Ear Reconstruction Simulation: From Handcrafting to 3D Printing
Abstract
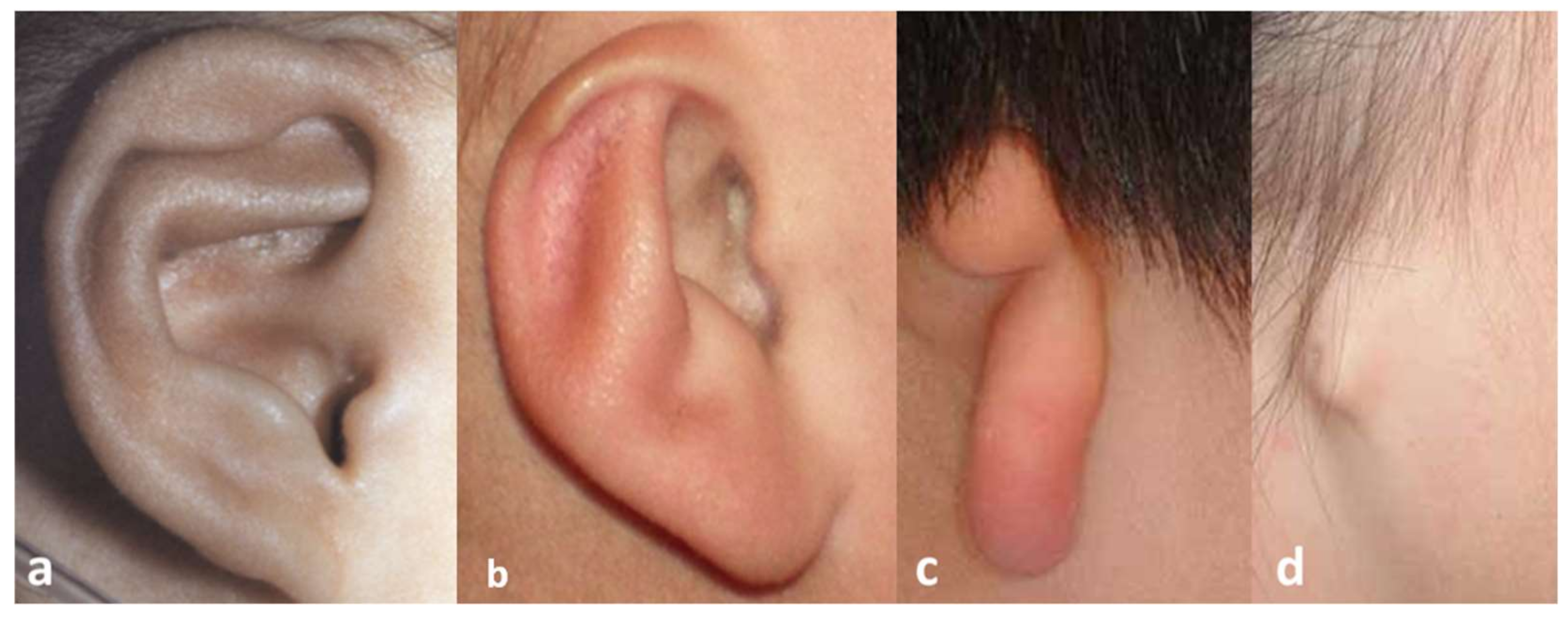
:1. Introduction
- Acquisition of a 3D structure of the ear by means of CT scan, photogrammetry or 3D scanner;
- 3D modelling of the prosthesis using CAD/CAM tools;
- Prosthesis mold manufacturing through 3D printers;
- Filling of the mold to obtain the silicone ear prosthesis.
2. Simulation and Preoperative Planning
- To study, evaluate the available costal cartilage of the patient, identify the best cartilage cutting strategy, and optimize the amount of cartilage taken reducing the donor site morbidity;
- To cut and carve costal cartilage to recreate a three-dimensional framework mimicking the curves and shape of a normal ear, giving it an aesthetical natural appear.
- To find the materials whose mechanical properties are similar to the ones of cartilages, and which could be shaped as the actual costal cartilages. This allows the surgeon to train on cutting, modelling, and carving in a realistic way;
- To find a fabrication method for the creation of anatomical replicas of both the costal cartilage and the ear to be used as reference to reconstruct the 3D framework.
