous tissue in vivo. J. Biomed. Mater. Res. Part A 2004, 68, 142–149. [Google Scholar] [CrossRef]
- Xu, G.; Wang, X.; Deng, C.; Teng, X.; Suuronen, E.J.; Shen, Z.; Zhong, Z. Injectable biodegradable hybrid hydrogels based on thiolated collagen and oligo(acryloyl carbonate)-poly(ethylene glycol)-oligo(acryloyl carbonate) copolymer for functional cardiac regeneration. Acta Biomater. 2015, 15, 55–64. [Google Scholar] [CrossRef]
- Singh, R.K.; Seliktar, D.; Putnam, A.J. Capillary morphogenesis in PEG-collagen hydrogels. Biomaterials 2013, 34, 9331–9340. [Google Scholar] [CrossRef]
- Kurimoto, A.; Tanabe, T.; Tachibana, A.; Yamauchi, K. Thiolated dermal bovine collagen as a novel support for bioactive substances—Conjugation with lysozyme. J. Biotechnol. 2001, 86, 1–8. [Google Scholar] [CrossRef]
- Pupkaite, J.; Rosenquist, J.; Hilborn, J.; Samanta, A. Injectable Shape-Holding Collagen Hydrogel for Cell Encapsulation and Delivery Cross-linked Using Thiol-Michael Addition Click Reaction. Biomacromolecules 2019, 20, 3475–3484. [Google Scholar] [CrossRef]
- Yamauchi, K.; Takeuchi, N.; Kurimoto, A.; Tanabe, T. Films of collagen crosslinked by S-S bonds: Preparation and characterization. Biomaterials 2001, 22, 855–863. [Google Scholar] [CrossRef]
- Drexler, J.W.; Powell, H.M. Dehydrothermal crosslinking of electrospun collagen. Tissue Eng. Part C Methods 2011, 17, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M.G. Physical crosslinking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Achilli, M.; Lagueux, J.; Mantovani, D. On the effects of UV-C and pH on the mechanical behavior, molecular conformation and cell viability of collagen-based scaffold for vascular tissue engineering. Macromol. Biosci. 2010, 10, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Menter, J.M.; Williamson, G.D.; Carlyle, K.; Moore, C.L.; Willis, I. Photochemistry of type I acid-soluble calf skin collagen: Dependence on excitation wavelength. Photochem. Photobiol. 1995, 62, 402–408. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaminska, A. Thermal helix-coil transition in UV irradiated collagen from rat tail tendon. Int. J. Biol. Macromol. 1999, 24, 337–340. [Google Scholar] [CrossRef]
- Ohan, M.P.; Weadock, K.S.; Dunn, M.G. Synergistic effects of glucose and ultraviolet irradiation on the physical properties of collagen. J. Biomed. Mater. Res. 2002, 60, 384–391. [Google Scholar] [CrossRef]
- Cornwell, K.G.; Lei, P.; Andreadis, S.T.; Pins, G.D. Crosslinking of discrete self-assembled collagen threads: Effects on mechanical strength and cell-matrix interactions. J. Biomed. Mater. Res. Part A 2007, 80, 362–371. [Google Scholar] [CrossRef]
