Inoculum Source Determines Acetate and Lactate Production during Anaerobic Digestion of Sewage Sludge and Food Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Inoculum
2.2. Experimental Set-Up
2.3. Analytical Methods
2.4. Calculation of Efficiency of Hydrolysis, Acidogenesis, Acetogenesis, and Methanogenesis
- DOCres = residual dissolved organic carbon,
- DOCin = ingoing dissolved organic carbon,
- TCGases = total carbon in produced gases,
- TOCVFA = total organic carbon in volatile fatty acids,
- TOCtot = total ingoing organic carbon.
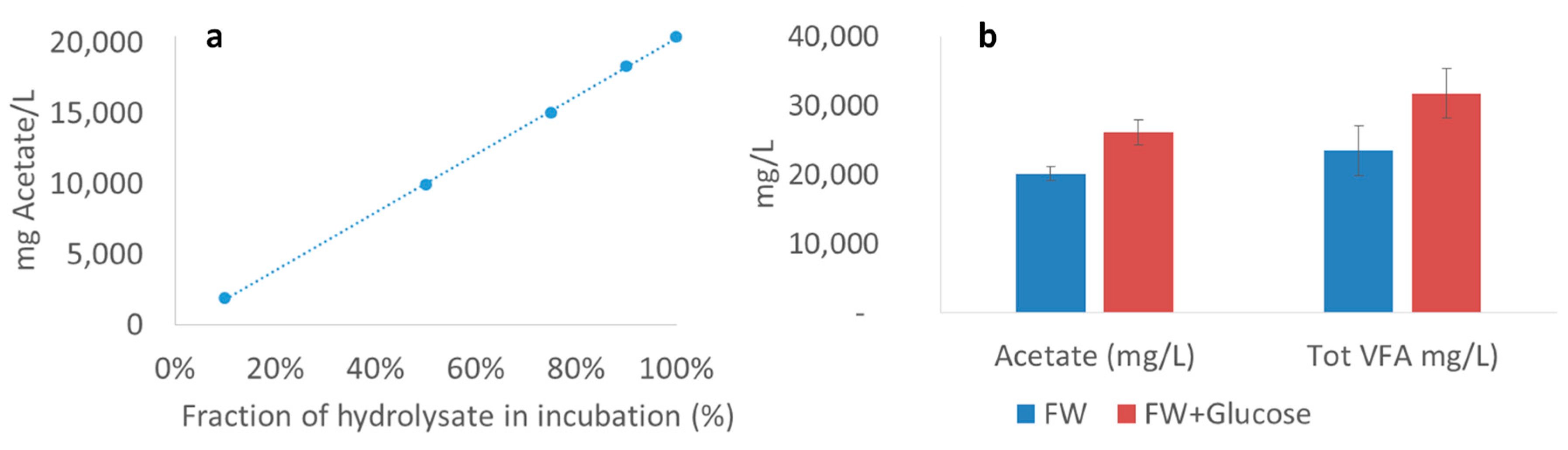
2.5. Additional Experiments to Determine Inhibitory Effects of VFA
2.6. Sample Collection, Molecular Analyses, and Sequence Data Processing
3. Results and Discussion
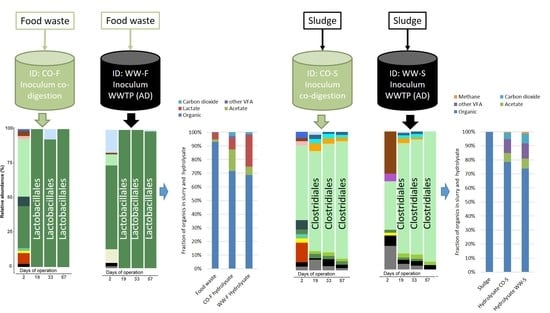
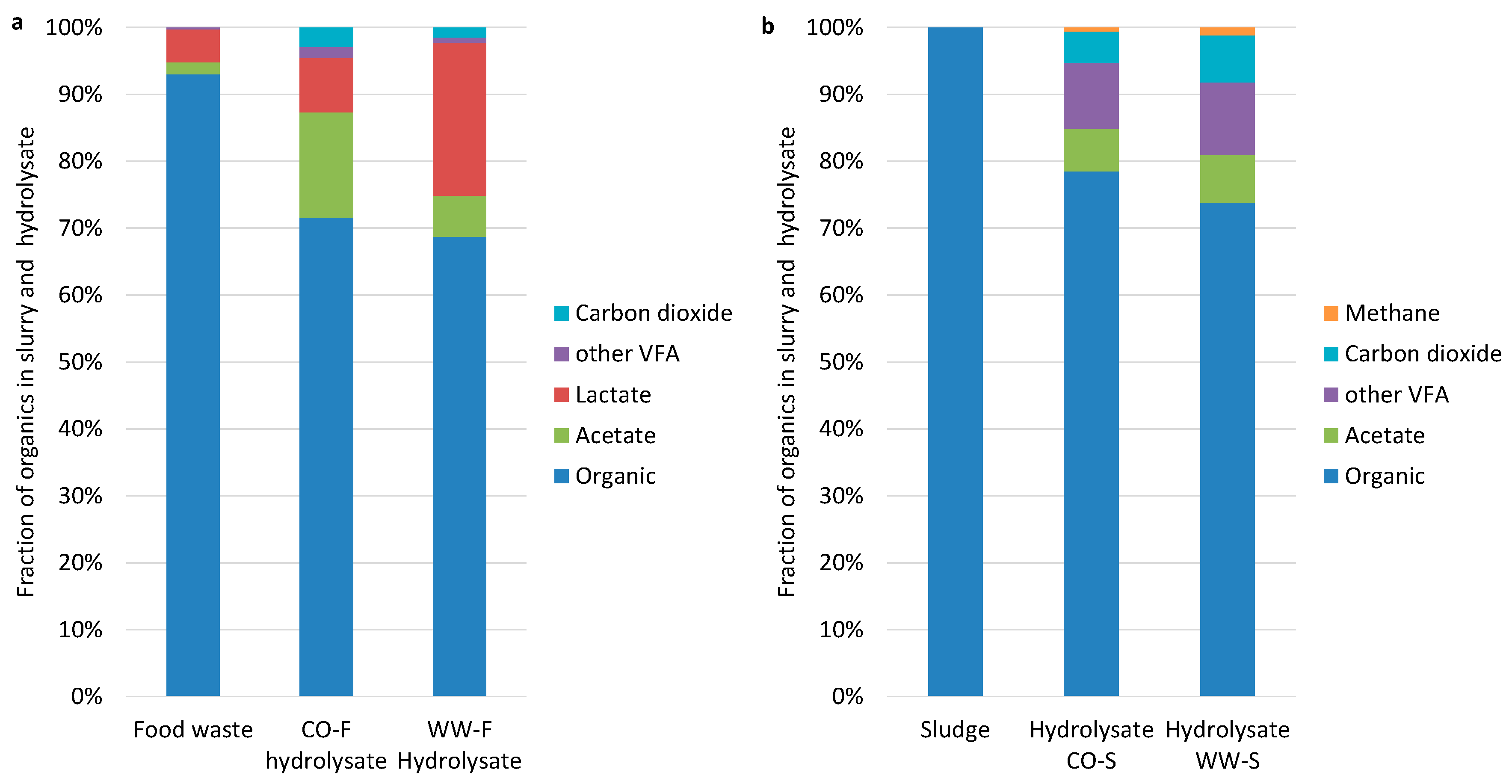
3.1. Hydrolysis Efficiency
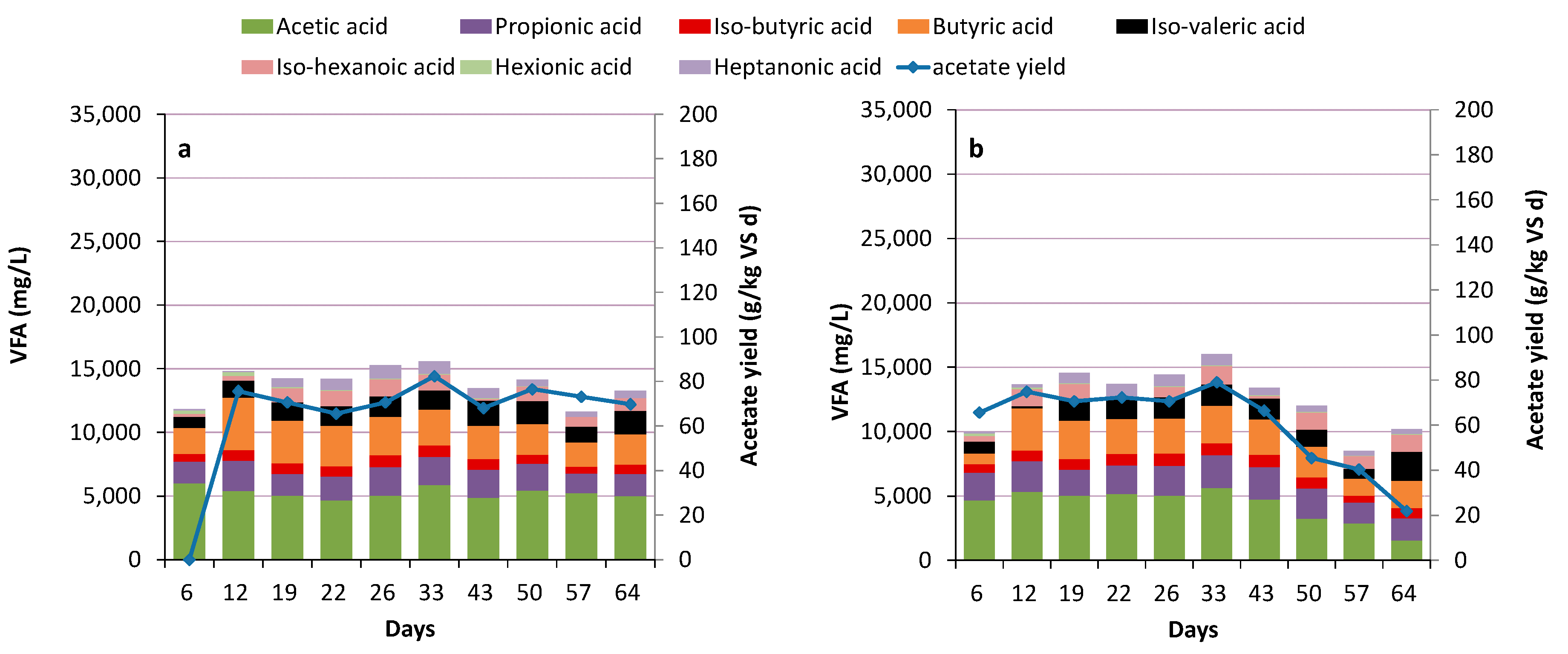
3.2. Acidogenesis and Acetogenesis
Limiting Factors—Undissociated Acetate and Ingoing DOC
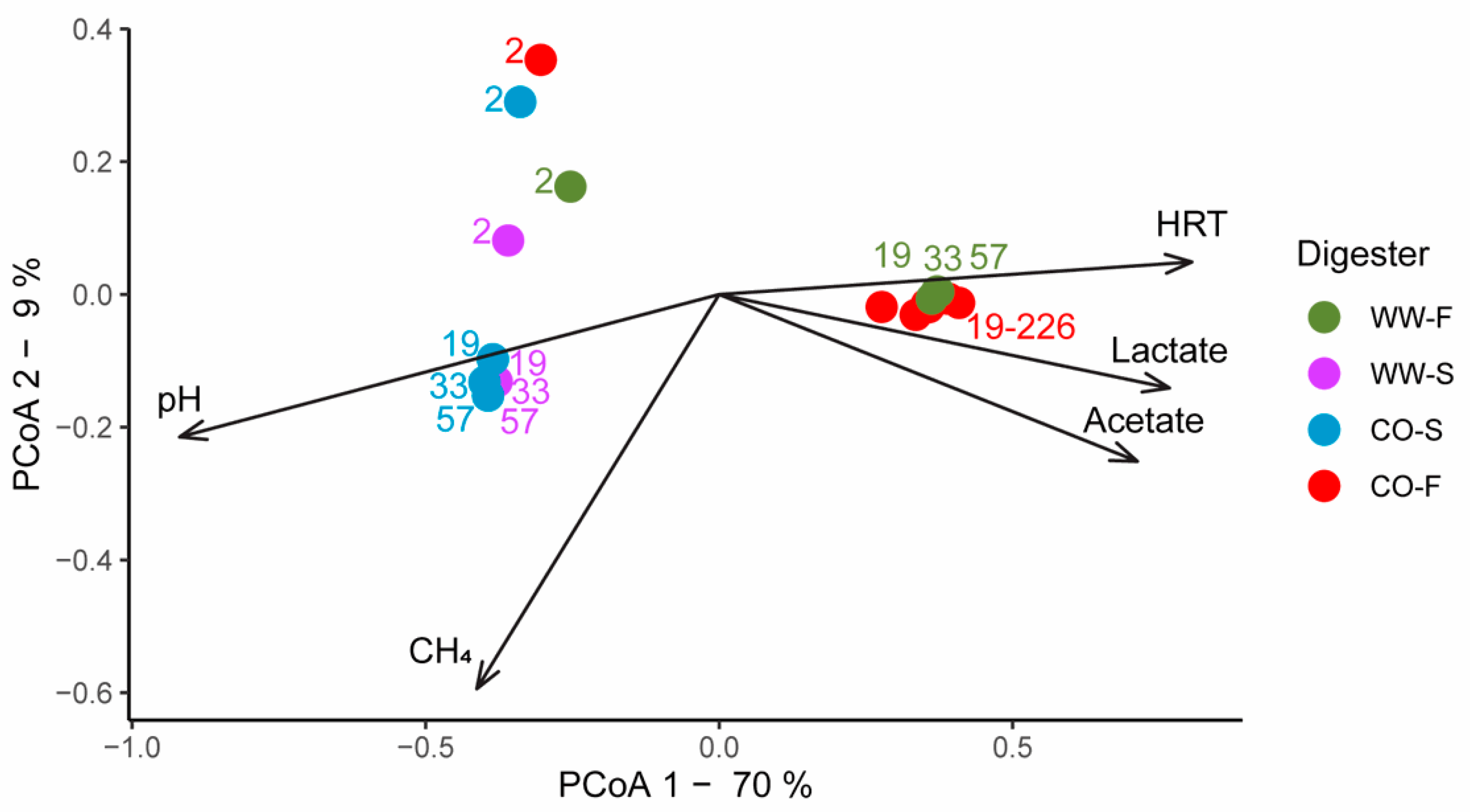
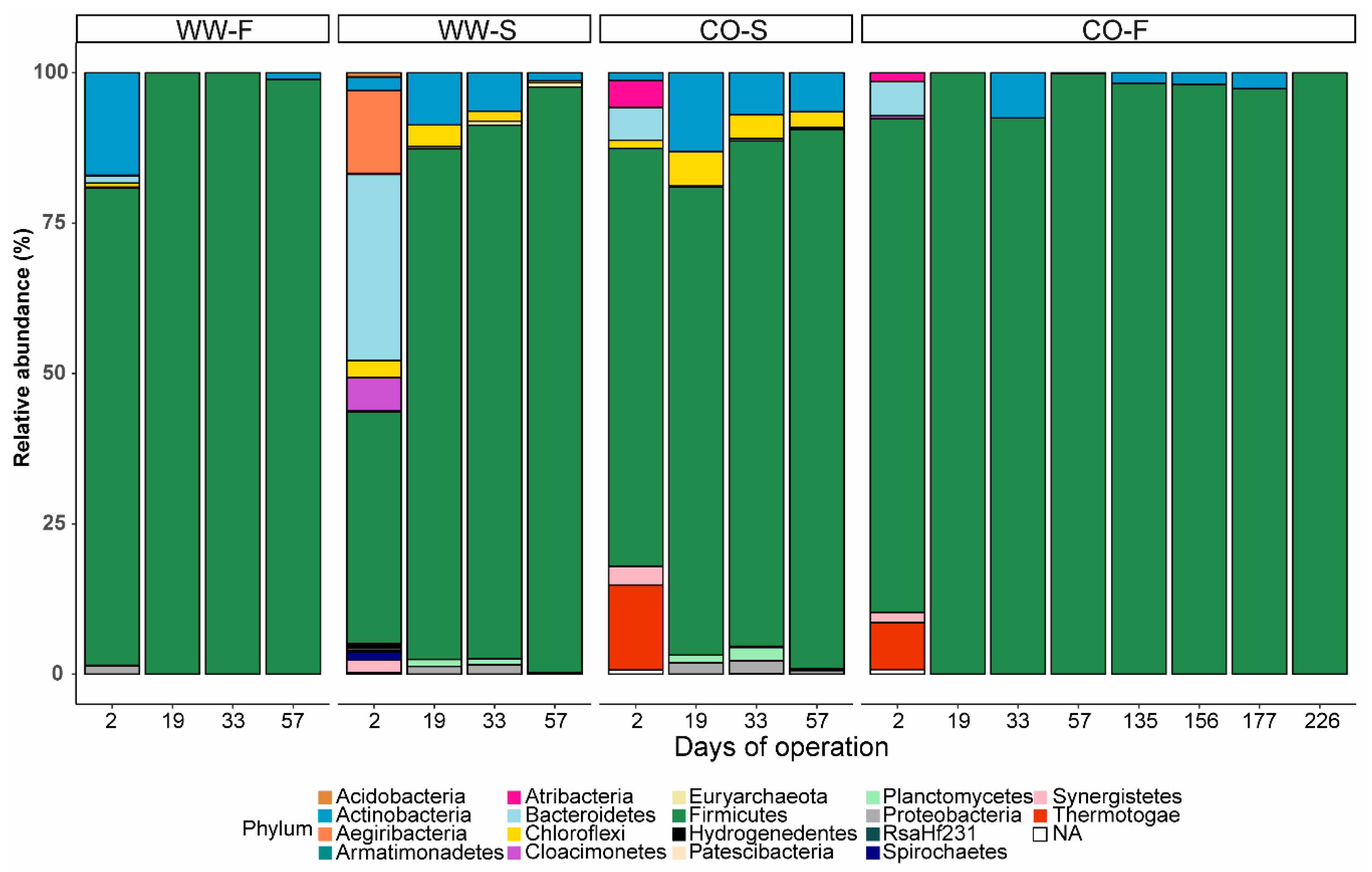
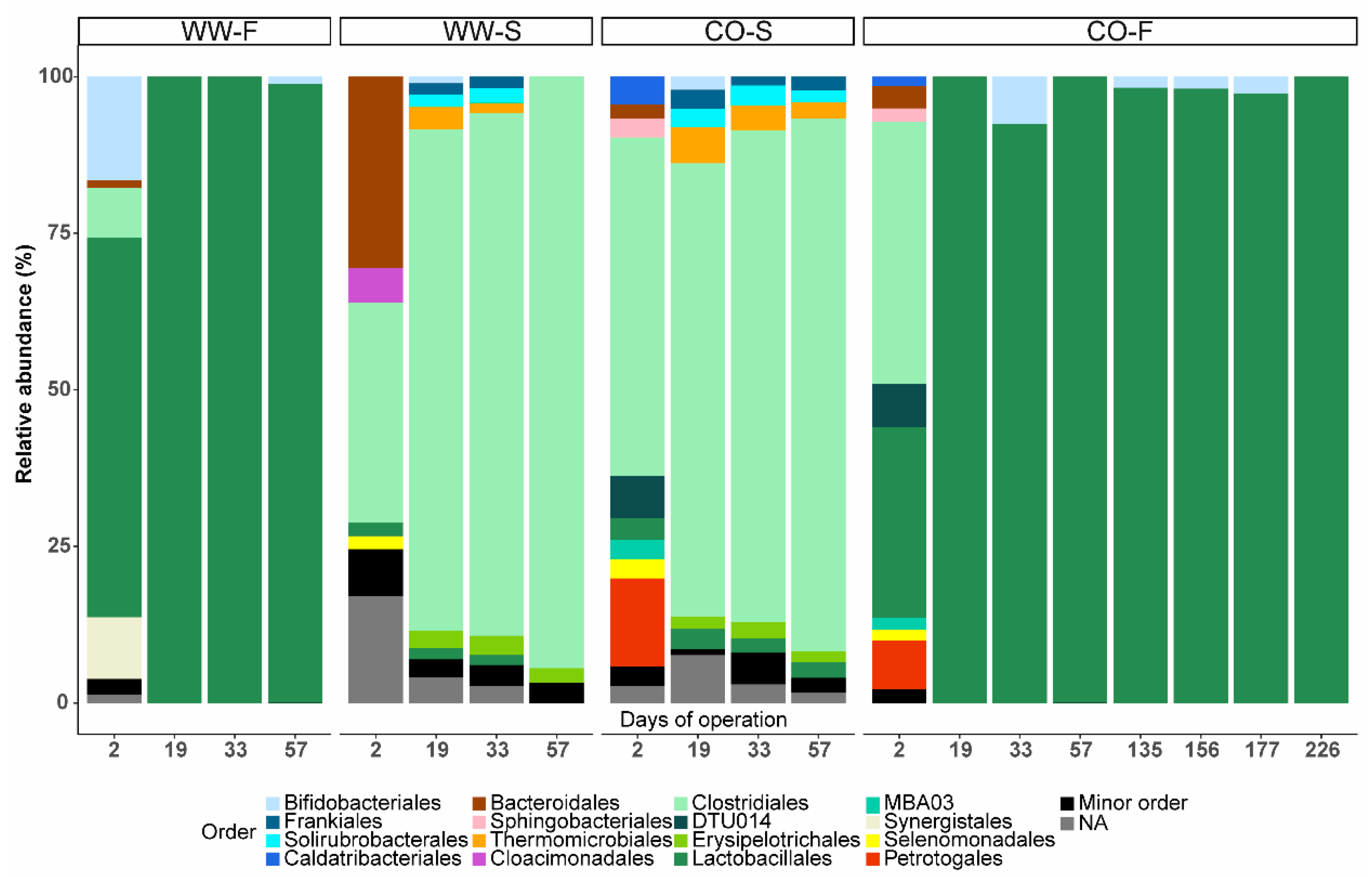
3.3. Microbial Community Structure
3.3.1. Importance of Inoculum Source
3.3.2. Effect of Substrate on Microbial Community Structure
3.3.3. Food Waste Reactors
