Hemodynamic Performance of Dysfunctional Prosthetic Heart Valve with the Concomitant Presence of Subaortic Stenosis: In Silico Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Numerical Simulation Setup
2.2. Validation
3. Results
3.1. Hemodynamics
3.1.1. Velocity Contours
3.1.2. Vortex Dynamics
3.1.3. Turbulent Shear Stress
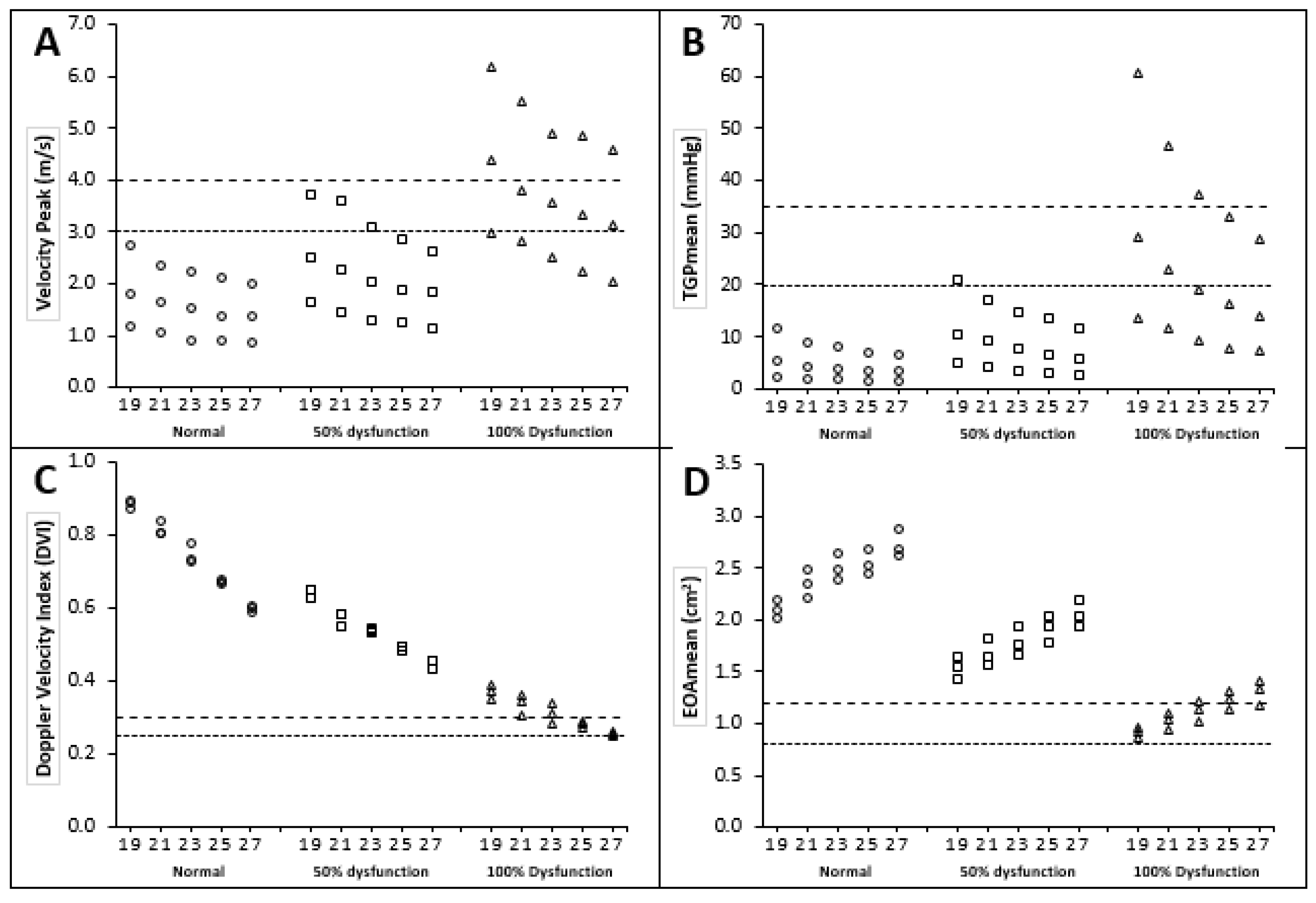
3.2. Doppler Echocardiographic Measurements
3.2.1. Peak Velocity and Mean Transvalvular Pressure Gradient
3.2.2. Doppler Velocity Index (DVI)
3.2.3. Effective Orifice Area (EOA)
3.3. Sensitivity and Specificity Analysis for Different Echo Doppler Parameters
4. Discussion
4.1. Flow Characteristics
4.2. Platelet Activation and Blood Components Damage
4.3. Evaluation of Doppler Parameters
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; O’Brien, K.D.; et al. Calcific Aortic Valve Disease: Not Simply a Degenerative Process A Review and Agenda for Research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Circulation 2011, 124, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Smadi, O.; Fenech, M.; Hassan, I.; Kadem, L. Flow through a defective mechanical heart valve: A steady flow analysis. Med. Eng. Phys. 2009, 31, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Aboulhosn, J.; Child, J.S. Left ventricular outflow obstruction: Subaortic stenosis, bicuspid aortic valve, supravalvar aortic stenosis, and coarctation of the aorta. Circulation 2006, 114, 2412–2422. [Google Scholar] [CrossRef] [PubMed]
- Ezon, D.S. Fixed subaortic stenosis: A clinical dilemma for clinicians and patients. Congenit. Heart Dis. 2013, 8, 450–456. [Google Scholar] [CrossRef]
- Aboulhosn, J.; Child, J.S. Echocardiographic evaluation of congenital left ventricular outflow obstruction. Echocardiography 2015, 32, 32–39. [Google Scholar] [CrossRef]