- Docherty, R.; Forrester, J.V.; Lackie, J.M.; Gregory, D.W. Glycosaminoglycans facilitate the movement of fibroblasts through three-dimensional collagen matrices. J. Cell Sci. 1989, 92, 263–270. [Google Scholar]
- Bittner, K.; Liszio, C.; Blumberg, P.; Schonherr, E.; Kresse, H. Modulation of collagen gel contraction by decorin. Biochem. J. 1996, 314, 159–166. [Google Scholar] [CrossRef]
- Casale, J.; Crane, J.S. Biochemistry, Glycosaminoglycans. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef]
- Sasisekharan, R.; Venkataraman, G. Heparin and heparan sulfate: Biosynthesis, structure and function. Curr. Opin. Chem. Biol. 2000, 4, 626–631. [Google Scholar] [CrossRef]
- Alphonsus, C.S.; Rodseth, R.N. The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia 2014, 69, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Masola, V.; Zaza, G.; Onisto, M.; Lupo, A.; Gambaro, G. Glycosaminoglycans, proteoglycans and sulodexide and the endothelium: Biological roles and pharmacological effects. Int. Angiol. 2014, 33, 243–254. [Google Scholar] [PubMed]
- Chappell, D.; Brettner, F.; Doerfler, N.; Jacob, M.; Rehm, M.; Bruegger, D.; Conzen, P.; Jacob, B.; Becker, B.F. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: An animal study. Eur. J. Anaesthesiol. 2014, 31, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Glant, T.T.; Buzas, E.I.; Finnegan, A.; Negroiu, G.; Cs-Szabo, G.; Mikecz, K. Critical roles of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J. Immunol. 1998, 160, 3812–3819. [Google Scholar] [PubMed]
- Walimbe, T.; Panitch, A.; Sivasankar, P.M. A Review of Hyaluronic Acid and Hyaluronic Acid-based Hydrogels for Vocal Fold Tissue Engineering. J. Voice 2017, 31, 416–423. [Google Scholar] [CrossRef]
- Parry, D.A.D.; Flint, M.H.; Gillard, G.C.; Craig, A.S. A role for glycosaminoglycans in the development of collagen fibrils. FEBS Lett. 1982, 149, 1–7. [Google Scholar] [CrossRef]
- Eng, D.; Caplan, M.; Preul, M.; Panitch, A. Hyaluronan scaffolds: A balance between backbone functionalization and bioactivity. Acta Biomater. 2010, 6, 2407–2414. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Thones, S.; Rother, S.; Wippold, T.; Blaszkiewicz, J.; Balamurugan, K.; Moeller, S.; Ruiz-Gomez, G.; Schnabelrauch, M.; Scharnweber, D.; Saalbach, A.; et al. Hyaluronan/collagen hydrogels containing sulfated hyaluronan improve wound healing by sustained release of heparin-binding EGF-like growth factor. Acta Biomater. 2019, 86, 135–147. [Google Scholar] [CrossRef]
