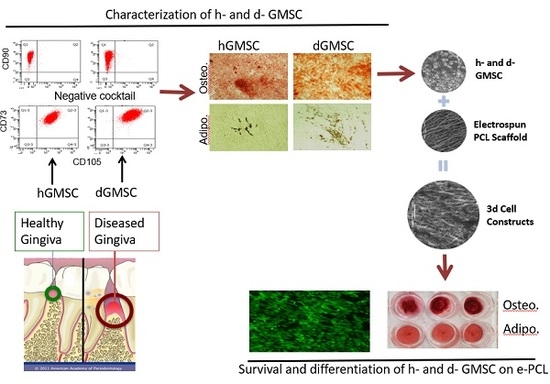
Mesenchymal Stem Cells Derived from Healthy and Diseased Human Gingiva Support Osteogenesis on Electrospun Polycaprolactone Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Sample Identification
2.2. Sample Collection and Establishment of Primary Clonal Cell Lines
2.3. Routine Cell Culture
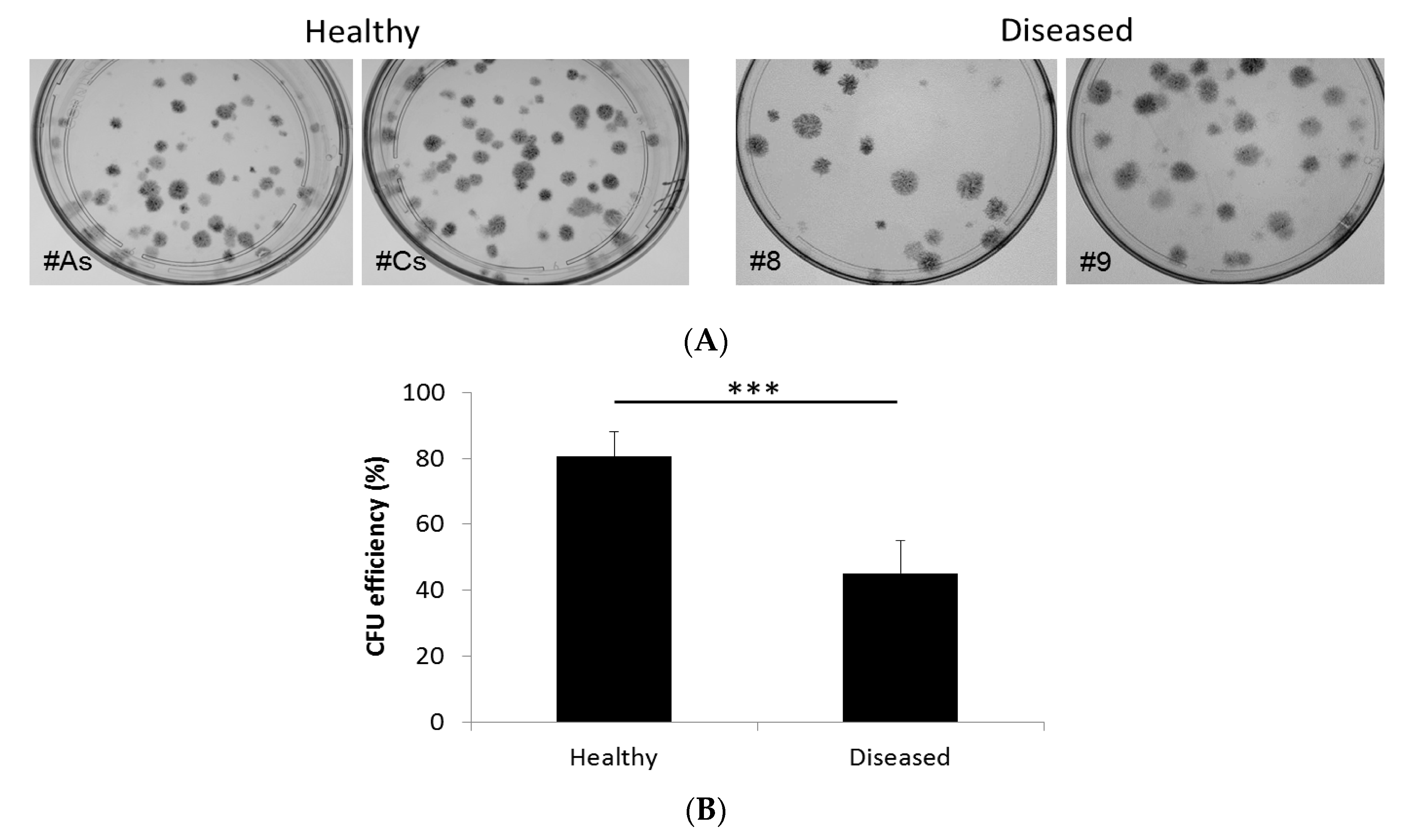