- Jonas, R.A. Modified Konno procedure for tunnel subaortic stenosis. Oper. Tech. Thorac. Cardiovasc. Surg. 2002, 7, 176–180. [Google Scholar] [CrossRef]
- Maron, B.J.; Redwood, D.R.; Roberts, W.C.; Henry, W.L.; Morrow, A.G.; Epstein, S.E. Tunnel Subaortic Stenosis Left Ventricular Outflow Tract Obstruction Produced by Fibromuscular Tubular Narrowing. Circulation 1976, 54, 404–416. [Google Scholar] [CrossRef]
- Opotowsky, A.R.; Pickard, S.S.; Geva, T. Imaging adult patients with discrete subvalvar aortic stenosis. Curr. Opin. Cardiol. 2017, 32, 513–520. [Google Scholar] [CrossRef]
- Hedayat, M.; Borazjani, I. Comparison of platelet activation through hinge vs bulk flow in bileaflet mechanical heart valves. J. Biomech. 2019, 83, 280–290. [Google Scholar] [CrossRef]
- Mashari, A.; Mahmood, F. Fixed versus dynamic left ventricular outflow tract obstruction: Res ipsa loquitur. J. Thorac. Cardiovasc. Surg. 2016, 151, 885–886. [Google Scholar] [CrossRef]
- Cilliers, A.M.; Gewillig, M. Rheology of discrete subaortic stenosis. Heart 2002, 88, 335–336. [Google Scholar] [CrossRef][Green Version]
- D’Andrea, A.; Padalino, R.; Cocchia, R.; Di Palma, E.; Riegler, L.; Scarafile, R.; Rossi, G.; Bianchi, R.; Tartaglione, D.; Cappelli Bigazzi, M.; et al. Effects of transcatheter aortic valve implantation on left ventricular and left atrial morphology and function. Echocardiography 2015, 32, 928–936. [Google Scholar] [CrossRef]