3.3.4. Sewage Sludge Reactors
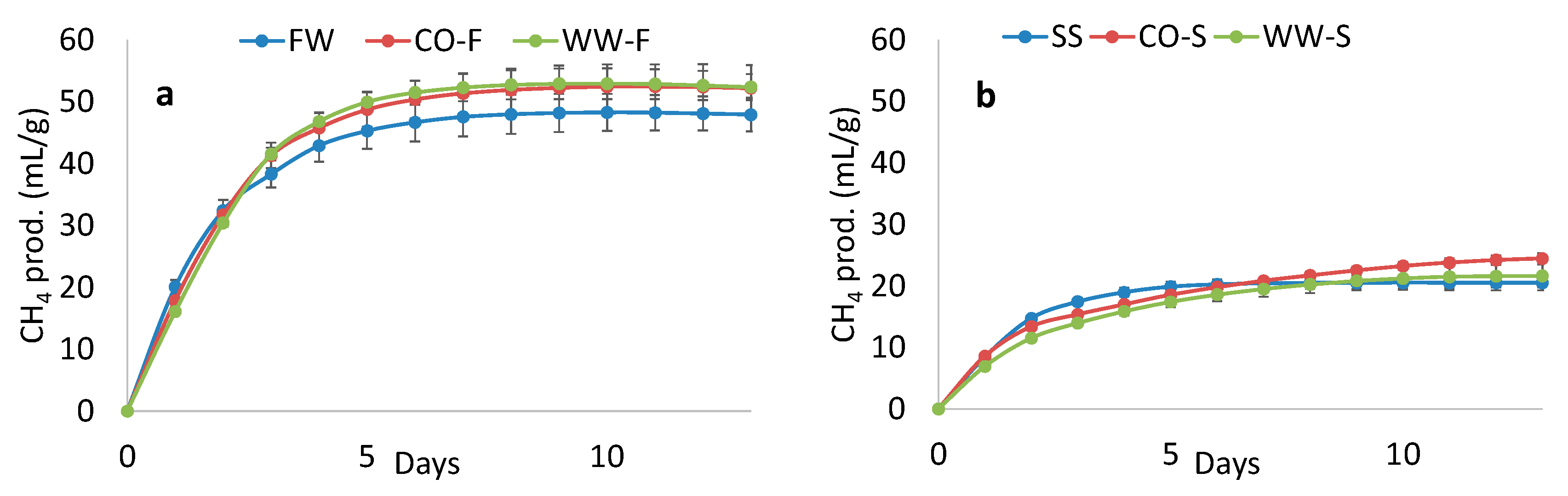
3.4. Methane Potential
3.5. Potential Acid Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hagman, L. The role of biogas solutions in sustainable biorefineries. J. Clean Prod. 2018, 172, 3982–3989. [Google Scholar] [CrossRef]
- Plugge, C.M. Biogas. Microb. Biotechnol. 2017, 10, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.L.; Guo, W.Q.; Zheng, H.S.; Luo, H.C.; Feng, X.; Yin, R.L.; Ren, N.Q. Enhancement of volatile fatty acid production by co-fermentation of food waste and excess sludge without pH control: The mechanism and microbial community analyses. Bioresour. Technol. 2016, 216, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yu, X.; Zhang, Y.; Shen, D.; Wang, M.; Long, Y.; Chen, T. Enhancement of acidogenic fermentation for volatile fatty acid production from food waste: Effect of redox potential and inoculum. Bioresour. Technol. 2016, 216, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.F.; Li, Y.Y.; Xu, K.Q.; Ebie, Y.; Inamori, Y.; Kong, H.N. A pH- and temperature-phased two-stage process for hydrogen and methane production from food waste. Int. J. Hydrog. Energy 2008, 33, 4739–4746. [Google Scholar] [CrossRef]