2.4. Colony Forming Unit (CFU) Assay
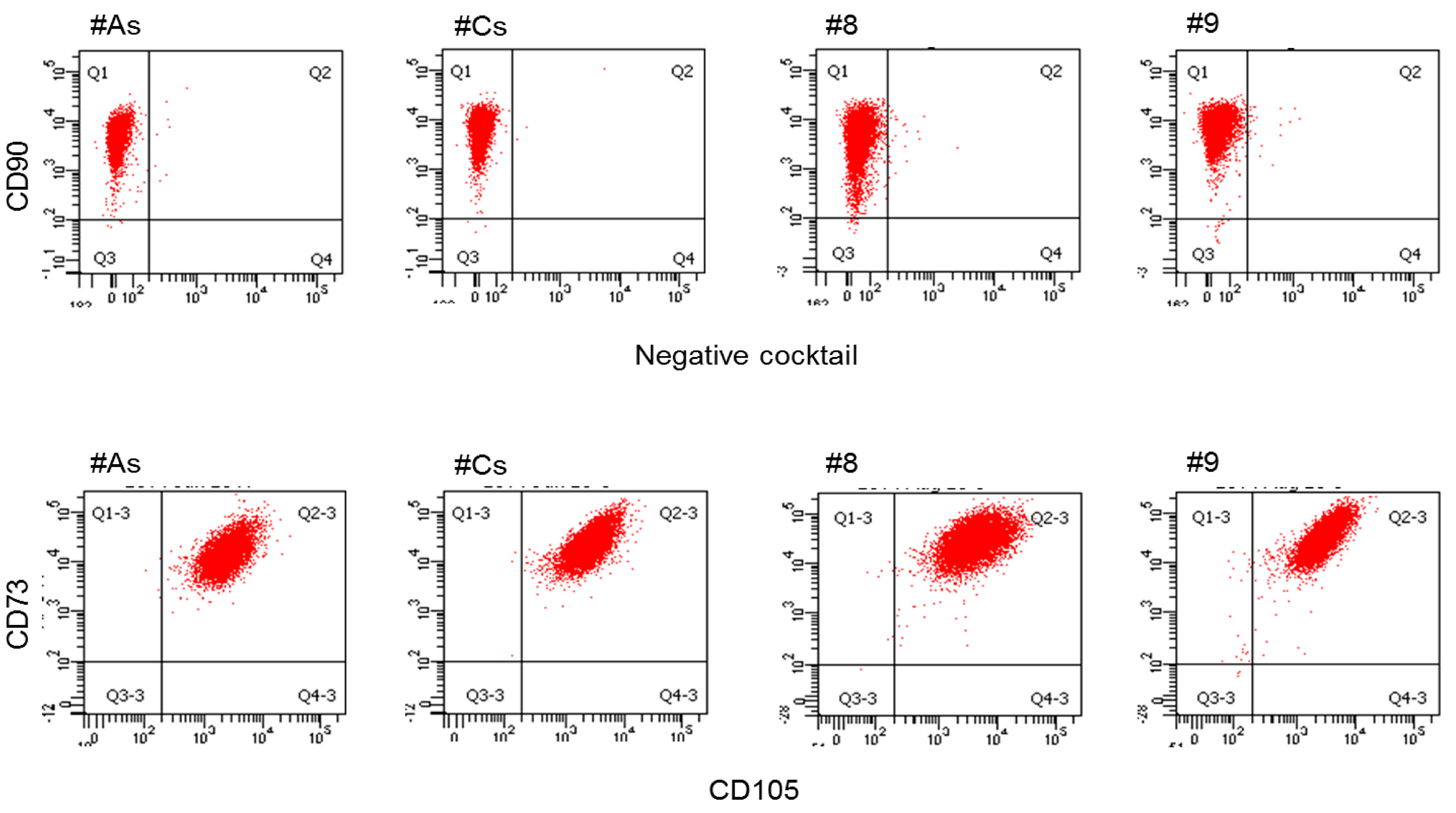
2.5. Flow Cytometric Analysis
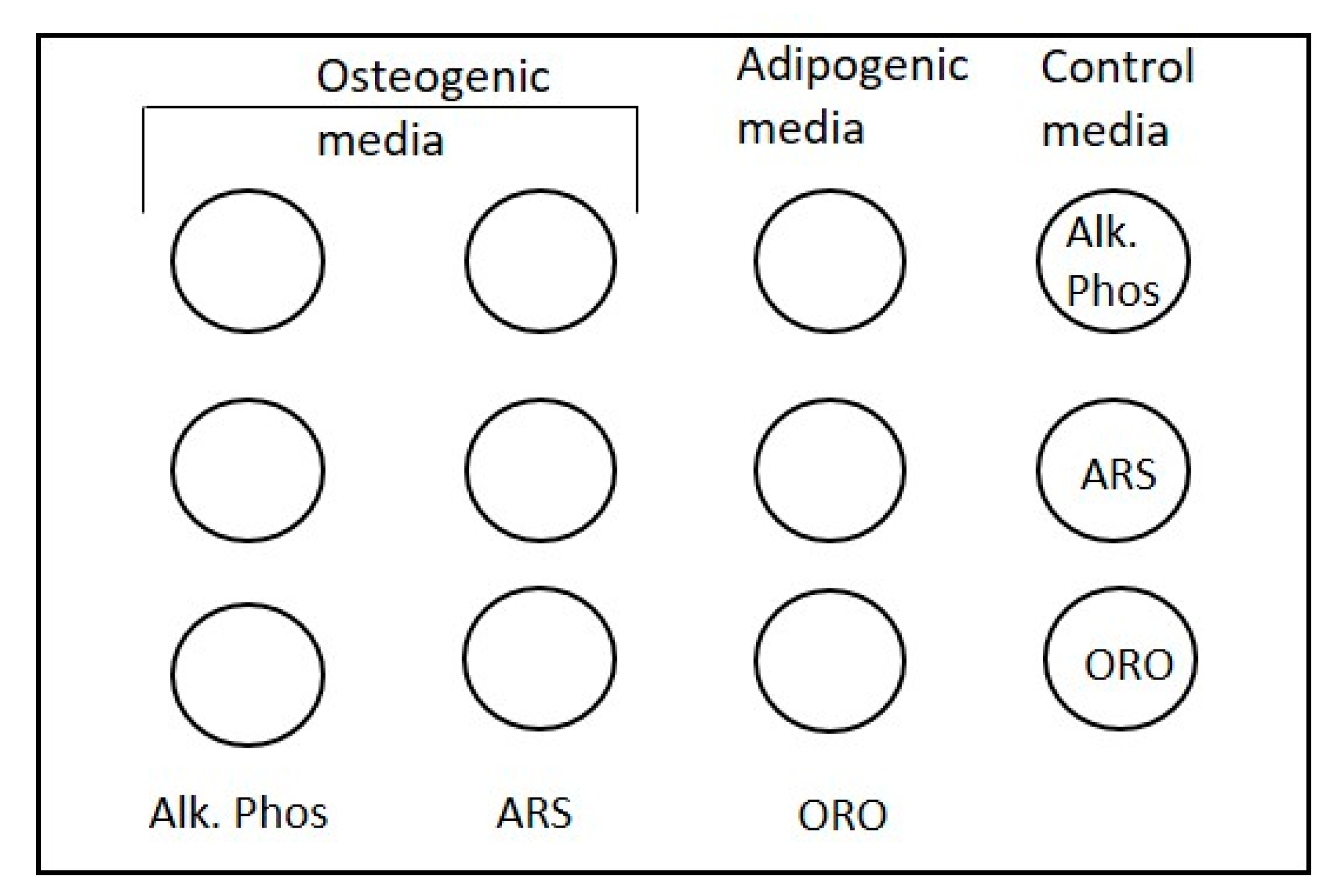
2.6. Differentiation Assays
2.6.1. Scale Used to Assess Osteogenesis
2.6.2. Scale Used to Assess Adipogenesis
2.7. Cell Proliferation
2.8. Measurement of Alkaline Phosphatase (ALP) Activity
2.9. Electrospinning PCL Scaffolds