- Ravindra, V.M.; Mazur, M.D.; Kumpati, G.S.; Park, M.S.; Patel, A.N.; Tandar, A.; Welt, F.G.; Bull, D.; Couldwell, W.T.; Taussky, P. Carotid Artery Stenosis in the Setting of Transcatheter Aortic Valve Replacement: Clinical and Technical Considerations of Carotid Stenting. World Neurosurg. 2016, 86, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.D.; Weitz, J.I.; Giustino, G.; Makkar, R.; Mehran, R. Prosthetic Heart Valve Thrombosis. J. Am. Coll. Cardiol. 2016, 68, 2670–2689. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, I.; Noly, P.E.; Ghoneim, A.; Stevens, L.M.; Cartier, R.; Poirier, N.; Bouchard, D.; Demers, P.; El-Hamamsy, I. Is the Ross procedure a riskier operation? Perioperative outcome comparison with mechanical aortic valve replacement in a propensity-matched cohort. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 41–47. [Google Scholar] [CrossRef]
- Darwazah, A.K. Recurrent pannus formation causing prosthetic aortic valve dysfunction: Is excision without valve re-replacement applicable? J. Cardiothorac. Surg. 2012, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.-S.; Bedeleanu, D.; Kovacs, E.; Ciumărnean, L.; Molnar, A. PANNUS-RELATED PROSTHETIC VALVE DYSFUNCTION. CASE REPORT. Clujul Med. 2016, 89, 169–175. [Google Scholar] [CrossRef]
- Roudaut, R.; Serri, K.; Lafitte, S. Thrombosis of prosthetic heart valves: Diagnosis and therapeutic considerations. Heart 2007, 93, 137–142. [Google Scholar] [CrossRef]
- Smadi, O.; Garcia, J.; Pibarot, P.; Gaillard, E.; Hassan, I.; Kadem, L. Accuracy of Doppler-echocardiographic parameters for the detection of aortic bileaflet mechanical prosthetic valve dysfunction. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 142–151. [Google Scholar] [CrossRef]
- Gaillard, E.; Kadem, L.; Pibarot, P.; Durand, L.G. Impact of a concomitant subaortic stenosis on the assessment of the severity of an aortic valve stenosis: An in-vitro study. In IFMBE Proceedings; Ratko, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 31, pp. 394–397. [Google Scholar]