- Rother, S.; Galiazzo, V.D.; Kilian, D.; Fiebig, K.M.; Becher, J.; Moeller, S.; Hempel, U.; Schnabelrauch, M.; Waltenberger, J.; Scharnweber, D.; et al. Hyaluronan/Collagen Hydrogels with Sulfated Hyaluronan for Improved Repair of Vascularized Tissue Tune the Binding of Proteins and Promote Endothelial Cell Growth. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Rother, S.; Kronert, V.; Hauck, N.; Berg, A.; Moeller, S.; Schnabelrauch, M.; Thiele, J.; Scharnweber, D.; Hintze, V. Hyaluronan/collagen hydrogel matrices containing high-sulfated hyaluronan microgels for regulating transforming growth factor-beta1. J. Mater. Sci. Mater. Med. 2019, 30, 65. [Google Scholar] [CrossRef] [PubMed]
- Hempel, U.; Hintze, V.; Moller, S.; Schnabelrauch, M.; Scharnweber, D.; Dieter, P. Artificial extracellular matrices composed of collagen I and sulfated hyaluronan with adsorbed transforming growth factor beta1 promote collagen synthesis of human mesenchymal stromal cells. Acta Biomater. 2012, 8, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Hempel, U.; Moller, S.; Noack, C.; Hintze, V.; Scharnweber, D.; Schnabelrauch, M.; Dieter, P. Sulfated hyaluronan/collagen I matrices enhance the osteogenic differentiation of human mesenchymal stromal cells in vitro even in the absence of dexamethasone. Acta Biomater. 2012, 8, 4064–4072. [Google Scholar] [CrossRef]
- Kliemt, S.; Lange, C.; Otto, W.; Hintze, V.; Moller, S.; von Bergen, M.; Hempel, U.; Kalkhof, S. Sulfated hyaluronan containing collagen matrices enhance cell-matrix-interaction, endocytosis, and osteogenic differentiation of human mesenchymal stromal cells. J. Proteome Res. 2013, 12, 378–389. [Google Scholar] [CrossRef]
- Hempel, U.; Matthaus, C.; Preissler, C.; Moller, S.; Hintze, V.; Dieter, P. Artificial matrices with high-sulfated glycosaminoglycans and collagen are anti-inflammatory and pro-osteogenic for human mesenchymal stromal cells. J. Cell Biochem. 2014, 115, 1561–1571. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Palumbo, F.; Prestwich, G.D. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: A covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials 2003, 24, 3825–3834. [Google Scholar] [CrossRef]
- Wirostko, B.; Mann, B.K.; Williams, D.L.; Prestwich, G.D. Ophthalmic Uses of a Thiol-Modified Hyaluronan-Based Hydrogel. Adv. Wound Care 2014, 3, 708–716. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef]
- Walimbe, T.; Calve, S.; Panitch, A.; Sivasankar, M.P. Incorporation of types I and III collagen in tunable hyaluronan hydrogels for vocal fold tissue engineering. Acta Biomater. 2019, 87, 97–107. [Google Scholar] [CrossRef]