- Moestedt, J.; Nordell, E.; Hallin, S.; Schnürer, A. Two stage anaerobic digestion for reduced hydrogen sulphide production. J. Chem. Technol. Biotechnol. 2015, 91, 1055–1062. [Google Scholar]
- Luo, G.; Xie, L.; Zhou, Q.; Angelidaki, I. Enhancement of bioenergy production from organic wastes by two-stage anaerobic hydrogen and methane production process. Bioresour. Technol. 2011, 102, 8700–8706. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Feng, L.Y.; Yan, Y.Y.; Chen, Y.G. Co-fermentation of waste activated sludge with food waste for short-chain fatty acids production: Effect of pH at ambient temperature. Front. Environ. Sci. Eng. China 2011, 5, 623–632. [Google Scholar] [CrossRef]
- Jiang, J.G.; Zhang, Y.J.; Li, K.M.; Wang, Q.; Gong, C.X.; Li, M.L. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Yin, J.; Yu, X.; Wang, M.; Long, Y.; Shentu, J.; Chen, T. Acidogenic fermentation characteristics of different types of protein-rich substrates in food waste to produce volatile fatty acids. Bioresour. Technol. 2017, 227, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, K.; Yang, Y.Q.; Shen, D.S.; Wang, M.Z.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.W.; Liu, D.P.; Zeng, R.J.; Angelidaki, I. Hydrogen and methane production from household solid waste in the two-stage fermentation process. Water Res. 2006, 40, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Moestedt, J.; Müller, B.; Nagavara, N.Y.; Schnürer, A. Acetate and lactate production during two-stage anaerobic digestion of food waste driven by Lactobacilli and Aeriscardovia. Bioenergy Biofuels. (Submitted 2019, under review).
- Tang, J.; Wang, X.; Hu, Y.; Zhang, Y.; Li, Y. Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR. Waste Manag. 2016, 52, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Eyice, O.; Schnurer, A.; Cetecioglu, Z. Volatile fatty acids production via mixed culture fermentation: Revealing the link between pH, inoculum type and bacterial composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, W.; Liu, H.; Wang, G.; Liu, H. Evaluation of volatile fatty acids production and dewaterability of waste activated sludge with different thermo-chemical pretreatments. Int. Biodeterior. Biodegrad. 2018, 129, 170–178. [Google Scholar] [CrossRef]
- Feng, K.; Li, H.; Zheng, C. Shifting product spectrum by pH adjustment during long-term continuous anaerobic fermentation of food waste. Bioresour. Technol. 2018, 270, 180–188. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.C.; Hu, Y.; Zhang, Y.; Li, Y. Effect of pH on lactic acid production from acidogenic fermentation of food waste with different types of inocula. Bioresour. Technol. 2017, 224, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Yang, Y.H. Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Bio/Technol. 2017, 16, 327–345. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Nghiem, L.D.; Hai, F.I.; Deng, L.J.; Wang, J.; Wu, Y. Optimization of process parameters for production of volatile fatty acid, biohydrogen and methane from anaerobic digestion. Bioresour. Technol. 2016, 219, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.G.; Lee, J.H.; Noh, M.H.; Jung, G.Y. Rediscovering Acetate Metabolism: Its Potential Sources and Utilization for Biobased Transformation into Value-Added Chemicals. J. Agric. Food Chem. 2018, 66, 3998–4006. [Google Scholar] [CrossRef] [PubMed]
- Vidra, A.; Németh, Á. Bio-produced Acetic Acid: A Review. Period. Polytech. Chem. Eng. 2018, 62, 245–256. [Google Scholar] [CrossRef]
- Hawkes, F.R.; Hussy, I.; Kyazze, G.; Dinsdale, R.; Hawkes, D.L. Continuous dark fermentative hydrogen production by mesophilic microflora: Principles and progress. Int. J. Hydrog. Energy. 2007, 32, 172–184. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Energetics and Application of Heterotrophy in Acetogenic Bacteria. Appl. Environ. Microbiol. 2016, 82, 4056–4069. [Google Scholar] [CrossRef]