3. Costal Cartilage Simulator Materials: from Potatoes to Silicone
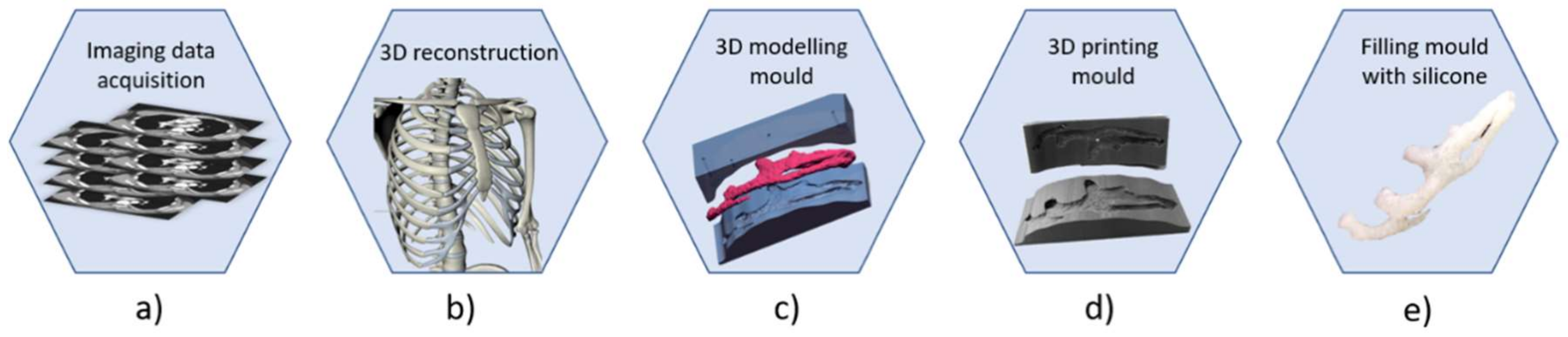
4. Methods to Simulate Ear Reconstruction
- The costal cartilage, i.e., the component from which to extract the basic material for the realization of the ear framework;
- The reference ear, i.e., the template to copy to obtain the ideal ear.
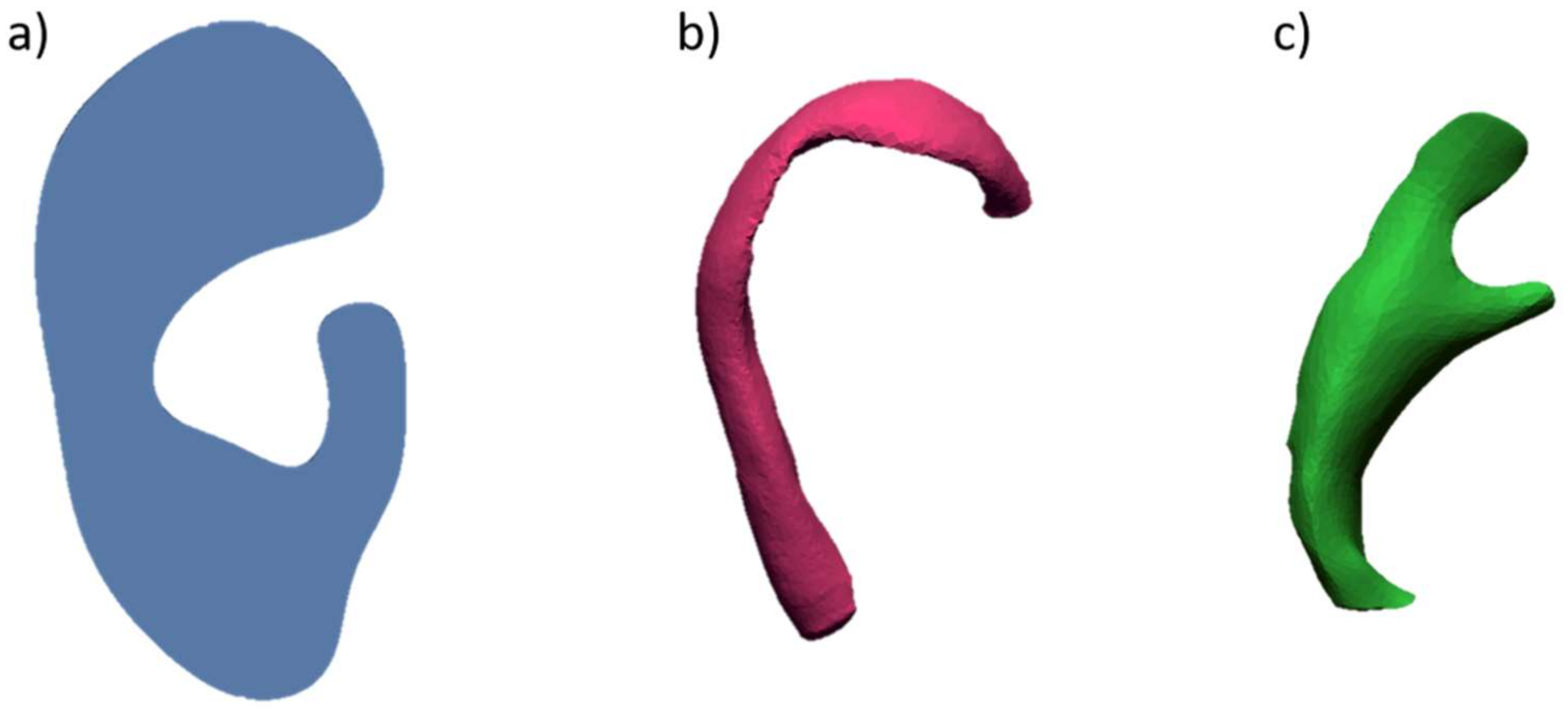
4.1. Costal Cartilage Fabrication Methods
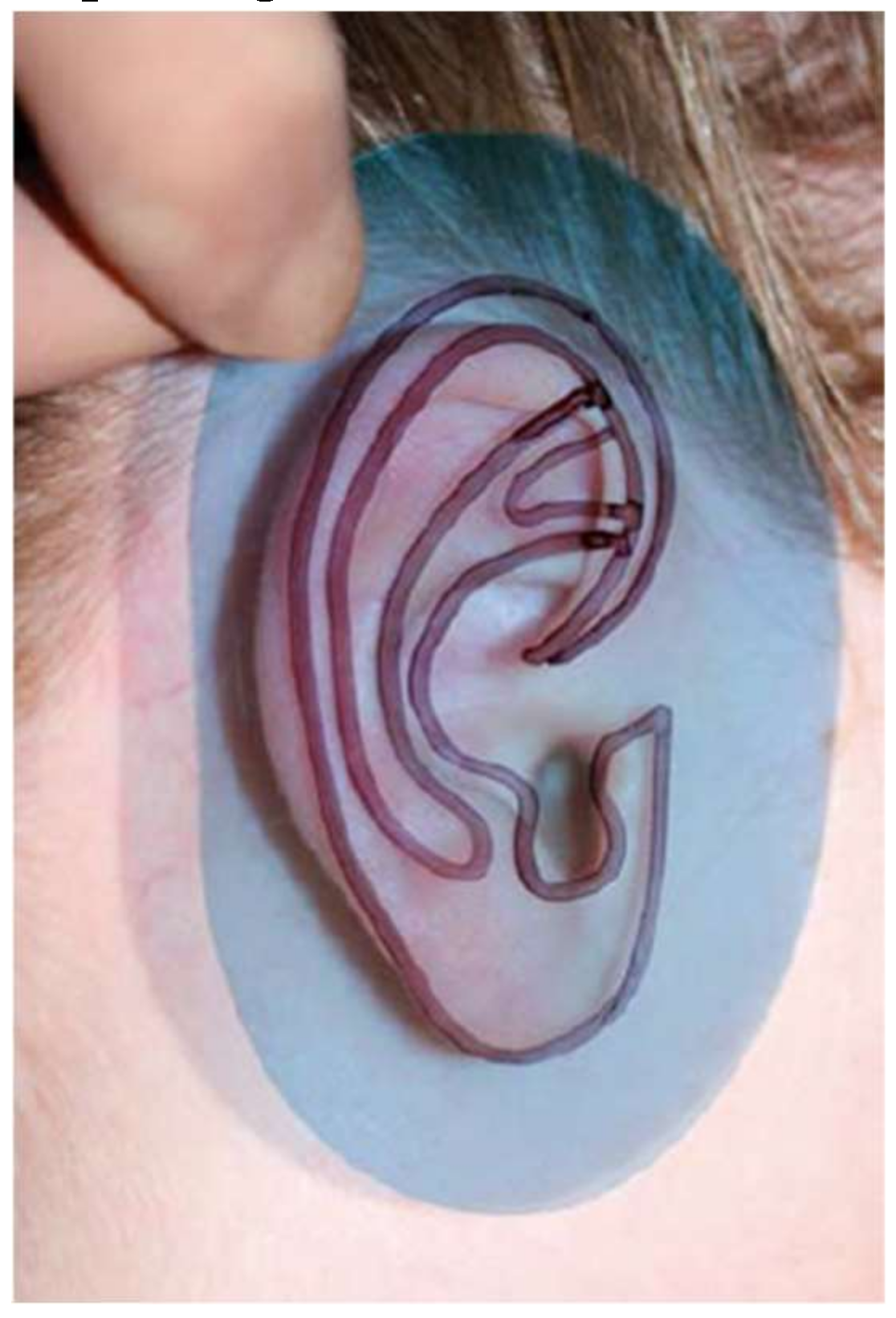
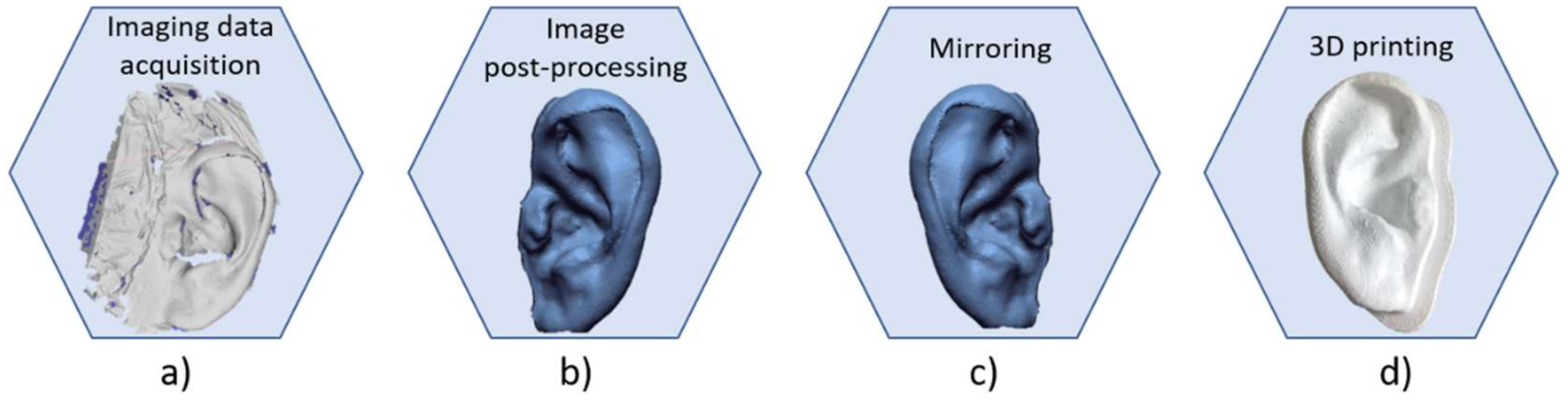
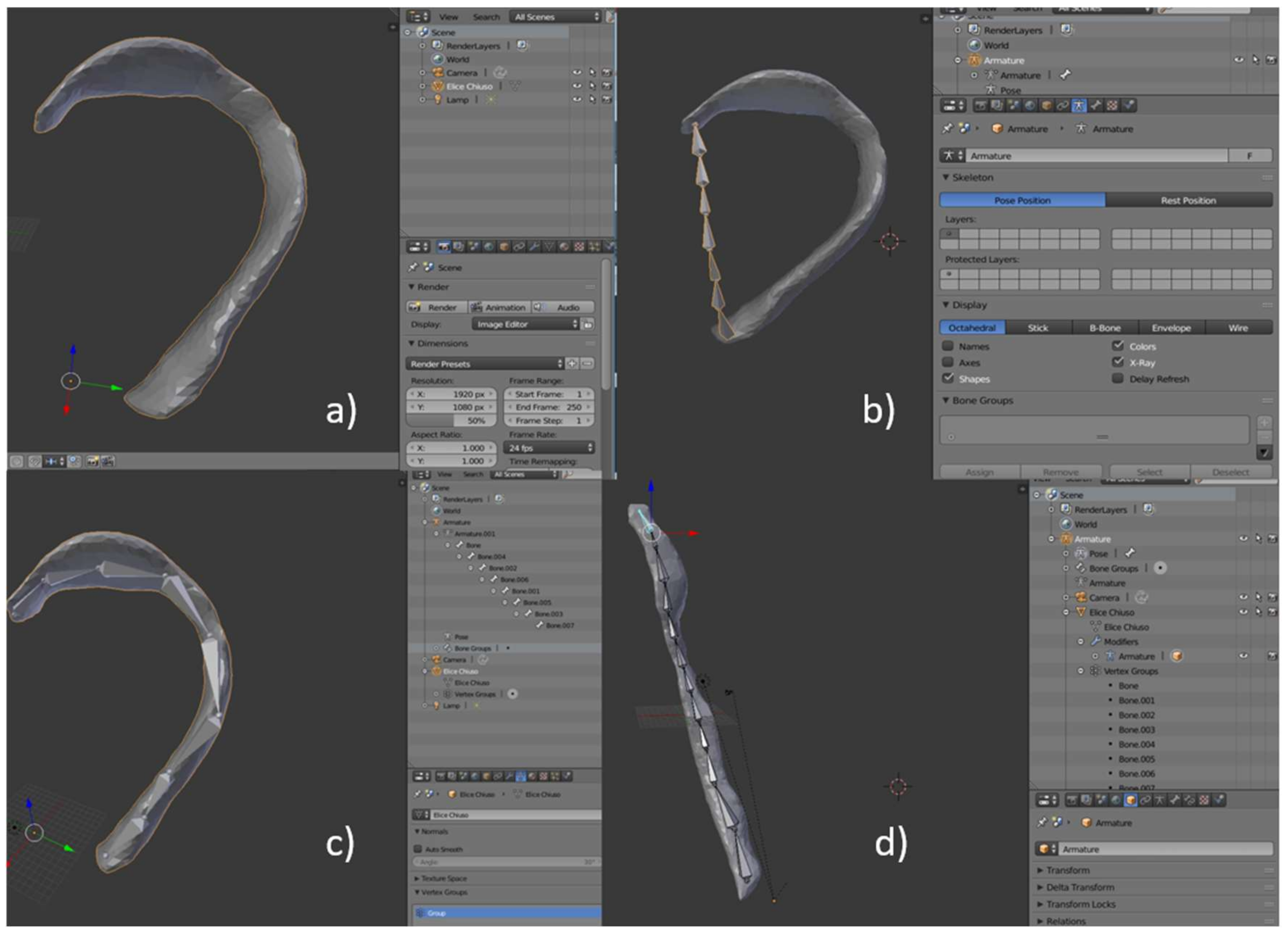
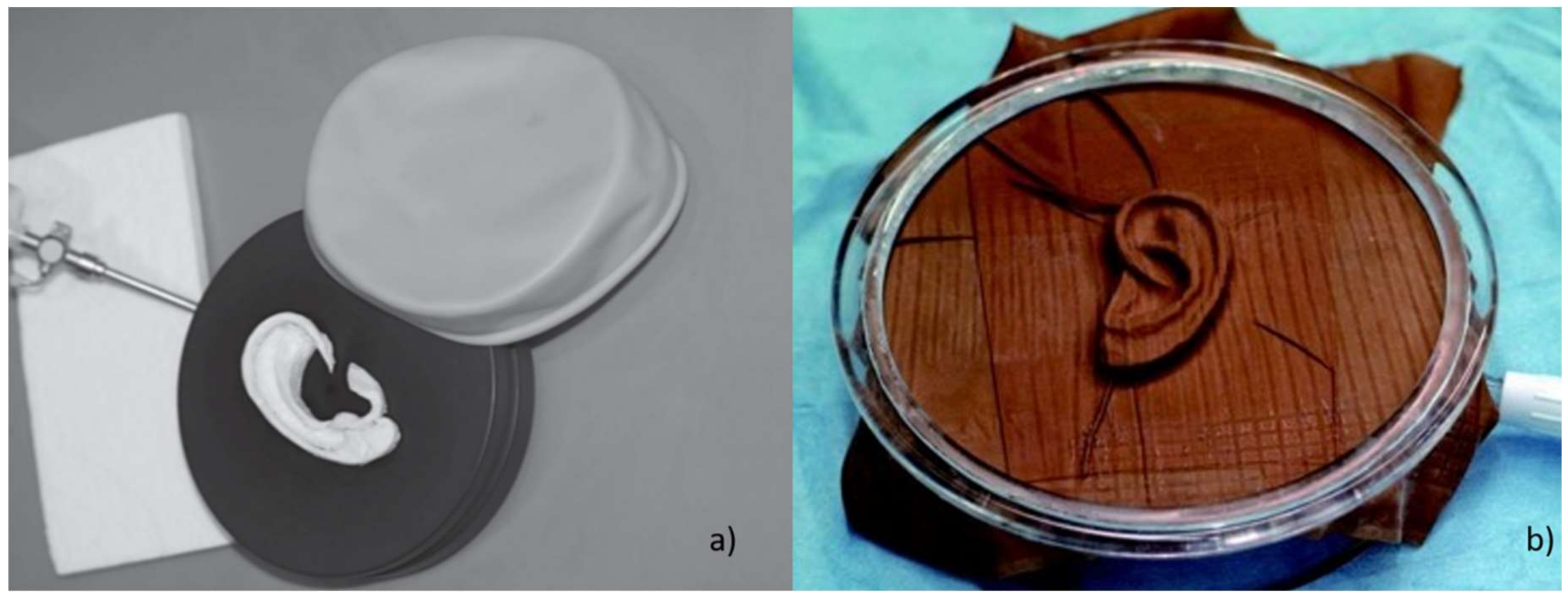
4.2. Reference Ear and Tools Fabrication Methods
5. Methods to Evaluate a Surgeon’s Performance
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heike, C.L.; Luquetti, D.V.; Hing, A.V. Craniofacial Microsomia Overview; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Luquetti, D.V.; Leoncini, E.; Mastroiacovo, P. Microtia-anotia: A global review of prevalence rates. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 813–822. [Google Scholar] [CrossRef]
- Ross, M.T.; Cruz, R.; Hutchinson, C.; Arnott, W.L.; Woodruff, M.A.; Powell, S.K. Aesthetic reconstruction of microtia: A review of current techniques and new 3D printing approaches. Virtual Phys. Prototyp. 2018, 13, 117–130. [Google Scholar] [CrossRef]
- Ishimoto, S.; Ito, K.; Karino, S.; Takegoshi, H.; Kaga, K.; Yamasoba, T. Hearing levels in patients with microtia: Correlation with temporal bone malformation. Laryngoscope 2007, 117, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Llano-Rivas, I.; González-del Angel, A.; del Castillo, V.; Reyes, R.; Carnevale, A. Microtia: A clinical and genetic study at the National Institute of Pediatrics in Mexico City. Arch. Med. Res. 1999, 30, 120–124. [Google Scholar] [CrossRef]