- Suri, S.; Schmidt, C.E. Photopatterned collagen-hyaluronic acid interpenetrating polymer network hydrogels. Acta Biomater. 2009, 5, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Brigham, M.D.; Bick, A.; Lo, E.; Bendali, A.; Burdick, J.A.; Khademhosseini, A. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng. Part A 2009, 15, 1645–1653. [Google Scholar] [CrossRef]
- Suri, S.; Schmidt, C.E. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng. Part A 2010, 16, 1703–1716. [Google Scholar] [CrossRef] [PubMed]
- Her, G.J.; Wu, H.C.; Chen, M.H.; Chen, M.Y.; Chang, S.C.; Wang, T.W. Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages. Acta Biomater. 2013, 9, 5170–5180. [Google Scholar] [CrossRef]
- Murphy, C.M.; Matsiko, A.; Haugh, M.G.; Gleeson, J.P.; O’Brien, F.J. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J. Mech. Behav. Biomed. Mater. 2012, 11, 53–62. [Google Scholar] [CrossRef]
- Matsiko, A.; Levingstone, T.J.; O’Brien, F.J.; Gleeson, J.P. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 11, 41–52. [Google Scholar] [CrossRef]
- Koh, R.H.; Jin, Y.; Kang, B.J.; Hwang, N.S. Chondrogenically primed tonsil-derived mesenchymal stem cells encapsulated in riboflavin-induced photocrosslinking collagen-hyaluronic acid hydrogel for meniscus tissue repairs. Acta Biomater. 2017, 53, 318–328. [Google Scholar] [CrossRef]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.G.; Hwang, N.S. Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, M.; Lu, W.; Zhang, X.; Ma, D.; Rong, X.; Yu, C.; Jin, Y. Cross-linked collagen-chondroitin sulfate-hyaluronic acid imitating extracellular matrix as scaffold for dermal tissue engineering. Tissue Eng. Part C Methods 2010, 16, 269–279. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, L.; Wang, Y. Crosslinked collagen-gelatin-hyaluronic acid biomimetic film for cornea tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 196–201. [Google Scholar] [CrossRef]
- Kirk, J.F.; Ritter, G.; Finger, I.; Sankar, D.; Reddy, J.D.; Talton, J.D.; Nataraj, C.; Narisawa, S.; Millan, J.L.; Cobb, R.R. Mechanical and biocompatible characterization of a cross-linked collagen-hyaluronic acid wound dressing. Biomatter 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Stowers, R.; Nam, S.; Xia, Y.; Chaudhuri, O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 2018, 154, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Lin, P.; Schmidt, C.E. Biodegradable hydrogels composed of oxime crosslinked poly(ethylene glycol), hyaluronic acid and collagen: A tunable platform for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 2015, 26, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, Y.; Jiang, B.; Fan, H.; Zhang, X. Effect of adipic dihydrazide modification on the performance of collagen/hyaluronic acid scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 307–316. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, T.; Xiao, Z.; Tang, P.; Xiao, Y.; Fan, Y.; Zhang, X. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2267–2279. [Google Scholar] [CrossRef]