- Schink, B. Energetics of Syntrophic Cooperation in Methanogenic Degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. [Google Scholar]
- Garcia-Aguirre, J.; Esteban-Gutiérrez, M.; Irizar, I.; González-Mtnez, J.; Aymerich, E. Continuous acidogenic fermentation: Narrowing the gap between laboratory testing and industrial application. Bioresour. Technol. 2019, 282, 407–416. [Google Scholar] [CrossRef]
- Jonsson, S.; Borén, H. Analysis of mono- and diesters of o-phthalic acid by solid-phase extractions with polystyrene-divinylbenzene-based polymers. J. Chromatogr. A 2002, 963, 393–400. [Google Scholar] [CrossRef]
- Westerholm, M.; Hansson, M.; Schnurer, A. Improved biogas production from whole stillage by co-digestion with cattle manure. Bioresour. Technol. 2012, 114, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, X.; Xiao, K.; Shen, N.; Zeng, R.J.; Zhou, Y. Enhanced volatile fatty acids (VFAs) production in a thermophilic fermenter with stepwise pH increase–Investigation on dissolved organic matter transformation and microbial community shift. Water Res. 2017, 112, 261–268. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food Energy-Methods of Analysis and Conversion Factors. Available online: http://www.fao.org/3/Y5022E/Y5022E00.htm (accessed on 15 November 2019).