- Billings, K.R.; Qureshi, H.; Gouveia, C.; Ittner, C.; Hoff, S.R. Management of hearing loss and the normal ear in cases of unilateral Microtia with aural atresia. Laryngoscope 2016, 126, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Chen, S. Clinical outcomes following ear reconstruction with adjuvant 3D template model. Acta Otolaryngol. 2016, 136, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chin, W.; Wu, J.; Zhang, Q.; Xu, F.; Xu, Z.; Zhang, R. Psychosocial outcomes among Microtia patients of different ages and genders before ear reconstruction. Aesthetic Plast. Surg. 2010, 34, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Horlock, N.; Vogelin, E.; Bradbury, E.T.; Grobbelaar, A.O.; Gault, D.T. Psychosocial outcome of patients after ear reconstruction. Ann. Plast. Surg. 2005, 54, 517–524. [Google Scholar] [CrossRef]
- Soukup, B.; Mashhadi, S.A.; Bulstrode, N.W. Health-related quality-of-life assessment and surgical outcomes for auricular reconstruction using autologous costal cartilage. Plast. Reconstr. Surg. 2012, 129, 632–640. [Google Scholar] [CrossRef]
- Baluch, N.; Nagata, S.; Park, C.; Wilkes, G.H.; Reinisch, J.; Kasrai, L.; Fisher, D. Auricular reconstruction for microtia: A review of available methods. Plast. Surg. 2014, 22, 39–43. [Google Scholar] [CrossRef]
- Federspil, P.A. Auricular prostheses in Microtia. Facial Plast. Surg. Clin. North. Am. 2018, 26, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Artioli, B.O.; Kunkel, M.E.; Mestanza, S.N. Feasibility Study of a Methodology Using Additive Manufacture to Produce Silicone Ear Prostheses. In World Congress on Medical Physics and Biomedical Engineering 2018; Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G.S., Eds.; Springer: Singapore, 2018; pp. 211–215. [Google Scholar]
- Subburaj, K.; Nair, C.; Rajesh, S.; Meshram, S.M.; Ravi, B. Rapid development of auricular prosthesis using CAD and rapid prototyping technologies. Int. J. Oral Maxillofac. Surg. 2007, 36, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, L.; Mingucci, R.; Gassino, G.; Scotti, R. CAD/CAM ear model and virtual construction of the mold. J. Prosthet. Dent. 2007, 98, 339–343. [Google Scholar] [CrossRef]
