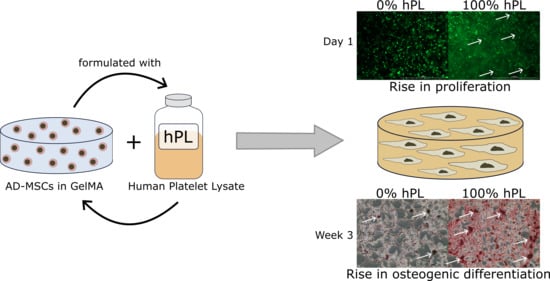
Gelatin-Methacryloyl (GelMA) Formulated with Human Platelet Lysate Supports Mesenchymal Stem Cell Proliferation and Differentiation and Enhances the Hydrogel’s Mechanical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. GelMA Synthesis and Hydrogel Preparation
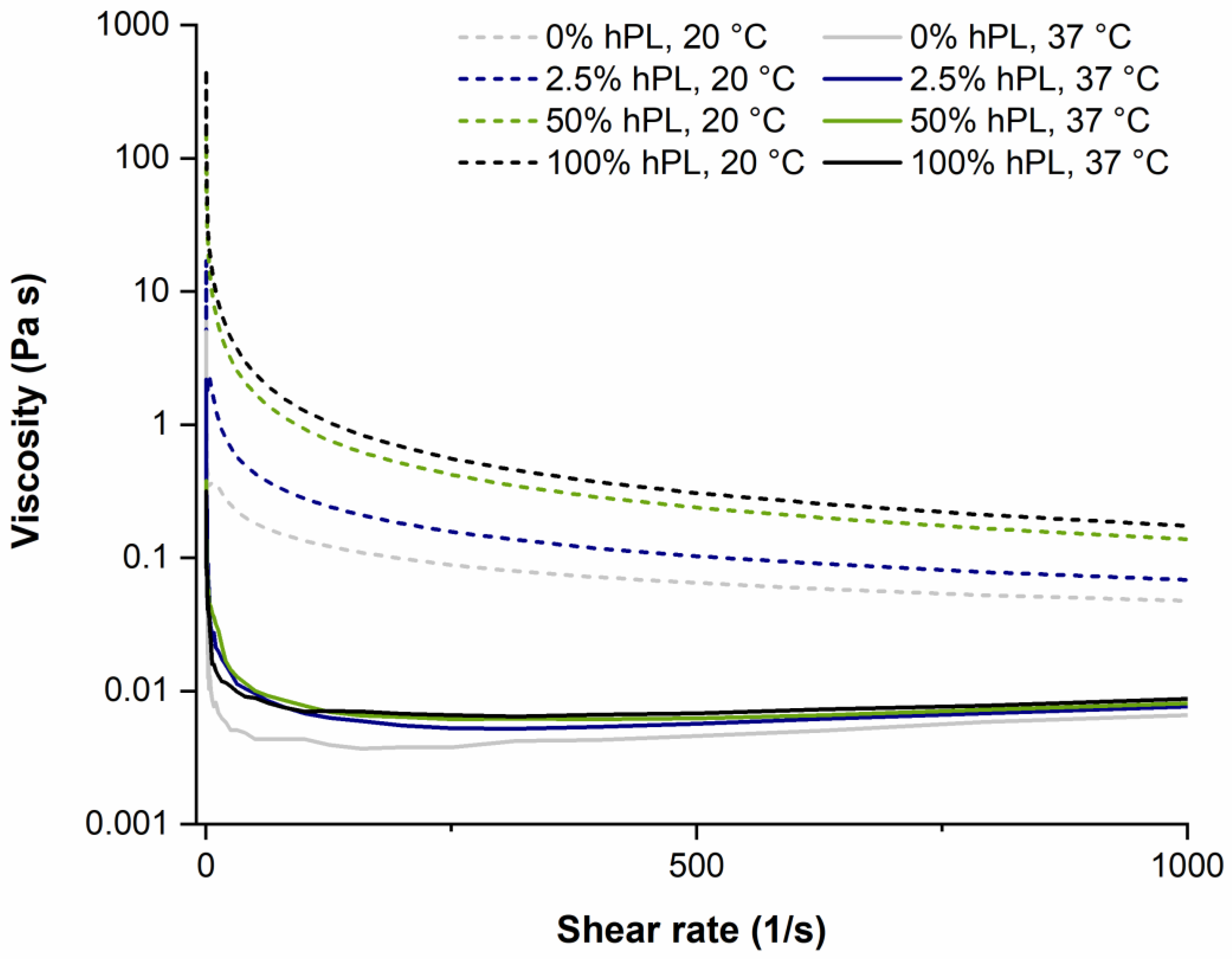
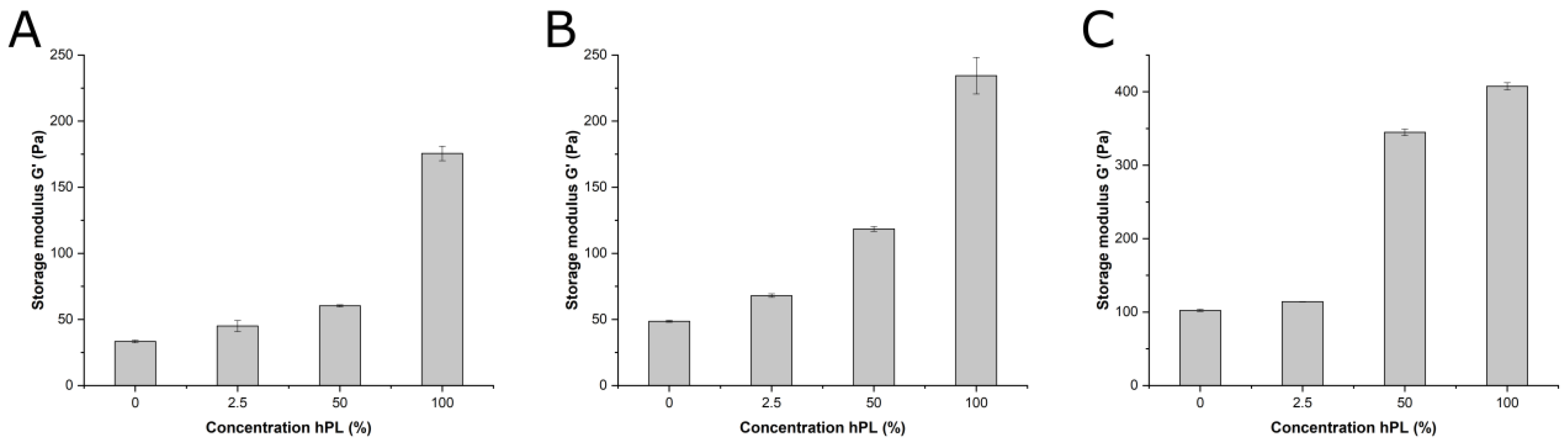
2.2. Rheological Characterization
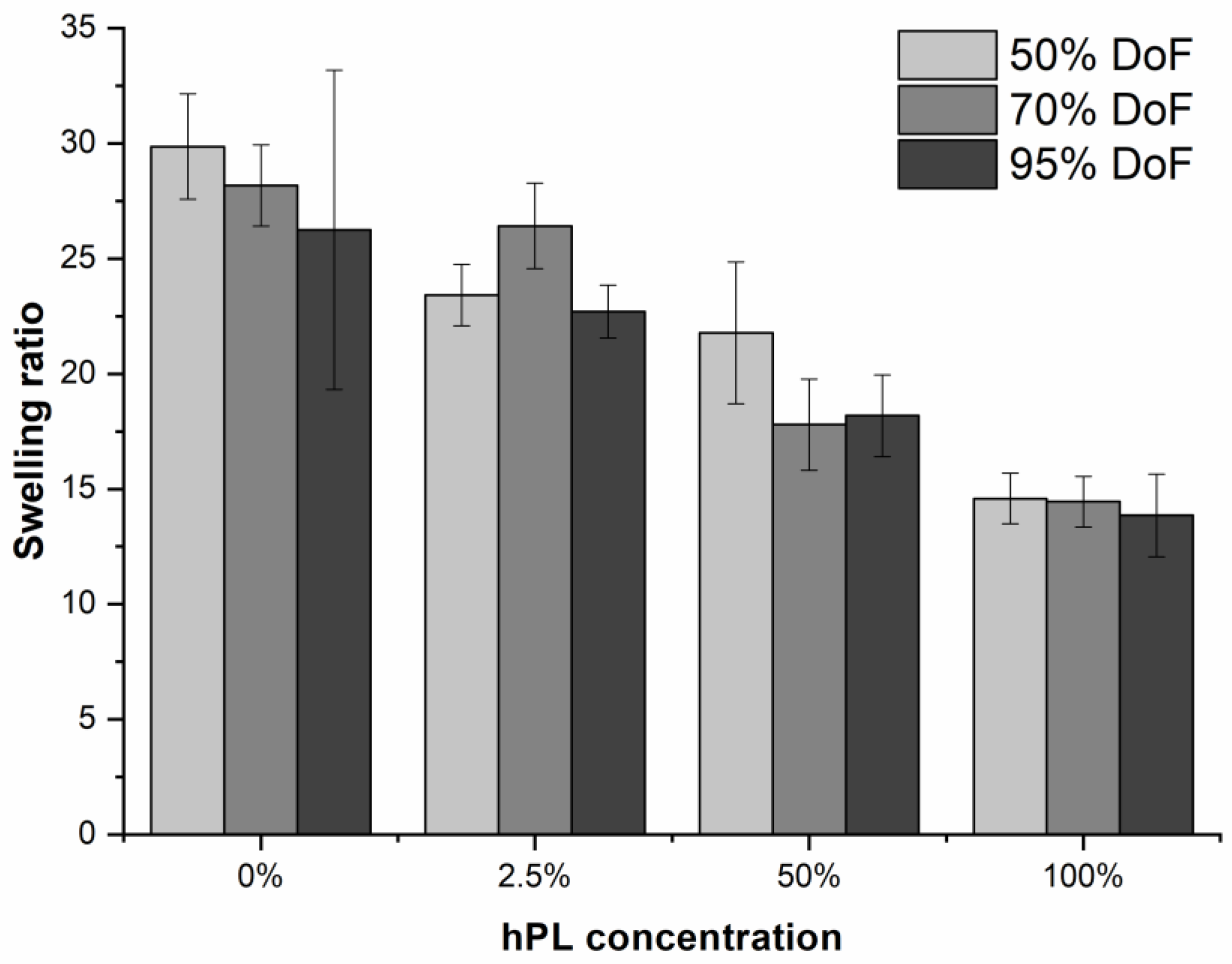
2.3. Hydrogel Swelling
2.4. Cell Culture
2.5. Encapsulation and Cultivation of AD-MSCs in Hydrogels
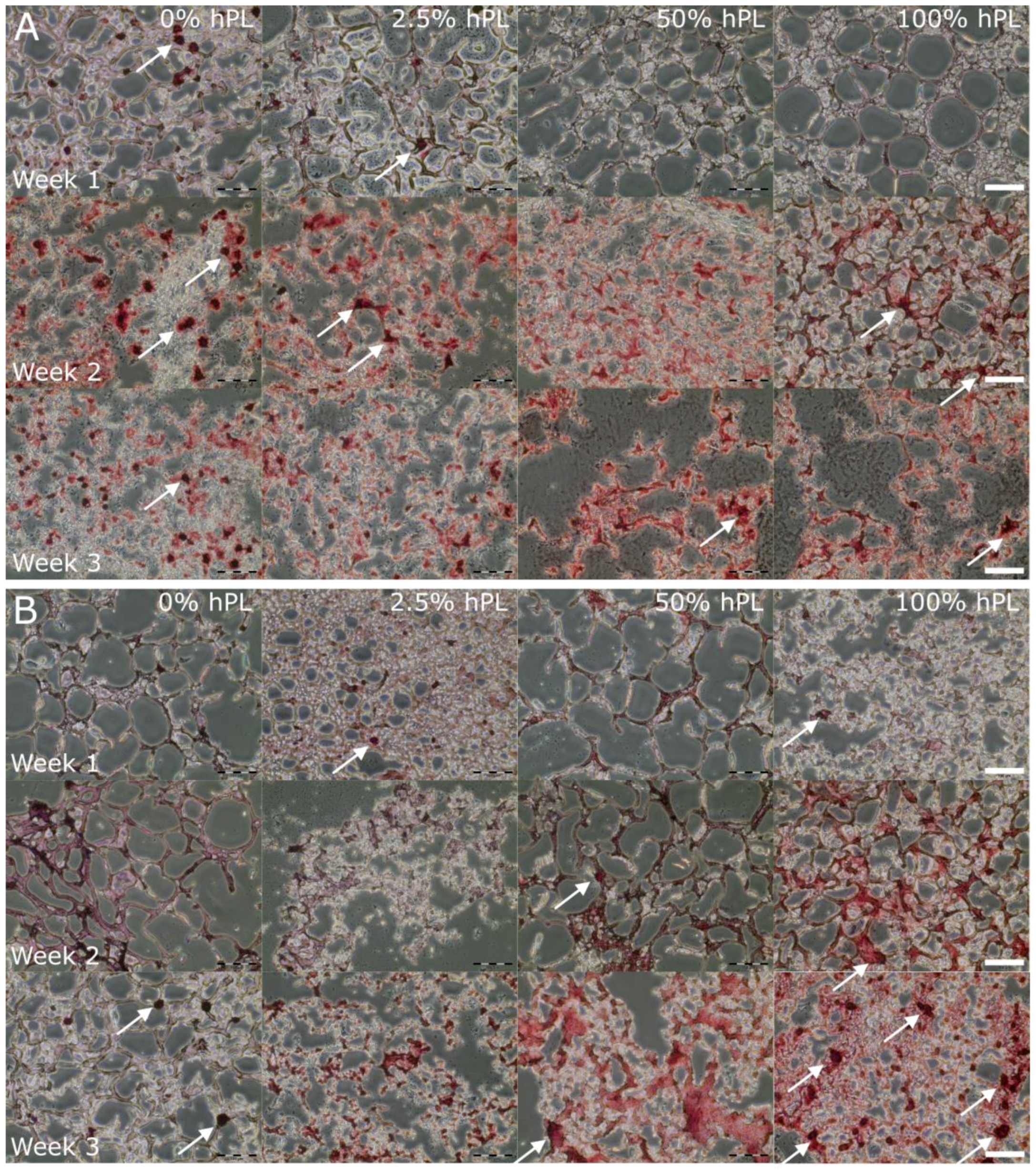
2.6. Osteogenic Differentiation, Cryosections, and Alizarin Red Staining
3. Results
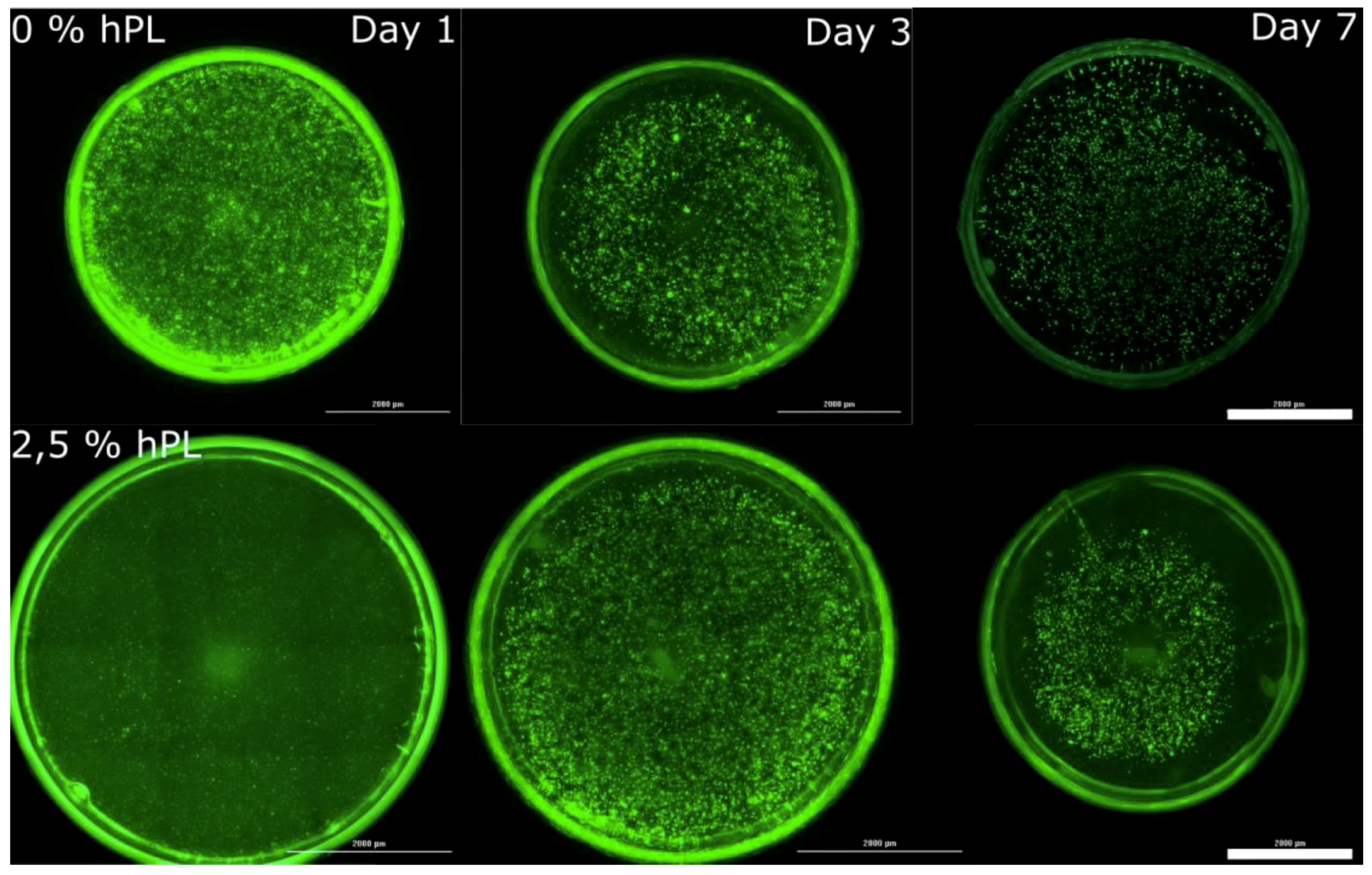
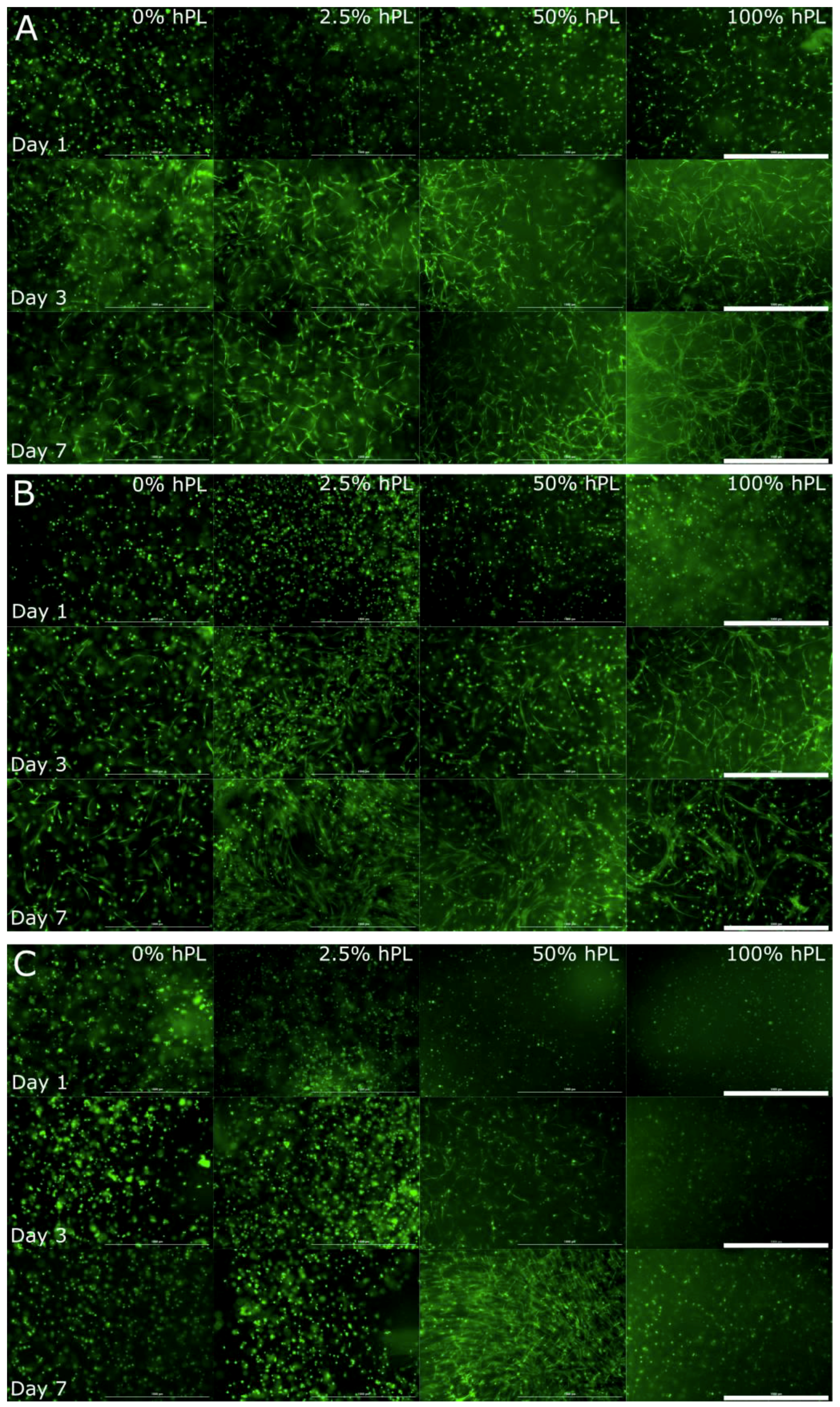
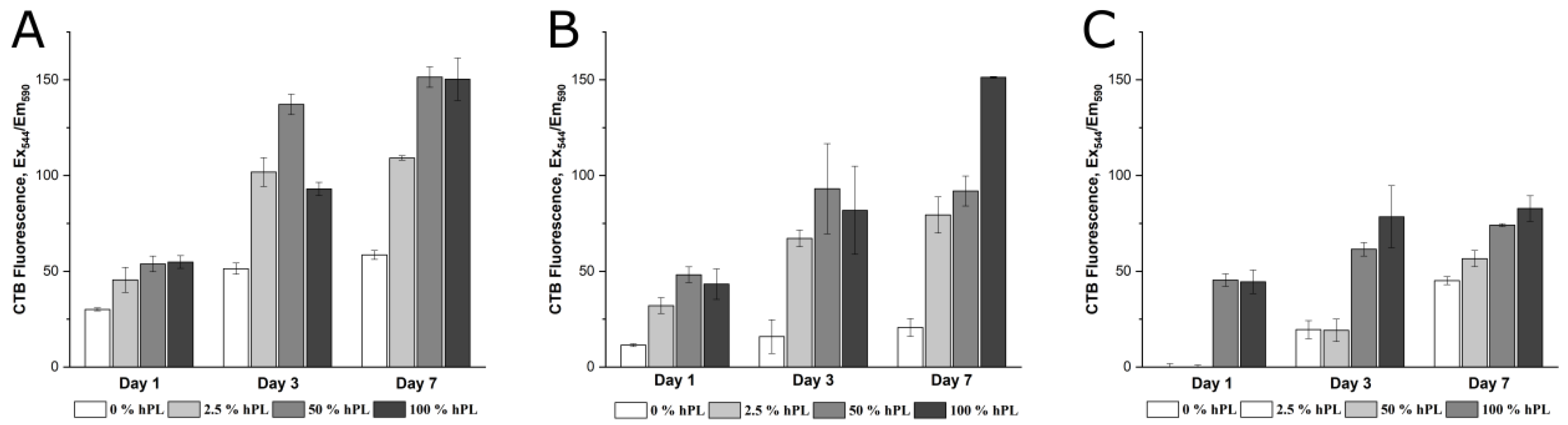
3.1. AD-MSCs’ Viability in GelMA-Hydrogels
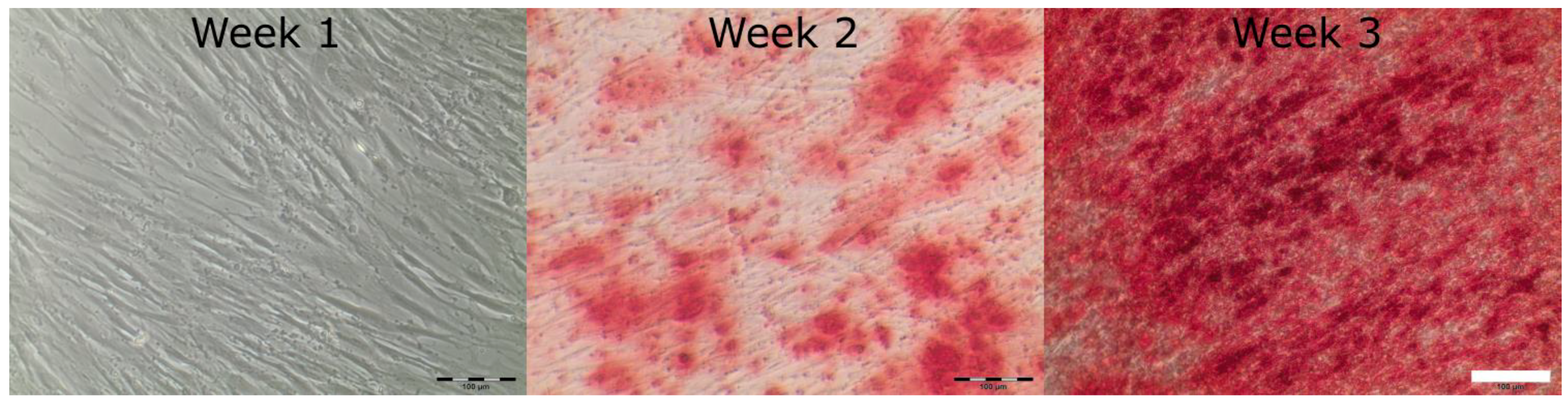
3.2. AD-MSC Differentiation in GelMA-Hydrogels
3.3. Influence of hPL on GelMA Properties
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Booij, T.H.; Price, L.S.; Danen, E.H.J. 3D cell-based assays for drug screens: Challenges in imaging, image analysis, and high-content analysis. SLAS Discov. Adv. Life Sci. RD 2019, 24, 615–627. [Google Scholar] [CrossRef]
- Di Modugno, F.; Colosi, C.; Trono, P.; Antonacci, G.; Ruocco, G.; Nisticò, P. 3D models in the new era of immune oncology: Focus on T cells, CAF and ECM. J. Exp. Clin. Cancer Res. 2019, 38, 117. [Google Scholar] [CrossRef]
- Sambale, F.; Lavrentieva, A.; Stahl, F.; Blume, C.; Stiesch, M.; Kasper, C.; Bahnemann, D.; Scheper, T. Three dimensional spheroid cell culture for nanoparticle safety testing. J. Biotechnol. 2015, 205, 120–129. [Google Scholar] [CrossRef]