- Stein, U.H.; Wimmer, B.; Ortner, M.; Fuchs, W.; Bochmann, G. Maximizing the production of butyric acid from food waste as a precursor for ABE-fermentation. Sci. Total. Environ. 2017, 598, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Sun, L.; Westerholm, M.; Schnürer, A. Bacterial community composition and fhs profiles of low- and high-ammonia biogas digesters reveal novel syntrophic acetate-oxidising bacteria. Biotechnol. Biofuels. 2016, 9, 1–18. [Google Scholar] [CrossRef]
- Westerholm, M.; Isaksson, S.; Karlsson-Lindsjö, O.; Schnürer, A. Microbial community adaptability to altered temperature conditions determines the potential for process optimisation in biogas processes. Appl. Energy 2018, 226, 838–848. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Available online: https://www.researchgate.net/publication/282247686_Vegan_Community_Ecology_Package_R_package_version_20-2 (accessed on 15 November 2019).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- Kakar, F.; Koupaie, E.H.; Hafez, H.; Elbeshbishy, E. Effect of Hydrothermal Pretreatment on Volatile Fatty Acids Production from Source-Separated Organics. Processes 2019, 7, 576. [Google Scholar] [CrossRef]
- Liu, H.; Han, P.; Liu, H.; Zhou, G.; Fu, B.; Zheng, Z. Full-scale production of VFAs from sewage sludge by anaerobic alkaline fermentation to improve biological nutrients removal in domestic wastewater. Bioresour. Technol. 2018, 260, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Khiewwijit, R.; Keesman, K.J.; Rijnaarts, H.; Temmink, H. Volatile fatty acids production from sewage organic matter by combined bioflocculation and anaerobic fermentation. Bioresour. Technol. 2015, 193, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, H. Volatile fatty acids productions by mesophilic and thermophilic sludge fermentation: Biological responses to fermentation temperature. Bioresour. Technol. 2015, 175, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chen, X.; Liu, H.; Liu, H.; Fu, B. Improved volatile fatty acids anaerobic production from waste activated sludge by pH regulation: Alkaline or neutral pH? Waste Manag. 2016, 48, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhou, Y.; Guo, C.; Maspolim, Y.; Ng, W.J. Impact of undissociated volatile fatty acids on acidogenesis in a two-phase anaerobic system. J. Environ. Sci. 2016, 42, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Infantes, D.; del Campo, A.G.; Villasenor, J.; Fernandez, F.J. Kinetic model and study of the influence of pH, temperature and undissociated acids on acidogenic fermentation. Biochem. Eng. J. 2012, 66, 66–72. [Google Scholar] [CrossRef]
- Sundberg, C.; Al-Soud, W.A.; Larsson, M.; Alm, E.; Yekta, S.S.; Svensson, B.H.; Sørensen, S.J.; Karlsson, A. 454 pyrosequencing analyses of bacterial and archaeal richness in 21 full-scale biogas digesters. FEMS Microbiol. Ecol. 2013, 85, 612–626. [Google Scholar] [CrossRef]
- Sun, L.; Müller, B.; Westerholm, M.; Schnürer, A. Syntrophic acetate oxidation in industrial CSTR biogas digesters. J. Biotechnol. 2014, 171, 39–44. [Google Scholar] [CrossRef]
- Westerholm, M.; Moestedt, J.; Schnürer, A. Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl. Energy 2016, 179, 124–135. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degréve, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Worm, P.; Müller, N.; Plugge, C.M.; Stams, A.J.M.; Schink, B. Syntrophy in Methanogenic Degradation. In (Endo)symbiotic Methanogenic Archaea; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Bohak, I.; Back, W.; Richter, L.; Ehrmann, M.; Ludwig, W.; Schleifer, K.H. Lactobacillus amylolyticus sp. nov., isolated from beer malt and beer wort. Syst. Appl. Microbiol. 1998, 21, 360–364. [Google Scholar] [PubMed]
- Reddy, G.; Altaf, M.; Naveena, B.J.; Venkateshwar, M.; Kumar, E.V. Amylolytic bacterial lactic acid fermentation—A review. Biotechnol. Adv. 2008, 6, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xie, S.; Yu, L.; Zhen, Y.; Zhao, Q.; Frear, C.; Chen, S.; Wang, Z.W.; Shi, Z. pH shaped kinetic characteristics and microbial community of food waste hydrolysis and acidification. Biochem. Eng. J. 2019, 146, 52–59. [Google Scholar] [CrossRef]
- Lindgren, S.E.; Axelsson, L.T.; McFeeters, R.F. Anaerobic l-lactate degradation by Lactobacillus plantarum. FEMS Microbiol. Lett. 1990, 66, 209–213. [Google Scholar] [CrossRef][Green Version]
- Grause, G.; Igarashi, M.; Kameda, T.; Yoshioka, T. Lactic acid as a substrate for fermentative hydrogen production. Int. J. Hydrog. Energy 2012, 37, 16967–16973. [Google Scholar] [CrossRef]
- Cselovszky, J.; Wolf, G.; Hammes, W.P. Production of formate, acetate, and succinate by anaerobic fermentation of Lactobacillus pentosus in the presence of citrate. Appl. Microbiol. Biotechnol. 1992, 37, 94–97. [Google Scholar] [CrossRef]