- Liacouras, P.; Garnes, J.; Roman, N.; Petrich, A.; Grant, G.T. Designing and manufacturing an auricular prosthesis using computed tomography, 3-dimensional photographic imaging, and additive manufacturing: A clinical report. J. Prosthet. Dent. 2011, 105, 78–82. [Google Scholar] [CrossRef]
- He, Y.; Xue, G.; Fu, J. Fabrication of low cost soft tissue prostheses with the desktop 3D printer. Sci. Rep. 2015, 4, 6973. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Hatamleh, M.M. Complete integration of technology for improved reproduction of auricular prostheses. J. Prosthet. Dent. 2014, 111, 430–436. [Google Scholar] [CrossRef]
- Rajion, Z.A.; Mohamed, N.A. Prosthetic ear reconstruction applying computer tomographic (CT) data and additive manufacturing technologies. J. Teknol. 2015, 76, 63–68. [Google Scholar]
- Yadav, S.; Narayan, A.I.; Choudhry, A.; Balakrishnan, D. CAD/CAM-assisted auricular prosthesis fabrication for a quick, precise, and more retentive outcome: A clinical report. J. Prosthodont. 2017, 26, 616–621. [Google Scholar] [CrossRef]
- Cabin, J.A.; Bassiri-Tehrani, M.; Sclafani, A.P.; Romo, T. Microtia reconstruction. Facial Plast. Surg. Clin. North. Am. 2014, 22, 623–638. [Google Scholar] [CrossRef]
- Williams, J.D.; Romo, T.; Sclafani, A.P.; Cho, H. Porous high-density polyethylene implants in auricular reconstruction. Arch. Otolaryngol. Head Neck Surg. 1997, 123, 578–583. [Google Scholar] [CrossRef]
- Romo, T.; Reitzen, S.D. Aesthetic Microtia reconstruction with Medpor. Facial Plast Surg. 2008, 24, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.; Wang, T.; Stephan, S. Alloplastic reconstruction of the microtic ear. Oper. Tech. Otolaryngol. Neck Surg. 2017, 28, 97–104. [Google Scholar] [CrossRef]