- Ge, L.; Leo, H.L.; Sotiropoulos, F.; Yoganathan, A.P. Flow in a mechanical bileaflet heart valve at laminar and near-peak systole flow rates: CFD simulations and experiments. J. Biomech. Eng. 2005, 127, 782–797. [Google Scholar] [CrossRef]
- Hellmeier, F.; Nordmeyer, S.; Yevtushenko, P.; Bruening, J.; Berger, F.; Kuehne, T.; Goubergrits, L.; Kelm, M. Hemodynamic Evaluation of a Biological and Mechanical Aortic Valve Prosthesis Using Patient-Specific MRI-Based CFD. Artif. Organs 2018, 42, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.H.; Schubert, K.; Jamali, A.; Scharfschwerdt, M. The influence of different inflow configurations on computational fluid dynamics in a novel three-leaflet mechanical heart valve prosthesis. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Smadi, O.; Hassan, I.; Pibarot, P.; Kadem, L. Numerical and experimental investigations of pulsatile blood flow pattern through a dysfunctional mechanical heart valve. J. Biomech. 2010, 43. [Google Scholar] [CrossRef] [PubMed]
- Khalili, F.; Gamage, P.P.T.; Sandler, R.H.; Mansy, H.A. Adverse hemodynamic conditions associated with mechanical heart valve leaflet immobility. Bioengineering 2018, 5, 74. [Google Scholar] [CrossRef]
- Jahandardoost, M.; Fradet, G.; Mohammadi, H. Effect of heart rate on the hemodynamics of bileaflet mechanical heart valves’ prostheses (St. Jude Medical) in the aortic position and in the opening phase: A computational study. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2016, 230, 175–190. [Google Scholar] [CrossRef]