- STANTON, T.B.; SAVAGE, D.C. Roseburia cecicola gen. nov., sp. nov., a Motile, Obligately Anaerobic Bacterium from a Mouse Cecum. Int. J. Syst. Evol. Microbiol. 1983, 33, 618–627. [Google Scholar] [CrossRef]
- Duncan, S.H.; Hold, G.L.; Barcenilla, A.; Stewart, S.S.; Flint, H.J. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int. J. Syst. Evol. Microbiol. 2002, 52, 1615–1620. [Google Scholar]
- Duncan, S.H.; Aminov, R.I.; Scott, K.P.; Louis, P.; Stanton, T.B.; Flint, H.J. Proposal of Roseburia faecis sp. nov., Roseburia hominis sp. nov. and Roseburia inulinivorans sp. nov., based on isolates from human faeces. Int. J. Syst. Evol. Microbiol. 2006, 56, 2437–2441. [Google Scholar] [CrossRef]
- Falsen, E.; Collins, M.D.; Welinder-Olsson, C.; Song, Y.; Finegold, S.M.; Lawson, P.A. Fastidiosipila sanguinis gen. nov., sp. nov., a new Gram-positive, coccus-shaped organism from human blood. Int. J. Syst. Evol. Microbiol. 2005, 55, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, Z.; Wang, Q.; Zhu, C.; Wu, Z. An anaerobic dynamic membrane bioreactor (AnDMBR) for landfill leachate treatment: Performance and microbial community identification. Bioresour. Technol. 2014, 161, 29–39. [Google Scholar] [CrossRef]
- He, Q.; Li, L.; Zhao, X.; Qu, L.; Wu, D.; Peng, X. Investigation of foaming causes in three mesophilic food waste digesters: Reactor performance and microbial analysis. Sci. Rep. 2017, 7, 13701. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lee, J.; Han, G.; Hwang, S. Comprehensive analysis of microbial communities in full-scale mesophilic and thermophilic anaerobic digesters treating food waste-recycling wastewater. Bioresour. Technol. 2018, 259, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Cardinali-Rezende, J.; Rojas-Ojeda, P.; Nascimento, A.M.; Sanz, J.L. Proteolytic bacterial dominance in a full-scale municipal solid waste anaerobic reactor assessed by 454 pyrosequencing technology. Chemosphere 2016, 146, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Dong, B.; Dai, X.; Wang, H.; Li, N.; Yang, D. Effects of thermal hydrolysis on the metabolism of amino acids in sewage sludge in anaerobic digestion. Waste Manag. 2019, 88, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Slobodkin, A. The Family Peptostreptococcaceae. In The Prokaryotes; Rosenberg, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Stackebrandt, E. The Family Eubacteriaceae. In The Prokaryotes-Firmicutes and Tenericutes; Rosenberg, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]

| WW-S | CO-S | WW-F | CO-F | |
|---|---|---|---|---|
| Inoculum source | WWTP | Co-digestion | WWTP | Co-digestion |
| Substrate | Sewage sludge | Sewage sludge | Food waste | Food waste |
| OLR (kg VS/m3/d) | 14.2 (23.7) | 14.2 (23.7) | 27.0 (13.5) | 27.0 (13.5) |
| HRT (days) | 5 (3) | 5 (3) | 5 (10) | 5 (10) |
| WW-S | CO-S | WW-F | CO-F | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time period | 0–50 | 50–65 | 0–50 | 50–65 | 0–20 | 20–65 | 0–20 | 20–200 | days |
| HRT a | 5 | 3 | 5 | 3 | 5 | 10 | 5 | 0 | days |
| Hydrolysis | 15% | 10% | 15% | 15% | 1% | 2% | 6% | 7% | of TOC in |
| Acidogenesis | 66% | 48% | 71% | 61% | 38% | 65% | 61% | 56% | of DOC |
| Acetogenesis | 38% | 25% | 37% | 40% | 37% | 21% | 40% | 58% | of VFA |
| Protein hydrolysis | 53% | 59% | 65% | 57% | - | 15% | - | 17% | of raw protein in |
| Methanogenesis (1st stage) | 4% | 7% | 2% | 2% | 1% | 0% | 1% | 0% | of TOC in |
| Methanogenesis (2nd stage) | nd | 59% | nd | 60% | nd | 44% | nd | 46% | of TOC in |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moestedt, J.; Westerholm, M.; Isaksson, S.; Schnürer, A. Inoculum Source Determines Acetate and Lactate Production during Anaerobic Digestion of Sewage Sludge and Food Waste. Bioengineering 2020, 7, 3. https://doi.org/10.3390/bioengineering7010003
Moestedt J, Westerholm M, Isaksson S, Schnürer A. Inoculum Source Determines Acetate and Lactate Production during Anaerobic Digestion of Sewage Sludge and Food Waste. Bioengineering. 2020; 7(1):3. https://doi.org/10.3390/bioengineering7010003
Chicago/Turabian StyleMoestedt, Jan, Maria Westerholm, Simon Isaksson, and Anna Schnürer. 2020. "Inoculum Source Determines Acetate and Lactate Production during Anaerobic Digestion of Sewage Sludge and Food Waste" Bioengineering 7, no. 1: 3. https://doi.org/10.3390/bioengineering7010003
APA StyleMoestedt, J., Westerholm, M., Isaksson, S., & Schnürer, A. (2020). Inoculum Source Determines Acetate and Lactate Production during Anaerobic Digestion of Sewage Sludge and Food Waste. Bioengineering, 7(1), 3. https://doi.org/10.3390/bioengineering7010003