- Cronin, T.D. Use of a silastic frame for total and subtotal reconstruction of the external ear. Plast. Reconstr. Surg. 1966, 37, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Sevin, K.; Askar, I.; Saray, A.; Yormuk, E. Exposure ofhigh-density porous polyethylene (Medpor®) used for contour restoration and treatment. Br. J. Oral Maxillofac. Surg. 2000, 38, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Nayyer, L.; Birchall, M.; Seifalian, A.M.; Jell, G. Design and development of nanocomposite scaffolds for auricular reconstruction. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 235–246. [Google Scholar] [CrossRef]
- Firmin, F. State-of-the-Art Autogenous Ear Reconstruction in Cases of Microtia. In Aesthetics and Functionality in Ear Reconstruction; Karger: Basel, Switzerland, 2010; Volume 68, pp. 25–52. [Google Scholar]
- Im, D.D.; Paskhover, B.; Staffenberg, D.A.; Jarrahy, R. Current management of Microtia: A national survey. Aesthetic Plast. Surg. 2013, 37, 402–408. [Google Scholar] [CrossRef]
- Reiffel, A.J.; Kafka, C.; Hernandez, K.A.; Popa, S.; Perez, J.L.; Zhou, S.; Pramanik, S. High-fidelity tissue engineering of patient-specific auricles for reconstruction of pediatric Microtia and other auricular deformities. PLoS ONE 2013, 8, e56506. [Google Scholar] [CrossRef]
- Schroeder, M.J.; Lloyd, M.S. Tissue engineering strategies for auricular reconstruction. J. Craniofac. Surg. 2017, 28, 2007–2011. [Google Scholar] [CrossRef]
- Cohen, P.; Bernstein, J.L.; Morrison, K.A.; Spector, J.A.; Bonassar, L.J. Tissue engineering the human auricle by auricular chondrocyte-mesenchymal stem cell co-implantation. PLoS ONE 2018, 13, e0202356. [Google Scholar] [CrossRef]
- Otto, I.A.; Melchels, F.P.; Zhao, X.; Randolph, M.A.; Kon, M.; Breugem, C.C.; Malda, J. Auricular reconstruction using biofabrication-based tissue engineering strategies. Biofabrication 2015, 7, 032001. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, H.; Yin, Z.; Liu, Y.; Zhang, Q.; Zhang, C.; Pan, B.; Zhou, J.; Zhou, X.; Sun, H.; et al. In vitro regeneration of patient-specific ear-shaped cartilage and its first clinical application for auricular reconstruction. EBioMedicine 2018, 28, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Daniel, M. Medical error-the third leading cause of death in the US. BMJ 2016, 353. [Google Scholar] [CrossRef] [PubMed]