- Van Griensven, M.; Diederichs, S.; Roeker, S.; Boehm, S.; Peterbauer, A.; Wolbank, S.; Riechers, D.; Stahl, F.; Kasper, C. Mechanical strain using 2D and 3D bioreactors induces osteogenesis: Implications for bone tissue engineering. In Bioreactor Systems for Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2009; Volume 112, pp. 95–123. [Google Scholar]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Ruedinger, F.; Lavrentieva, A.; Blume, C.; Pepelanova, I.; Scheper, T. Hydrogels for 3D mammalian cell culture: A starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 2015, 99, 623–636. [Google Scholar] [CrossRef]
- Lev, R.; Seliktar, D. Hydrogel biomaterials and their therapeutic potential for muscle injuries and muscular dystrophies. J. R. Soc. Interface 2018, 15, 20170380. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; Van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-Methacrylamide Hydrogels as Potential Biomaterials for Fabrication of Tissue-Engineered Cartilage Constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]
- Re, F.; Sartore, L.; Moulisova, V.; Cantini, M.; Almici, C.; Bianchetti, A.; Chinello, C.; Dey, K.; Agnelli, S.; Manferdini, C.; et al. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; A Levett, P.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Bulcke, A.I.V.D.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Home—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 15 July 2019).
- Tonsho, M.; Michel, S.; Ahmed, Z.; Alessandrini, A.; Madsen, J.C. Heart transplantation: Challenges facing the field. Cold Spring Harb. Perspect. Med. 2014, 4, a015636. [Google Scholar] [CrossRef]
- Orgill, D.P.; Carlos, B. Biomaterials for Treating Skin Loss; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Bieback, K.; Hecker, A.; Kocaömer, A.; Lannert, H.; Schallmoser, K.; Strunk, D.; Klüter, H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells 2009, 27, 2331–2341. [Google Scholar] [CrossRef]
- Müller, I.; Kordowich, S.; Holzwarth, C.; Spano, C.; Isensee, G.; Staiber, A.; Viebahn, S.; Gieseke, F.; Langer, H.; Gawaz, M.; et al. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy 2006, 8, 437–444. [Google Scholar] [CrossRef]