- Liang, W.H.; Kienitz, B.L.; Penick, K.J.; Welter, J.F.; Zawodzinski, T.A.; Baskaran, H. Concentrated collagen-chondroitin sulfate scaffolds for tissue engineering applications. J. Biomed. Mater. Res. A 2010, 94, 1050–1060. [Google Scholar] [CrossRef]
- Zhang, L.; Li, K.F.; Xiao, W.Q.; Zheng, L.; Xiao, Y.M.; Fan, H.S.; Zhang, X.D. Preparation of collagen-chondroitin sulfate-hyaluronic acid hybrid hydrogel scaffolds and cell compatibility in vitro. Carbohydr. Polym. 2011, 84, 118–125. [Google Scholar] [CrossRef]
- Pieper, J.S.; Oosterhof, A.; Dijkstra, P.J.; Veerkamp, J.H.; van Kuppevelt, T.H. Preparation and characterization of porous crosslinked collagenous matrices containing bioavailable chondroitin sulphate. Biomaterials 1999, 20, 847–858. [Google Scholar] [CrossRef]
- Pieper, J.S.; van Wachem, P.B.; van Luyn, M.J.A.; Brouwer, L.A.; Hafmans, T.; Veerkamp, J.H.; van Kuppevelt, T.H. Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials 2000, 21, 1689–1699. [Google Scholar] [CrossRef]
- Pietrucha, K. Physicochemical properties of 3D collagen-CS scaffolds for potential use in neural tissue engineering. Int. J. Biol. Macromol. 2015, 80, 732–739. [Google Scholar] [CrossRef]
- Pietrucha, K.; Szymański, J.; Drobnik, J. The Behavior of Embryonic Neural Cells within the 3D Micro-structured Collagen-Based Scaffolds. In Proceedings of the 6th European Conference of the International Federation for Medical and Biological Engineering, Dubrovnik, Croatia, 7–11 September 2014; pp. 549–552. [Google Scholar]
- Stuart, K.; Panitch, A. Influence of chondroitin sulfate on collagen gel structure and mechanical properties at physiologically relevant levels. Biopolymers 2008, 89, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Stuart, K.; Panitch, A. Characterization of gels composed of blends of collagen I, collagen III, and chondroitin sulfate. Biomacromolecules 2009, 10, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.F.; Shepherd, D.M.; West, G.B.; Stroud, S.W. Function of heparin. Nature 1955, 176, 1123. [Google Scholar] [CrossRef] [PubMed]