- Andel, C.; Davidow, S.L.; Hollander, M.; Moreno, D.A. The economics of health care quality and medical errors. J. Health Care Finance 2012, 39, 39–50. [Google Scholar] [PubMed]
- Ziv, A.; Small, S.D.; Root Wolpe, P. Patient safety and simulation-based medical education. Med. Teach. 2000, 22, 489–495. [Google Scholar]
- Furferi, R.; Governi, L.; Uccheddu, F.; Volpe, Y. A Rgb-D Based Instant Body-Scanning Solution For Compact Box Installation; Springer: Cham, Switzerland, 2017; pp. 819–828. [Google Scholar]
- Baronio, G.; Harran, S.; Signoroni, A. A critical analysis of a hand orthosis reverse engineering and 3D printing process. Appl. Bionics Biomech. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carfagni, M.; Facchini, F.; Furferi, R.; Ghionzoli, M.; Governi, L.; Messineo, A.; Servi, M.; Uccheddu, F.; Volpe, Y. A semi-automatic computer-aided method for personalized Vacuum Bell design. Comput. Aided. Des. Appl. 2018, 15, 247–255. [Google Scholar] [CrossRef]
- Di Angelo, L.; Di, P.; Spezzaneve, S.A. Symmetry line detection for non-erected postures. Int. J. Interact. Des. Manuf. 2013, 7, 271–276. [Google Scholar] [CrossRef]
- Hoehnke, C.; Eder, M.; Papadopulos, N.A.; Zimmermann, A.; Brockmann, G.; Biemer, E.; Kovacs, L. Minimal invasive reconstruction of posttraumatic hemi facial atrophy by 3D computer-assisted lipofilling. J. Plast. Reconstr. Aesthetic Surg. 2007, 60, 1138–1144. [Google Scholar] [CrossRef]
- Sharma, V.P.; Bella, H.; Cadier, M.M.; Pigott, R.W.; Goodacre, T.E.E.; Richard, B.M. Outcomes in facial aesthetics in cleft lip and palate surgery: A systematic review. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 1233–1245. [Google Scholar] [CrossRef]
- Volpe, Y.; Furferi, R.; Governi, L.; Uccheddu, F.; Carfagni, M.; Mussa, F.; Scagnet, M.; Genitori, L. Surgery of complex craniofacial defects: A single-step AM-based methodology. Comput. Methods Programs Biomed. 2018, 165, 225–233. [Google Scholar] [CrossRef]