- Fekete, N.; Rojewski, M.T.; Fürst, D.; Kreja, L.; Ignatius, A.; Dausend, J.; Schrezenmeier, H. GMP-Compliant isolation and large-scale expansion of bone marrow-derived MSC. PLOS ONE 2012, 7, e43255. [Google Scholar] [CrossRef]
- Fekete, N.; Gadelorge, M.; Fürst, D.; Maurer, C.; Dausend, J.; Fleury-Cappellesso, S.; Mailänder, V.; Lotfi, R.; Ignatius, A.; Sensebé, L.; et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: Production process, content and identification of active components. Cytotherapy 2012, 14, 540–554. [Google Scholar]
- Doucet, C.; Ernou, I.; Zhang, Y.; Begot, L.; Holy, X.; Llense, J.-R.; Lataillade, J.-J.; Llense, J.; Lataillade, J. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J. Cell. Physiol. 2005, 205, 228–236. [Google Scholar] [CrossRef]
- Shih, D.T.-B.; Chen, J.-C.; Chen, W.-Y.; Kuo, Y.-P.; Su, C.-Y.; Burnouf, T. Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate. Transfusion 2011, 51, 770–778. [Google Scholar] [CrossRef]
- Astori, G.; Amati, E.; Bambi, F.; Bernardi, M.; Chieregato, K.; Schäfer, R.; Sella, S.; Rodeghiero, F. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: Present and future. Stem Cell Res. Ther. 2016, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Cakiroglu, F.; Spiess, A.-N.; Dierlamm, J.; Zander, A.R.; Cappallo-Obermann, H.; Cappallo-Obermann, H. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J. Cell. Physiol. 2007, 213, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Schallmoser, K.; Bartmann, C.; Rohde, E.; Reinisch, A.; Kashofer, K.; Stadelmeyer, E.; Drexler, C.; Lanzer, G.; Linkesch, W.; Strunk, D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion 2007, 47, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Hemeda, H.; Giebel, B.; Wagner, W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy 2014, 16, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rebollo, E.; Mentrup, B.; Ebert, R.; Franzen, J.; Abagnale, G.; Sieben, T.; Ostrowska, A.; Hoffmann, P.; Roux, P.