- Xu, X.; Liu, T.; Li, C.; Zhu, L.; Li, S. A Numerical Analysis of Pressure Pulsation Characteristics Induced by Unsteady Blood Flow in a Bileaflet Mechanical Heart Valve. Processes 2019, 7, 232. [Google Scholar] [CrossRef]
- Guivier, C.; Deplano, V.; Pibarot, P. New insights into the assessment of the prosthetic valve performance in the presence of subaortic stenosis through a fluid-structure interaction model. J. Biomech. 2007, 40, 2283–2290. [Google Scholar] [CrossRef]
- Redaelli, A.; Bothorel, H.; Votta, E.; Soncini, M.; Morbiducci, U.; Del Gaudio, C.; Balducci, A.; Grigioni, M. 3-D Simulation of the st. Jude medical bileaflet valve opening process: Fluid-structure interaction study and experimental validation. J. Heart Valve Dis. 2004, 13, 804–813. [Google Scholar]
- Dumont, K.; Vierendeels, J.; Kaminsky, R.; Van Nooten, G.; Verdonck, P.; Bluestein, D. Comparison of the hemodynamic and thrombogenic performance of two bileaflet mechanical heart valves using a CFD/FSI model. J. Biomech. Eng. 2007, 129, 558–565. [Google Scholar] [CrossRef]
- Annerel, S.; Claessens, T.; Degroote, J.; Segers, P.; Vierendeels, J. Validation of a numerical FSI simulation of an aortic BMHV by in vitro PIV experiments. Med. Eng. Phys. 2014, 36, 1014–1023. [Google Scholar] [CrossRef]
- Yeh, H.H.; Barannyk, O.; Grecov, D.; Oshkai, P. The influence of hematocrit on the hemodynamics of artificial heart valve using fluid-structure interaction analysis. Comput. Biol. Med. 2019, 110, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Schima, H.; Kühn, P. Discrepancies between Doppler and catheter gradients across bileaflet aortic valve prostheses. Am. J. Cardiol. 1993, 71, 1241–1243. [Google Scholar] [CrossRef]