- Liaw, C.-Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kondiah, P.P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. 3D-printing and the effect on medical costs: A new era? Expert Rev. Pharmacoecon. Outcomes Res. 2016, 16, 23–32. [Google Scholar]
- Isogai, N.; Asamura, S.; Higashi, T.; Ikada, Y.; Morita, S.; Hillyer, J.; Jacquet, R.; Landis, W.J. Tissue engineering of an auricular cartilage model utilizing Cultured chondrocyte–poly(l-lactide-ε-caprolactone) scaffolds. Tissue Eng. 2004, 10, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Magritz, R.; Siegert, R. Auricular reconstruction: Surgical innovations, training methods, and an attempt for a look forward. Facial Plast. Surg. 2014, 30, 183–193. [Google Scholar] [PubMed]
- Murabit, A.; Anzarut, A.; Kasrai, L.; Fisher, D.; Wilkes, G. Teaching ear reconstruction using an alloplastic carving model. J. Craniofac. Surg. 2010, 21, 1719–1721. [Google Scholar] [CrossRef]
- Wilkes, G. Learning to perform ear reconstruction. Facial Plast. Surg. 2009, 25, 158–163. [Google Scholar] [CrossRef]
- The Vital Beat. Ear to Help: Volunteers at the iRSM Create Surgical Simulation Models for Surgeons-In-Training. Available online: https://www.thevitalbeat.ca/news/ear-help/ (accessed on 4 October 2018).
- Vadodaria, S.; Mowatt, D.; Giblin, V.; Gault, D. Mastering ear cartilage sculpture: The vegetarian option. Plast. Reconstr. Surg. 2005, 116, 2043–2044. [Google Scholar] [CrossRef]
- Agrawal, K. Bovine Cartilage: A near perfect training tool for carving ear cartilage framework. Cleft Palate Craniofac. J. 2015, 52, 758–760. [Google Scholar] [CrossRef]
- Shin, H.S.; Hong, S.C. A porcine rib cartilage model for practicing ear-framework fabrication. J. Craniofac. Surg. 2013, 24, 1756–1757. [Google Scholar] [CrossRef]
- Brent, B.D. Reconstruction of the Ear. Plastic Surgery Key. Available online: https://plasticsurgerykey.com/reconstruction-of-the-ear/ (accessed on 15 November 2018).