-F.; Rath, B.; et al. Human platelet lysate versus fetal calf serum: These supplements do not select for different mesenchymal stromal cells. Sci. Rep. 2017, 7, 5132. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, C.; Ibrahim, M.; Scafetta, G.; Napoletano, C.; Mangino, G.; Pierelli, L.; Frati, G.; De Falco, E. Optimization of the isolation and expansion method of human mediastinal-adipose tissue derived mesenchymal stem cells with virally inactivated GMP-grade platelet lysate. Cytotechnology 2015, 67, 165–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jonsdottir-Buch, S.M.; Lieder, R.; Sigurjonsson, O.E. Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells. PLoS ONE 2013, 8, e68984. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Xu, K.; Zheng, X. Using glucosamine to improve the properties of photocrosslinked gelatin scaffolds. J. Biomater. Appl. 2015, 29, 977–987. [Google Scholar] [CrossRef]
- Tancharoen, W.; Aungsuchawan, S.; Pothacharoen, P.; Bumroongkit, K.; Puaninta, C.; Pangjaidee, N.; Narakornsak, S.; Markmee, R.; Laowanitwattana, T.; Thaojamnong, C. Human platelet lysate as an alternative to fetal bovine serum for culture and endothelial differentiation of human amniotic fluid mesenchymal stem cells. Mol. Med. Rep. 2019, 19, 5123–5132. [Google Scholar] [CrossRef]
- Bernardo, M.; Avanzini, M.; Perotti, C.; Cometa, A.; Moretta, A.; Lenta, E.; Del Fante, C.; Novara, F.; De Silvestri, A.; Amendola, G.; et al. Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: Further insights in the search for a fetal calf serum substitute. J. Cell. Physiol. 2007, 211, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kore-Grodzicki, B.; Tauber-Finkelstein, M.; Chain, D.; Shaltiel, S. Vitronectin is phosphorylated by a cAMP-dependent protein kinase released by activation of human platelets with thrombin. Biochem. Biophys. Res. Commun. 1988, 157, 1131–1138. [Google Scholar] [CrossRef]