- Copes, F.; Chevallier, P.; Loy, C.; Pezzoli, D.; Boccafoschi, F.; Mantovani, D. Heparin-Modified Collagen Gels for Controlled Release of Pleiotrophin: Potential for Vascular Applications. Front. Bioeng. Biotechnol. 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Guidry, C.; Grinnell, F. Heparin modulates the organization of hydrated collagen gels and inhibits gel contraction by fibroblasts. J. Cell Biol. 1987, 104, 1097–1103. [Google Scholar] [CrossRef]
- Salchert, K.; Streller, U.; Pompe, T.; Herold, N.; Grimmer, M.; Werner, C. In vitro reconstitution of fibrillar collagen type I assemblies at reactive polymer surfaces. Biomacromolecules 2004, 5, 1340–1350. [Google Scholar] [CrossRef]
- Watarai, A.; Schirmer, L.; Thones, S.; Freudenberg, U.; Werner, C.; Simon, J.C.; Anderegg, U. TGFbeta functionalized starPEG-heparin hydrogels modulate human dermal fibroblast growth and differentiation. Acta Biomater. 2015, 25, 65–75. [Google Scholar] [CrossRef]
- Fahimipour, F.; Dashtimoghadam, E.; Mahdi Hasani-Sadrabadi, M.; Vargas, J.; Vashaee, D.; Lobner, D.C.; Jafarzadeh Kashi, T.S.; Ghasemzadeh, B.; Tayebi, L. Enhancing cell seeding and osteogenesis of MSCs on 3D printed scaffolds through injectable BMP2 immobilized ECM-Mimetic gel. Dent. Mater. 2019, 35, 990–1006. [Google Scholar] [CrossRef]
- Binner, M.; Bray, L.J.; Friedrichs, J.; Freudenberg, U.; Tsurkan, M.V.; Werner, C. Cell-instructive starPEG-heparin-collagen composite matrices. Acta Biomater. 2017, 53, 70–80. [Google Scholar] [CrossRef][Green Version]
- Smidsrod, O.; Skjakbrk, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Liu, C.; Lewin Mejia, D.; Chiang, B.; Luker, K.E.; Luker, G.D. Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion. Acta Biomater. 2018, 75, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Moxon, S.R.; Corbett, N.J.; Fisher, K.; Potjewyd, G.; Domingos, M.; Hooper, N.M. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109904. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.S.; Teply, B.A.; Stevens, M.M.; Zeitels, S.M.; Langer, R. Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials 2006, 27, 1104–1109. [Google Scholar] [CrossRef]
- Jin, G.Z.; Kim, H.W. Efficacy of collagen and alginate hydrogels for the prevention of rat chondrocyte dedifferentiation. J. Tissue Eng. 2018, 9, 2041731418802438. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, C.; Jin, G.Z.; Kim, H.W. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes and their phenotype maintenance. Tissue Eng. Regen. Med. 2016, 13, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Mei, E.C.; Li, S.K.; Song, J.W.; Xing, R.R.; Li, Z.M.; Yan, X.H. Self-assembling Collagen/Alginate hybrid hydrogels for combinatorial photothermal and immuno tumor therapy. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 577, 570–575. [Google Scholar] [CrossRef]