- Thadani, S.M.; Ladani, P.S. A new method for training of ear framework creation by silicon dental impression material. Indian J. Plast. Surg. 2012, 45, 134–137. [Google Scholar] [CrossRef]
- Erdogan, B.; Morioka, D.; Hamada, T.; Kusano, T.; Win, K.M. Use of a plastic eraser for ear reconstruction training. Indian J. Plast. Surg. 2018, 51, 66–69. [Google Scholar] [PubMed]
- Manickavachakan, N.; Shetty, N.; Mathyoo Joseph, V.T. Novel use of 3D reconstruction technology for ear framework construction-the cartilage model. Orig. Res. Artic. J. Evid. Based Med. Heal. 2018, 5, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Imai, K.; Fujimoto, T.; Morimoto, K.; Niitsuma, K.; Matsumoto, H. New training method of creating ear framework by using precise copy of costal cartilage. J. Craniofac. Surg. 2009, 20, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Miyamoto, S.; Nagasao, T.; Kasai, S.; Kishi, K. Preoperative modeling of costal cartilage for the auricular reconstruction of Microtia. Plast. Reconstr. Surg. 2011, 128, 23e–24e. [Google Scholar] [CrossRef] [PubMed]
- Berens, A.M.; Newman, S.; Bhrany, A.D.; Murakami, C.; Sie, K.C.Y.; Zopf, D.A. Computer-aided design and 3D printing to produce a costal cartilage model for simulation of auricular reconstruction. Otolaryngol. Neck Surg. 2016, 155, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.F.; Morrison, R.J.; Green, G.E.; Zopf, D.A. Computer-aided design and 3-dimensional printing for costal cartilage simulation of airway graft carving. Otolaryngol. Neck Surg. 2017, 156, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Pan, B.; Yang, Q.; Zhao, Y.; He, L.; Lin, L.; Sun, H.; Song, Y.; Yu, X.; Sun, Z.; et al. Three-dimensional autologous cartilage framework fabrication assisted by new additive manufactured ear-shaped templates for microtia reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 1436–1444. [Google Scholar] [CrossRef]
- Jeon, B.; Lee, C.; Kim, M.; Choi, T.H.; Kim, S.; Kim, S. Fabrication of three-dimensional scan-to-print ear model for microtia reconstruction. J. Surg. Res. 2016, 206, 490–497. [Google Scholar] [CrossRef]
- Flores, R.L.; Liss, H.; Raffaelli, S.; Humayun, A.; Khouri, K.S.; Coelho, P.G.; Witek, L. The technique for 3D printing patient-specific models for auricular reconstruction. J. Craniomaxillofac. Surg. 2017, 45, 937–943. [Google Scholar] [CrossRef]
- Bos, E.J.; Scholten, T.; Song, Y.; Verlinden, J.C.; Wolff, J.; Forouzanfar, T.; Helder, M.N.; van Zuijlen, P. Developing a parametric ear model for auricular reconstruction: A new step towards patient-specific implants. J. Craniomaxillofac. Surg. 2015, 43, 390–395. [Google Scholar] [CrossRef]
- Oyama, A.; Fujimori, H.; Funayama, E.; Yamamoto, Y. Intraoperative simulation device using negative pressure for construction of framework in Microtia reconstruction. Plast. Reconstr. Surg. 2008, 121, 129e–130e. [Google Scholar] [CrossRef] [PubMed]



| Material | Advantages | Disadvantages | Price |
|---|---|---|---|
| Soap bar [50] |
|
| 1.20 € |
| Vegetables [51,52] |
|
| 0.20 € |
| Animals [53,54] |
|
| 5.00 € |
| Human cadavers [55] |
|
| 2000 € |
| Plastic eraser [57] |
|
| 4.00 € |
| Acrylic polyurethane [60] |
|
| 1.00 € |
| Silicone [58,59,61] |
| 0.60 € |
| Components | Fabrication Methods | Facilities and Software | Material | Manpower |
|---|---|---|---|---|
| Costal cartilages |
|
|
|
|
|
|
|
| |
| Reference ear and tools |
|
|
|
|
|
|
|
| |
|
|
|
| |
|
|
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussi, E.; Furferi, R.; Volpe, Y.; Facchini, F.; McGreevy, K.S.; Uccheddu, F. Ear Reconstruction Simulation: From Handcrafting to 3D Printing. Bioengineering 2019, 6, 14. https://doi.org/10.3390/bioengineering6010014
Mussi E, Furferi R, Volpe Y, Facchini F, McGreevy KS, Uccheddu F. Ear Reconstruction Simulation: From Handcrafting to 3D Printing. Bioengineering. 2019; 6(1):14. https://doi.org/10.3390/bioengineering6010014
Chicago/Turabian StyleMussi, Elisa, Rocco Furferi, Yary Volpe, Flavio Facchini, Kathleen S. McGreevy, and Francesca Uccheddu. 2019. "Ear Reconstruction Simulation: From Handcrafting to 3D Printing" Bioengineering 6, no. 1: 14. https://doi.org/10.3390/bioengineering6010014
APA StyleMussi, E., Furferi, R., Volpe, Y., Facchini, F., McGreevy, K. S., & Uccheddu, F. (2019). Ear Reconstruction Simulation: From Handcrafting to 3D Printing. Bioengineering, 6(1), 14. https://doi.org/10.3390/bioengineering6010014