- Arnett, T.R.; Henderson, B. Methods in Bone Biology; Springer US: Boston, MA, USA, 1997; ISBN 978-0-412-75770-9. [Google Scholar]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, E.; Melchels, F.P.; Holzapfel, B.M.; Meckel, T.; Hutmacher, D.W.; Loessner, D. Gelatine methacrylamide-based hydrogels: An alternative three-dimensional cancer cell culture system. Acta Biomater. 2014, 10, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Dhert, W.J.A.; Hutmacher, D.W.; Malda, J. Development and characterisation of a new bioink for additive tissue manufacturing. J. Mater. Chem. B 2014, 2, 2282. [Google Scholar] [CrossRef]
- Pang, Z.; Deeth, H.; Sopade, P.; Sharma, R.; Bansal, N. Rheology, texture and microstructure of gelatin gels with and without milk proteins. Food Hydrocoll. 2014, 35, 484–493. [Google Scholar] [CrossRef]
- Kaji, H.; Camci-Unal, G.; Langer, R.; Khademhosseini, A. Engineering systems for the generation of patterned co-cultures for controlling cell-cell interactions. Biochim. Biophys. Acta 2011, 1810, 239–250. [Google Scholar] [CrossRef]
- Shi, J.; Xing, M.M.Q.; Zhong, W. Development of hydrogels and biomimetic regulators as tissue engineering scaffolds. Membranes 2012, 2, 70–90. [Google Scholar] [CrossRef]
- Schallmoser, K.; Strunk, D. Generation of a pool of human platelet lysate and efficient use in cell culture. Methods Mol. Biol. 2013, 946, 349–362. [Google Scholar] [CrossRef]
- Shih, D.T.-B.; Burnouf, T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. New Biotechnol. 2015, 32, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Rizki, A.; Mian, I.S. Tissue architecture: the ultimate regulator of breast epithelial function. Curr. Opin. Cell Boil. 2003, 15, 753–762. [Google Scholar] [CrossRef]
- Cukierman, E.; Blanco, P.; Palucka, A.K.; Gill, M.; Pascual, V.; Banchereau, J. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, E.; Pankov, R.; Yamada, K.M. Cell interactions with three-dimensional matrices. Curr. Opin. Cell Boil. 2002, 14, 633–640. [Google Scholar] [CrossRef]