- Wong, F.S.; Wong, C.C.; Chan, B.P.; Lo, A.C. Sustained Delivery of Bioactive GDNF from Collagen and Alginate-Based Cell-Encapsulating Gel Promoted Photoreceptor Survival in an Inherited Retinal Degeneration Model. PLoS ONE 2016, 11, e0159342. [Google Scholar] [CrossRef]
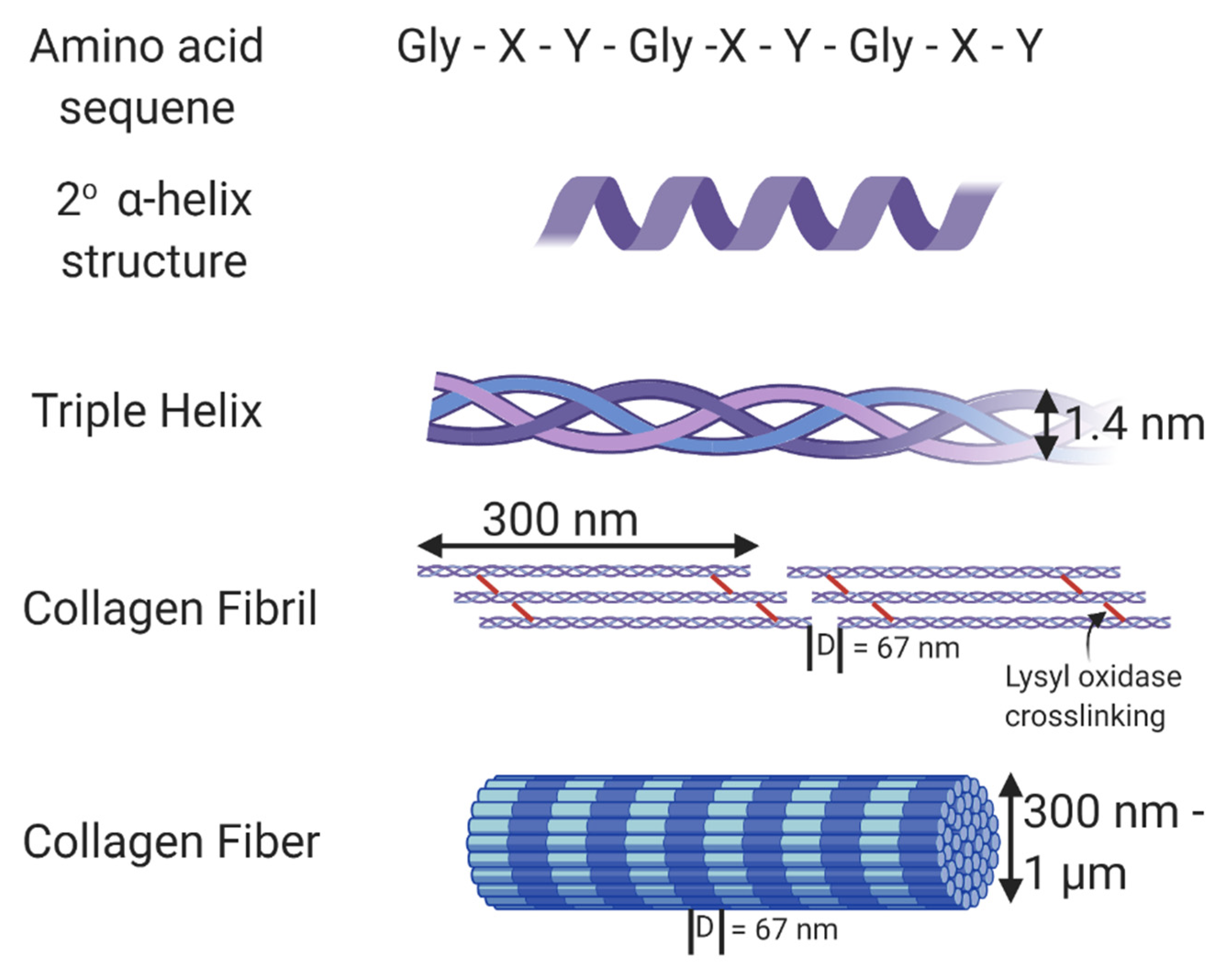
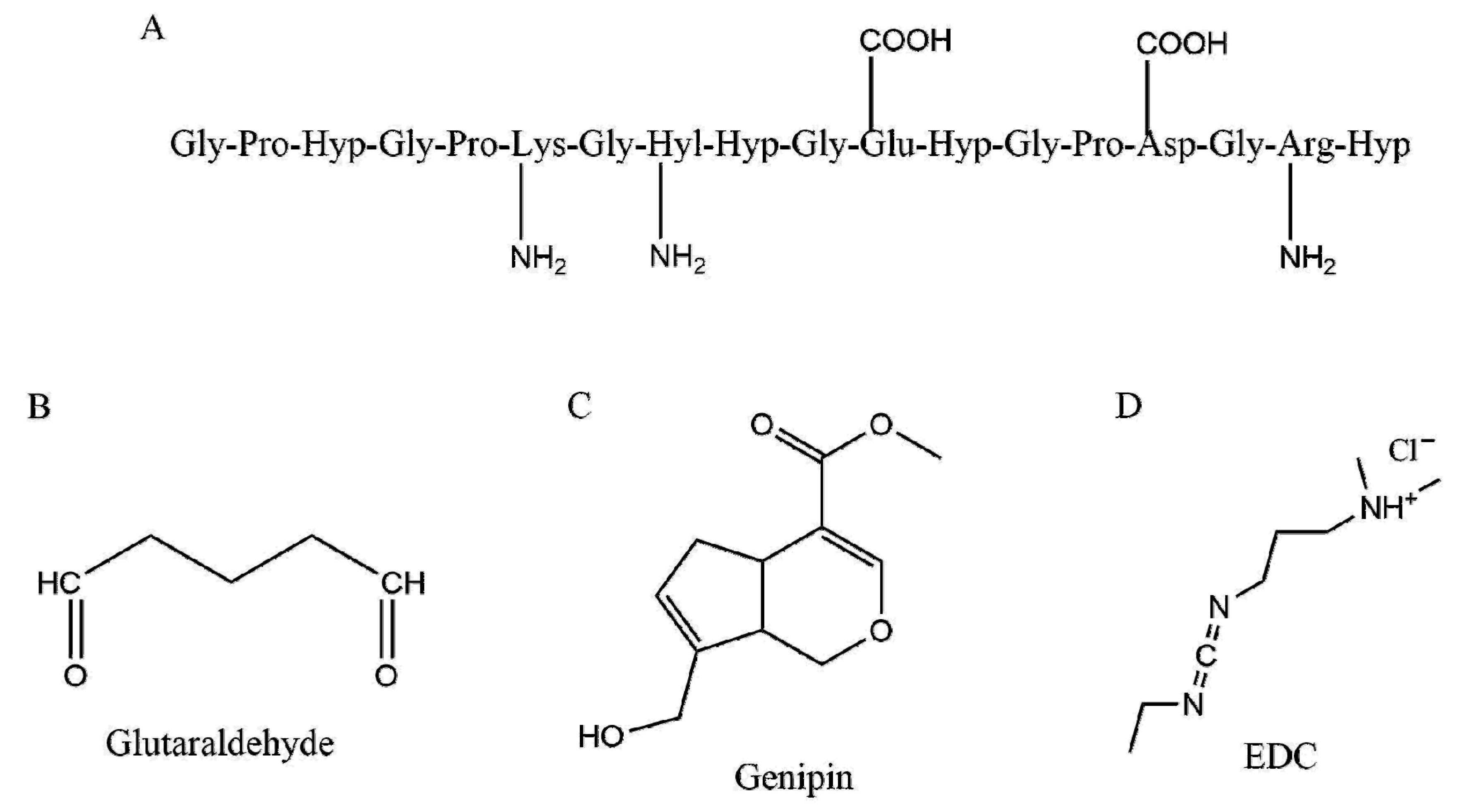
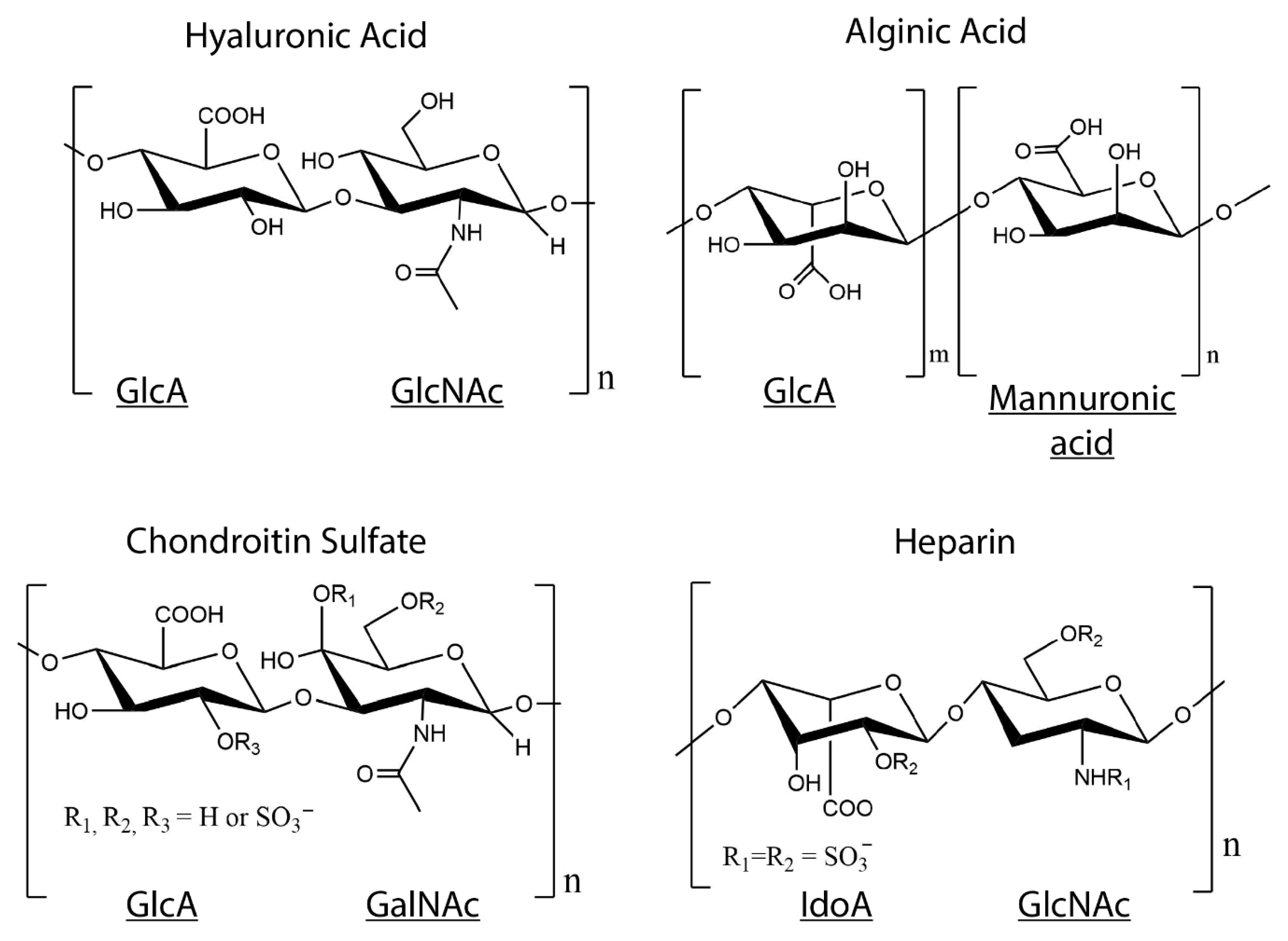
| Collagen Hydrogels with No Crosslinkers | |||
| Hydrogel | Collagen Concentration and Temperature | Application | Reference |
| Collagen type I | 3 mg/mL, 37 °C 8 mg/mL, 37 °C 7 mg/mL, 37 °C 3.45 mg/mL, 37 °C | 3D test bed for drug testing | [41] |
| 3D tumor model | [43,51] | ||
| Stem cell differentiation | [47,48,49,50] | ||
| Electrochemically or magnetically aligned collagen | 7 mg/mL, 37 °C 4 mg/mL, 37 °C 5 mg/mL, 37 °C 4 mg/mL, 30 °C 3 mg/mL, 25 °C | Tendon tissue engineering | [54,55] |
| Cartilage tissue engineering | [56] | ||
| Corneal tissue engineering | [57,58] | ||
| Neural tissue engineering | [61] | ||
| Collagen type I and/or type II | 4 mg/mL, 37 °C | Cartilage tissue engineering | [44,45,46,68] |
| Concentrated/compressed collagen | 2 mg/mL, 10 mg/mL, 15 mg/mL, 25 °C | Dermal tissue engineering | [63,64] |
| Crosslinked Collagen Hydrogels | |||
| Hydrogel | Collagen Concentration and Temperature | Application | Reference |
| EDC crosslinked collagen | 6.33 mg/mL, 4 °C | Corneal tissue engineering | [67] |
| Genipin crosslinked collagen | 2 mg/mL, 37 °C 6 mg/mL | Cartilage tissue engineering, stem cell differentiation | [69,72] |
| Dehydrothermal or UV crosslinked collagen | Unknown; Unknown | Vascular tissue engineering | [86] |
| Tendon tissue engineering | [88] | ||
| Thiol crosslinked collagen | 1% wt/v, 37 °C 3 mg/mL, 37 °C | Cardiovascular tissue engineering | [79] |
| Liver regeneration | [113] | ||
| Skin tissue engineering | [80] | ||
| Collagen HA Hydrogels | |||
| Hydrogel | Collagen Concentration and Temperature | Application | Reference |
| Sulfated HA–collagen | 1 mg/mL, 37 °C 0.5 mg/mL, 37 °C 1 mg/mL, 37 °C | Vascular tissue engineering | [105] |
| Skin tissue engineering | [106] | ||
| Bone tissue engineering | [107,108,109,110] | ||
| Thiolated HA–collagen IPN | 4 mg/mL, 37 °C | Vocal fold tissue engineering | [114] |
| HA hydrazine, HA aldehyde–collagen IPN | 2.5 mg/mL, 37 °C | Mimic in vivo microenvironment | [126] |
| Photocrosslinked HA–collagen IPN | 3 mg/mL, 37 °C 3 mg/mL, 37 °C | Regenerative medicine | [115,116] |
| Neural tissue engineering | [117] | ||