- Susin, F.M.; Espa, S.; Toninato, R.; Fortini, S.; Querzoli, G. Integrated strategy for in vitro characterization of a bileaflet mechanical aortic valve. Biomed. Eng. Online 2017, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Querzoli, G.; Fortini, S.; Cenedese, A. Effect of the prosthetic mitral valve on vortex dynamics and turbulence of the left ventricular flow. Phys. Fluids 2010, 22, 1–10. [Google Scholar] [CrossRef]
- Darwish, A.; Di Labbio, G.; Saleh, W.; Smadi, O.; Kadem, L. Experimental investigation of the flow downstream of a dysfunctional bileaflet mechanical aortic valve. Artif. Organs 2019, 43. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Chambers, J.B.; Dumesnil, J.G.; Foster, E.; Gottdiener, J.S.; Grayburn, P.A.; Khandheria, B.K.; Levine, R.A.; Marx, G.R.; Miller, F.A.; et al. Recommendations for Evaluation of Prosthetic Valves With Echocardiography and Doppler Ultrasound. A Report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, Developed in Conjunction. J. Am. Soc. Echocardiogr. 2009, 22, 975–1014. [Google Scholar] [CrossRef]
- Khalili, F. Hemodynamics of a Bileaflet Mechanical Heart Valve with Different Levels of Dysfunction. J. Appl. Biotechnol. Bioeng. 2017, 2, 2–7. [Google Scholar] [CrossRef]
- Bluestein, D.; Rambod, E.; Gharib, M. Vortex shedding as a mechanism for free emboli formation in mechanical heart valves. J. Biomech. Eng. 2000, 122, 125–134. [Google Scholar] [CrossRef]
- Grigioni, M.; Daniele, C.; D’Avenio, G.; Barbaro, V. The influence of the leaflets’ curvature on the flow field in two bileaflet prosthetic heart valves. J. Biomech. 2001, 34, 613–621. [Google Scholar] [CrossRef]
- De Tullio, M.D.; Cristallo, A.; Balaras, E.; Verzicco, R. Direct numerical simulation of the pulsatile flow through an aortic bileaflet mechanical heart valve. J. Fluid Mech. 2009, 622, 259–290. [Google Scholar] [CrossRef]