- Lee, J.; Lilly, G.D.; Doty, R.C.; Podsiadlo, P.; Kotov, N.A. In vitro toxicity testing of nanoparticles in 3D cell culture. Small 2009, 5, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cui, Z. Three-dimensional perfused cell culture. Biotechnol. Adv. 2014, 32, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Schmeichel, K.L.; Bissell, M.J. Modeling tissue-specific signaling and organ function in three dimensions. J. Cell Sci. 2003, 116, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A. Cell culture: Biology’s new dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Von Der Mark, K.; Gauss, V.; Von Der Mark, H.; Muller, P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef]
- Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef]
- Mironi-Harpaz, I.; Wang, D.Y.; Venkatraman, S.; Seliktar, D.; Venkatraman, S. Photopolymerization of cell-encapsulating hydrogels: Crosslinking efficiency versus cytotoxicity. Acta Biomater. 2012, 8, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.R.; Engler, A.J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Boil. 2010, 47, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mok, S.; Moraes, C. Micropocket hydrogel devices for all-in-one formation, assembly, and analysis of aggregate-based tissues. Biofabrication 2019, 11, 045013. [Google Scholar] [CrossRef] [PubMed]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and characterization of hybrid hyaluronic acid-gelatin hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef]
- Alsberg, E.; Anderson, K.W.; Albeiruti, A.; Rowley, J.A.; Mooney, D.J. Engineering growing tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 12025–12030. [Google Scholar] [CrossRef]
- Yang, F.; Williams, C.G.; Wang, D.-A.; Lee, H.; Manson, P.N.; Elisseeff, J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 2005, 26, 5991–5998. [Google Scholar] [CrossRef]