| EDC crosslinked HA–collagen | 0.5 wt%, 1 wt% 5 mg/mL, 25 °C 1 mg/mL, 37 °C 6 mg/mL, 37 °C | Stem cell differentiation | [118,119] |
| Cartilage tissue engineering | [60,120] | ||
| Dermal tissue engineering | [123,125] | ||
| Corneal tissue engineering | [124] | ||
| HA aldehyde–aminooxy PEG-collagen | 50, 100, 200 μg/mL | Neural tissue engineering | [127] |
| AAD modified HA-collagen | 8 mg/mL, 25 °C | Cartilage tissue engineering | [128] |
| Collagen CS Hydrogels | |||
| Hydrogel | Application | Reference | |
| Photocrosslinked CS–collagen IPN | 5 mg/mL, 37 °C | Cartilage tissue engineering | [129] |
| Dehydrothermal crosslinked CS–collagen | 2.6 mg/mL, 25 °C | Cartilage and dermal tissue engineering | [130] |
| Genipin crosslinked CS–collagen | 1 mg/mL, 37 °C | Cartilage tissue engineering | [131] |
| EDC crosslinked CS–collagen | 2.5 mg/mL 11, 8, 6, 4, 3 mg/mL | Dermal tissue engineering | [132,133] |
| Cartilage tissue engineering | [60] | ||
| Neural tissue engineering | [134,135] | ||
| Non crosslinked CS–collagen | 4 mg/mL, 37 °C | Cartilage tissue engineering | [137] |
| Collagen Heparin Hydrogels | |||
| Hydrogel | Collagen Concentration and Temperature | Application | Reference |
| Non crosslinked Heparin-collagen | 4 mg/mL, 25 °C | Vascular tissue engineering | [139] |
| EDC crosslinked Heparin–collagen | 2.5 mg/mL, 37 °C | Bone tissue engineering | [143] |
| starPEG–heparin–collagen | unknown | Cell instruction and differentiation | [142] |
| Collagen–Alginate hydrogels | |||
| Hydrogel | Collagen Concentration and Temperature | Application | Reference |
| CaCl2 crosslinked alginate–collagen IPN | 3 mg/mL, 37 °C 2.5 mg/mL, 37 °C 5 mg/mL, 37 °C 1 mg/mL, 37 °C 2 mg/mL, 37 °C | 3D tumor model | [146] |
| Neural tissue engineering | [147] | ||
| Vocal fold tissue engineering | [148] | ||
| Cartilage tissue engineering | [149,150,151] | ||
| Corneal tissue engineering | [153] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walimbe, T.; Panitch, A. Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels. Bioengineering 2020, 7, 156. https://doi.org/10.3390/bioengineering7040156
Walimbe T, Panitch A. Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels. Bioengineering. 2020; 7(4):156. https://doi.org/10.3390/bioengineering7040156
Chicago/Turabian StyleWalimbe, Tanaya, and Alyssa Panitch. 2020. "Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels" Bioengineering 7, no. 4: 156. https://doi.org/10.3390/bioengineering7040156
APA StyleWalimbe, T., & Panitch, A. (2020). Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels. Bioengineering, 7(4), 156. https://doi.org/10.3390/bioengineering7040156