- Liu, J.S.; Lu, P.; Chu, S. Turbulence characteristics downstream of bileaflet aortic valve prostheses. J. Biomech. Eng. 2000, 122, 118–124. [Google Scholar] [CrossRef]
- Dasi, L.P.; Simon, H.A.; Sucosky, P.; Yoganathan, A.P. Fluid mechanics of artificial heart valves. Clin. Exp. Pharmacol. Physiol. 2009, 36, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Pedrizzetti, G.; Domenichini, F. Flow-driven opening of a valvular leaflet. J. Fluid Mech. 2006, 569, 321–330. [Google Scholar] [CrossRef]
- Li, C.P.; Chen, S.F.; Lo, C.W.; Lu, P.C. Turbulence characteristics downstream of a new trileaflet mechanical heart valve. ASAIO J. 2011, 57, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Soslau, G.; Schechner, A.J.; Alcasid, P.J.; Class, R. Influence of vortex speed on fresh versus stored platelet aggregation in the absence and presence of extracellular ATP. Thromb. Res. 2000, 97, 15–27. [Google Scholar] [CrossRef]
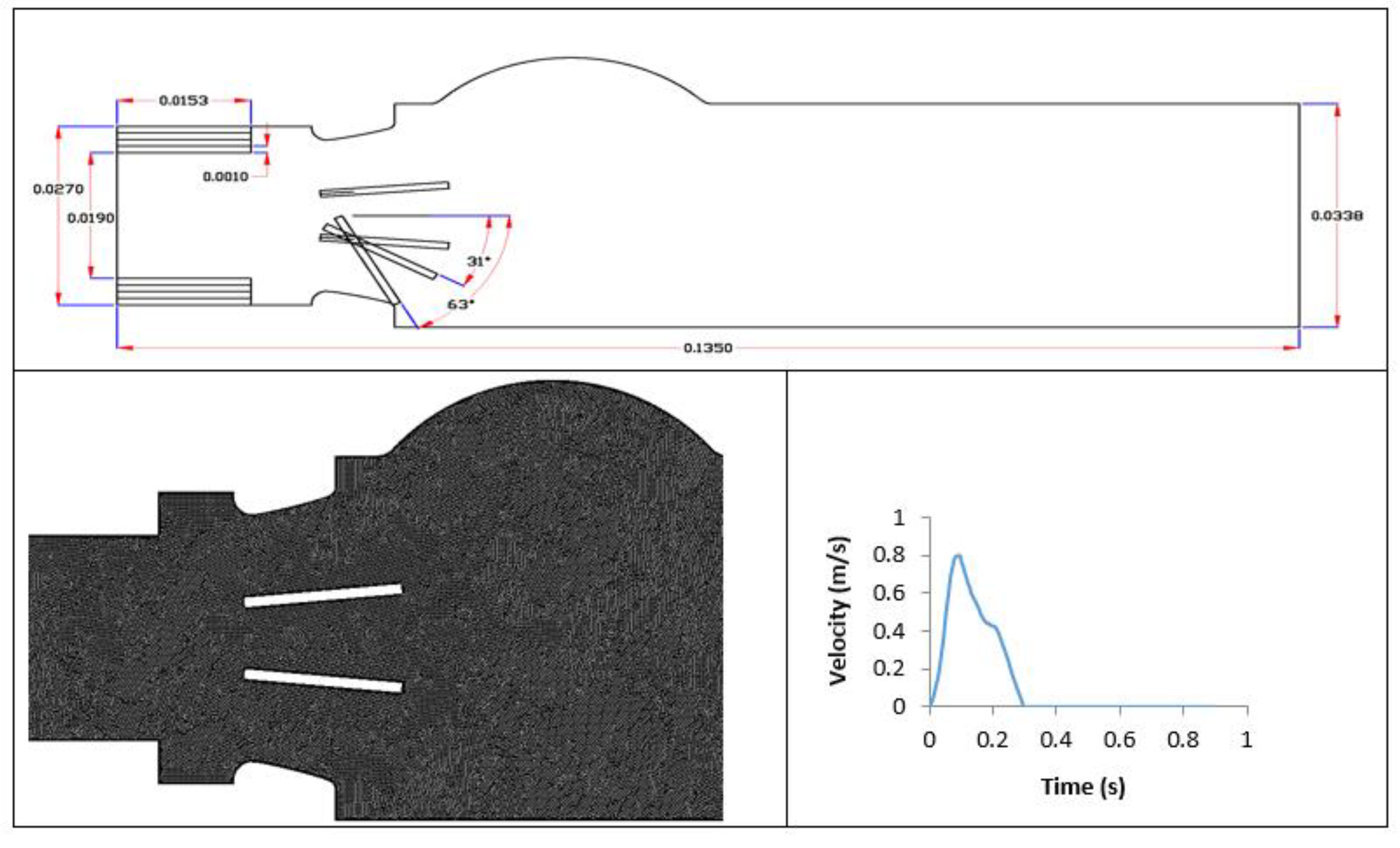
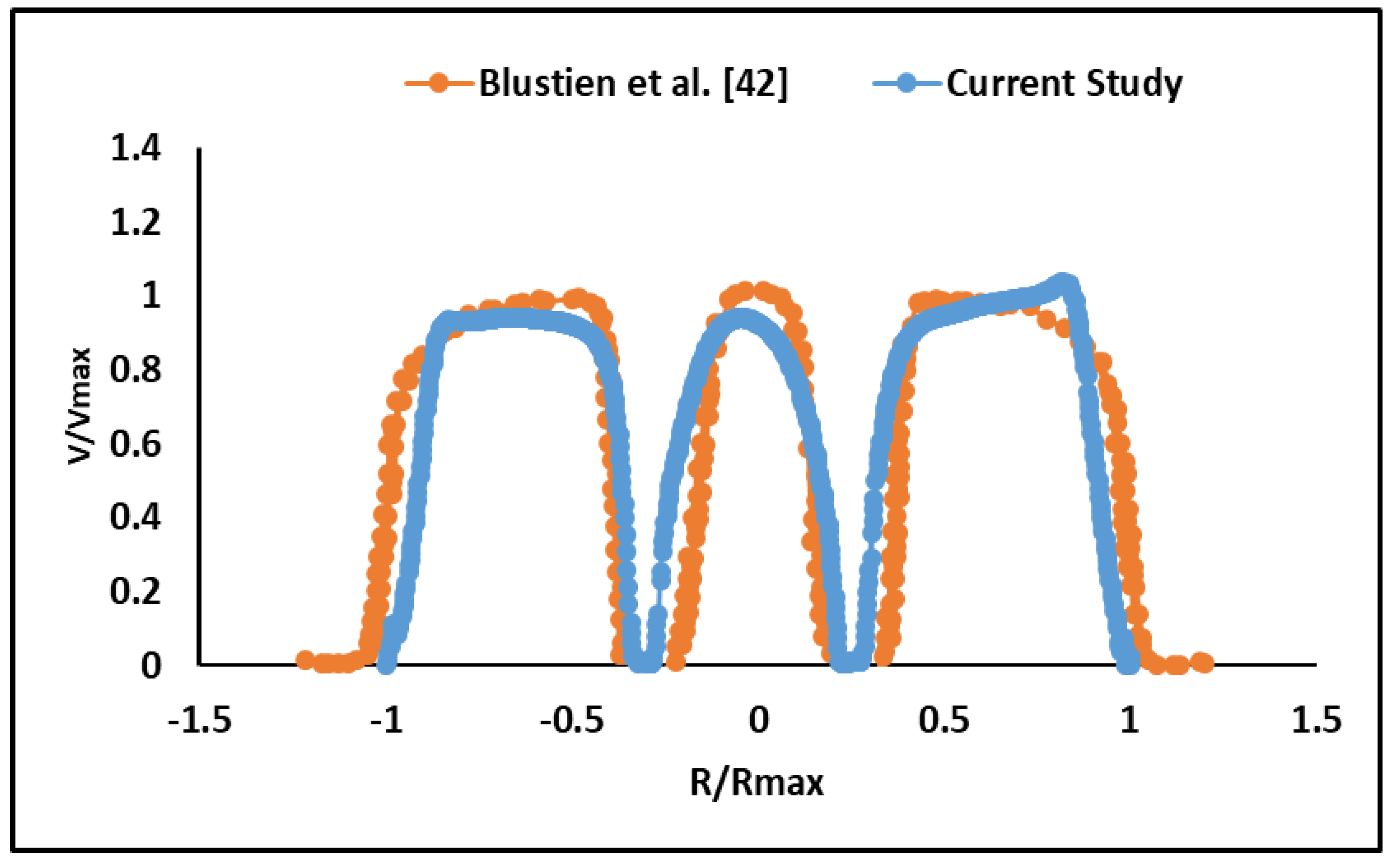

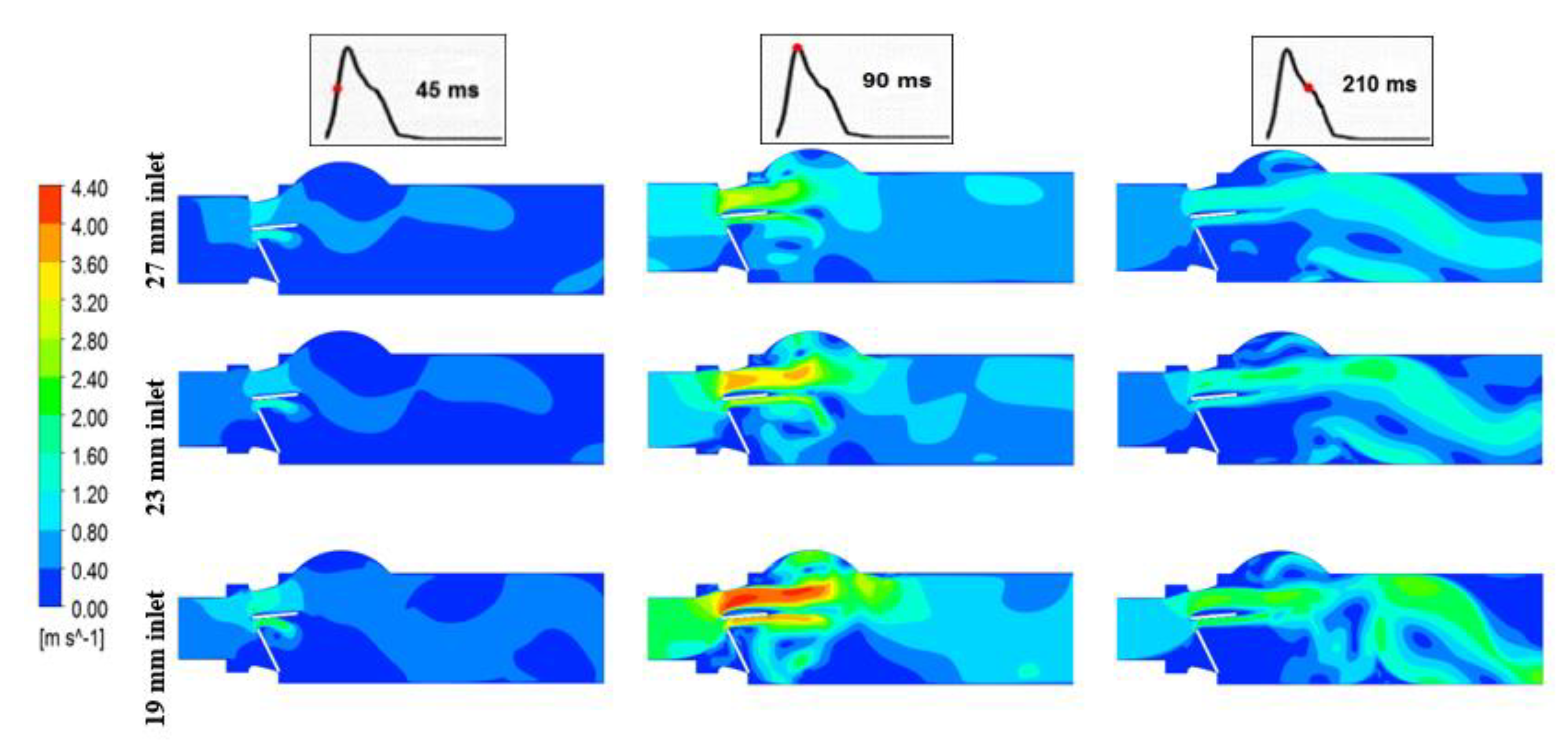
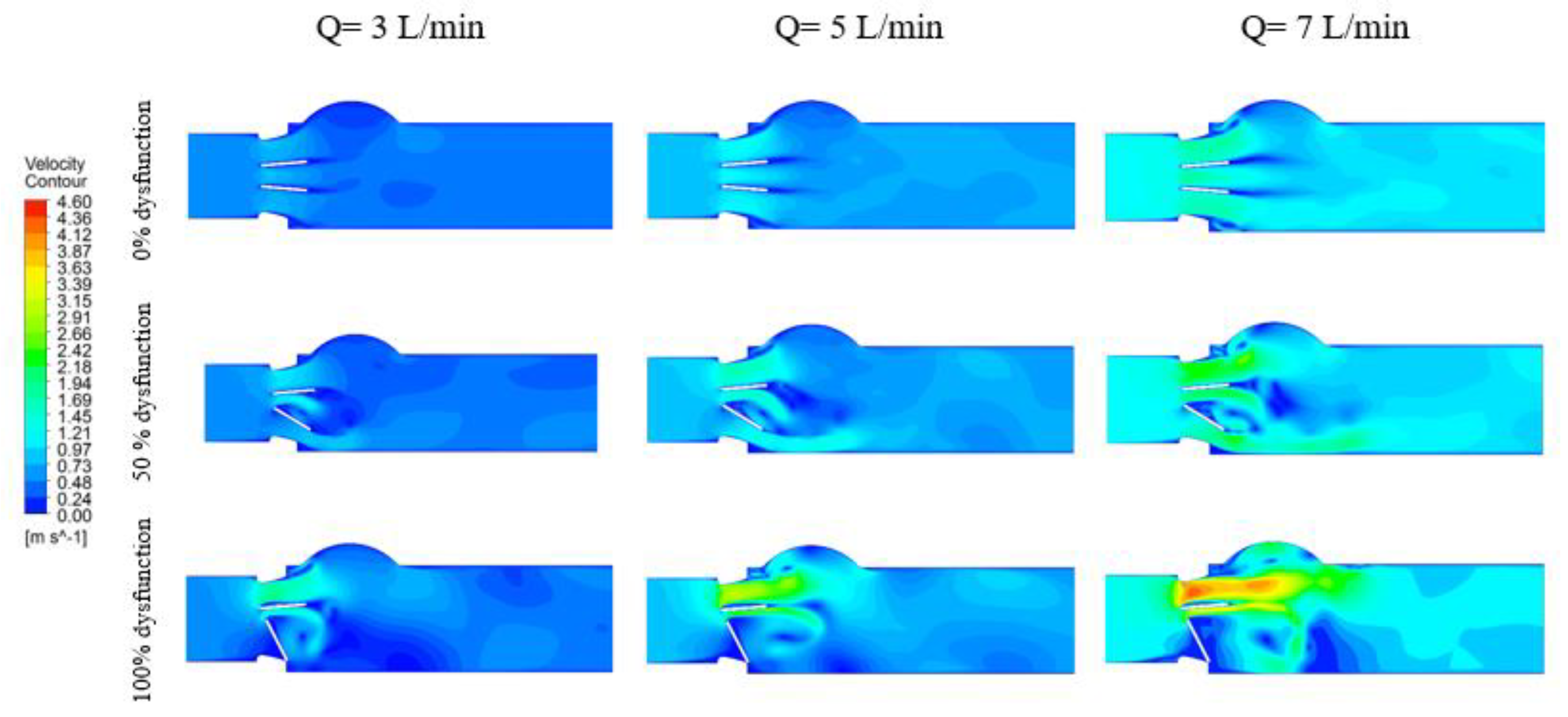



| Doppler Echocardiographic Parameter | LVOT Diameter | ||
|---|---|---|---|
| 27 mm | 23 mm | 19 mm | |
| Peak Velocity (m/s) | 2.17 ± 1.09 (0.86–4.58) | 2.45 ± 1.17 (0.91–4.89) | 3.02 ± 1.47 (1.19–6.20) |
| TGPmean (mmHg) | 9.03 ± 7.97 (1.42–28.71) | 11.73 ± 10.46 (1.71–37.40) | 17.68 ± 17.10 (2.44–60.53) |
| Doppler Velocity Index (DVI) | 0.43 ± 0.14 (0.25–0.61) | 0.53 ± 0.18 (0.28–0.78) | 0.63 ± 0.21 (0.35–0.89) |
| EOAmean (cm2) | 2.03 ± 0.59 (1.17–2.87) | 2.14 ± 0.37 (1.66–2.63) | 1.52 ± 0.49 (0.86–2.20) |