- Hern, D.L.; Hubbell, J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J. Biomed. Mater. Res. 1998, 39, 266–276. [Google Scholar] [CrossRef]
- Maheshwari, G.; Brown, G.; A Lauffenburger, D.; Wells, A.; Griffith, L.G. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 2000, 113, 1677–1686. [Google Scholar]
- Feng, Y.; Mrksich, M. The synergy peptide PHSRN and the adhesion peptide RGD mediate cell adhesion through a common mechanism. Biochemistry 2004, 43, 15811–15821. [Google Scholar] [CrossRef]
- Sander, H.J.; Slot, J.W.; Bouma, B.N.; A Bolhuis, P.; Pepper, D.S.; Sixma, J.J. Immunocytochemical localization of fibrinogen, platelet factor 4, and beta thromboglobulin in thin frozen sections of human blood platelets. J. Clin. Investig. 1983, 72, 1277–1287. [Google Scholar] [CrossRef]
- Heijnen, H.; van der Sluijs, P. Platelet secretory behaviour: As diverse as the granules…or not? J. Thromb. Haemost. 2015, 13, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Wencel-Drake, J.D.; Painter, R.G.; Zimmerman, T.S.; Ginsberg, M.H. Ultrastructural localization of human platelet thrombospondin, fibrinogen, fibronectin, and von Willebrand factor in frozen thin section. Blood 1985, 65, 929–938. [Google Scholar] [PubMed]
- Wang, X.; Hu, X.; Dulińska-Molak, I.; Kawazoe, N.; Yang, Y.; Chen, G. Discriminating the independent influence of cell adhesion and spreading area on stem cell fate determination using micropatterned surfaces. Sci. Rep. 2016, 6, 28708. [Google Scholar] [CrossRef] [PubMed]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Stuart, M.A.C.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef] [PubMed]
- A Lauffenburger, D.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Chowdhury, F.; Na, S.; Li, D.; Poh, Y.-C.; Tanaka, T.S.; Wang, F.; Wang, N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010, 9, 82–88. [Google Scholar] [CrossRef]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bönnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef]
- Solon, J.; Levental, I.; Sengupta, K.; Georges, P.C.; Janmey, P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 2007, 93, 4453–4461. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of matrix stiffness on the morphology, adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells. Int. J. Med Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Visser, J.; Melchels, F.P.; Jungst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirsch, M.; Birnstein, L.; Pepelanova, I.; Handke, W.; Rach, J.; Seltsam, A.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) Formulated with Human Platelet Lysate Supports Mesenchymal Stem Cell Proliferation and Differentiation and Enhances the Hydrogel’s Mechanical Properties. Bioengineering 2019, 6, 76. https://doi.org/10.3390/bioengineering6030076
Kirsch M, Birnstein L, Pepelanova I, Handke W, Rach J, Seltsam A, Scheper T, Lavrentieva A. Gelatin-Methacryloyl (GelMA) Formulated with Human Platelet Lysate Supports Mesenchymal Stem Cell Proliferation and Differentiation and Enhances the Hydrogel’s Mechanical Properties. Bioengineering. 2019; 6(3):76. https://doi.org/10.3390/bioengineering6030076
Chicago/Turabian StyleKirsch, Marline, Luise Birnstein, Iliyana Pepelanova, Wiebke Handke, Jessica Rach, Axel Seltsam, Thomas Scheper, and Antonina Lavrentieva. 2019. "Gelatin-Methacryloyl (GelMA) Formulated with Human Platelet Lysate Supports Mesenchymal Stem Cell Proliferation and Differentiation and Enhances the Hydrogel’s Mechanical Properties" Bioengineering 6, no. 3: 76. https://doi.org/10.3390/bioengineering6030076
APA StyleKirsch, M., Birnstein, L., Pepelanova, I., Handke, W., Rach, J., Seltsam, A., Scheper, T., & Lavrentieva, A. (2019). Gelatin-Methacryloyl (GelMA) Formulated with Human Platelet Lysate Supports Mesenchymal Stem Cell Proliferation and Differentiation and Enhances the Hydrogel’s Mechanical Properties. Bioengineering, 6(3), 76. https://doi.org/10.3390/bioengineering6030076