| Parameter | Diagnostic Criteria for Dysfunction | Detection Mild-to-Severe Dysfunction (both 50 % and 100 % Dysfunctional Leaflets are Considered) | Detection Moderate-to-Severe Dysfunction (only 100% Dysfunctional Leaflet) | ||
|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | ||
| Peak velocity | ≥4 m/s | 20 | 100 | 40 | 100 |
| ≥3 m/s | 47 | 100 | 73 | 90 | |
| TGPmean | ≥35 mmHg | 10 | 100 | 20 | 100 |
| ≥20 mmHg | 27 | 100 | 47 | 97 | |
| Doppler Velocity Index (DVI) | ≤0.35 | 40 | 100 | 80 | 100 |
| ≤0.30 | 27 | 100 | 53 | 100 | |
| ≤0.25 | 3 | 100 | 7 | 100 | |
| EOAmean | ≤1.2 cm2 | 33 | 100 | 67 | 100 |
| ≤0.8 cm2 | 0 | 100 | 0 | 100 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smadi, O.; Abdelkarim, A.; Awad, S.; D. Almomani, T. Hemodynamic Performance of Dysfunctional Prosthetic Heart Valve with the Concomitant Presence of Subaortic Stenosis: In Silico Study. Bioengineering 2020, 7, 90. https://doi.org/10.3390/bioengineering7030090
Smadi O, Abdelkarim A, Awad S, D. Almomani T. Hemodynamic Performance of Dysfunctional Prosthetic Heart Valve with the Concomitant Presence of Subaortic Stenosis: In Silico Study. Bioengineering. 2020; 7(3):90. https://doi.org/10.3390/bioengineering7030090
Chicago/Turabian StyleSmadi, Othman, Anas Abdelkarim, Samer Awad, and Thakir D. Almomani. 2020. "Hemodynamic Performance of Dysfunctional Prosthetic Heart Valve with the Concomitant Presence of Subaortic Stenosis: In Silico Study" Bioengineering 7, no. 3: 90. https://doi.org/10.3390/bioengineering7030090
APA StyleSmadi, O., Abdelkarim, A., Awad, S., & D. Almomani, T. (2020). Hemodynamic Performance of Dysfunctional Prosthetic Heart Valve with the Concomitant Presence of Subaortic Stenosis: In Silico Study. Bioengineering, 7(3), 90. https://doi.org/10.3390/bioengineering7030090