2.10. Scanning Electron Microscopy
2.11. Scaffold Disinfection and Cell Seeding
2.12. Cell Survival Using Live/Dead Assay
2.13. Cell Proliferation Using MTS Assay
2.14. GMSC Differentiation on Scaffolds
2.15. Statistical Analyses
3. Results
3.1. Adherent Cells Isolated from Healthy and Diseased Gingiva Showed Characteristics of MSC
- Must be plastic-adherent under standard culture conditions
- Must express CD105, CD73, and CD90. MSCs should not express CD45, CD34, CD14, or CD11b, CD79alpha, or HLA-DR surface molecules and,
- Must differentiate into multiple lineages in vitro
3.1.1. Adherent Cells from Both Diseased and Healthy Gingiva Exhibit CFU Activity, Although to Different Degree
3.1.2. Flow Cytometry: Adherent Cells from Both Tissues Express Cell Surface Markers for Adult MSC
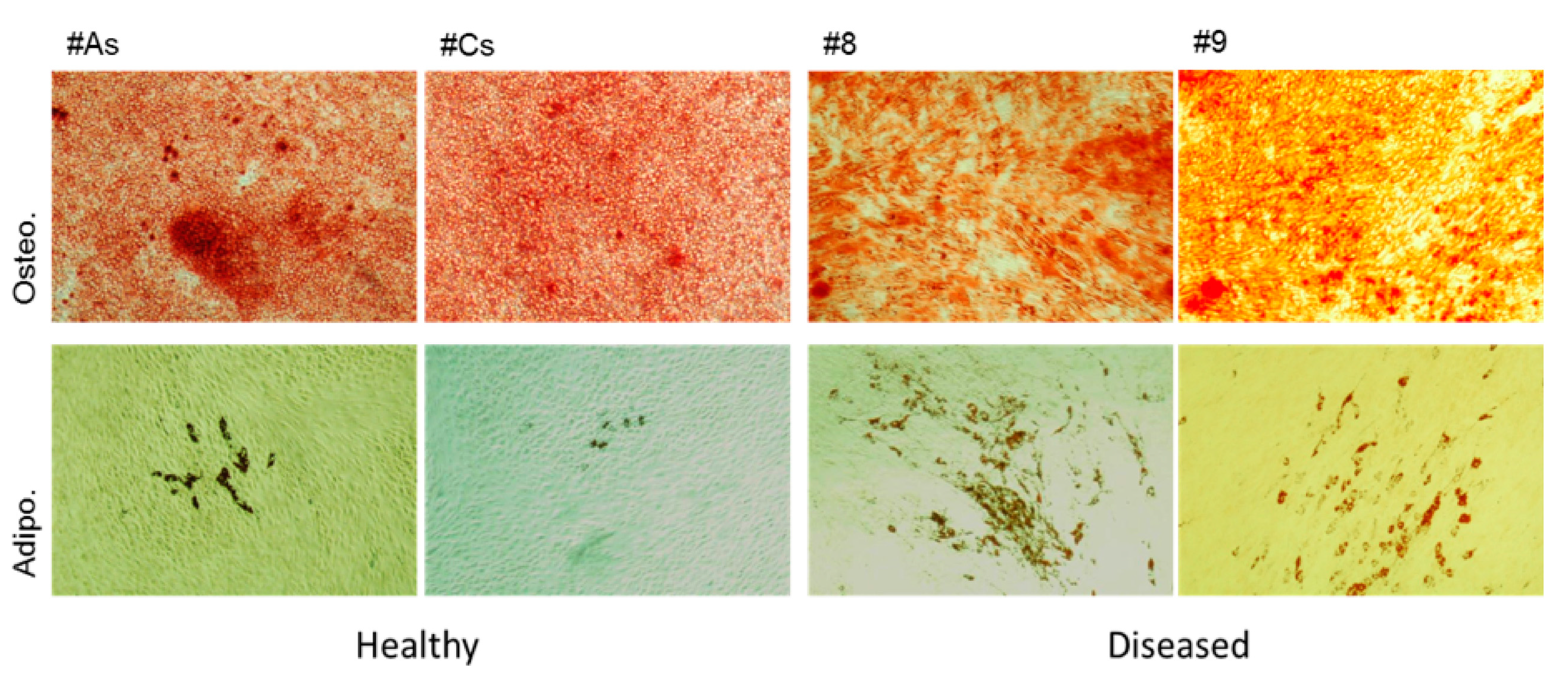
3.1.3. Adherent Cells from Healthy Gingiva Showed Higher Osteogenicity, while Cells from Diseased Gingiva Showed Increased Adipogenesis
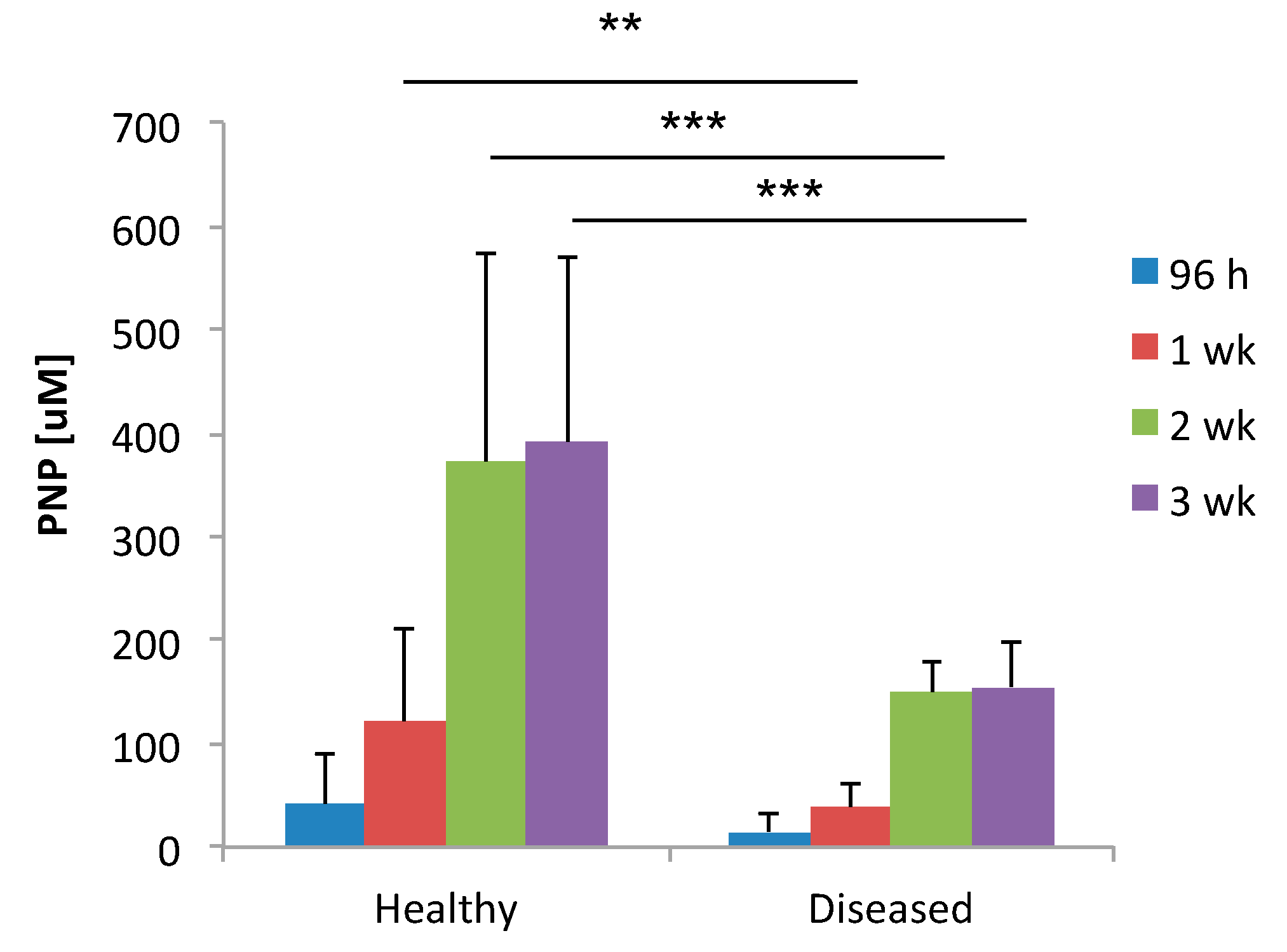
3.2. Alkaline Phosphatase Is Produced in Higher Levels in Healthy GMSCs (hGMSCs) Compared to Diseased GMSCs (dGMSCs)
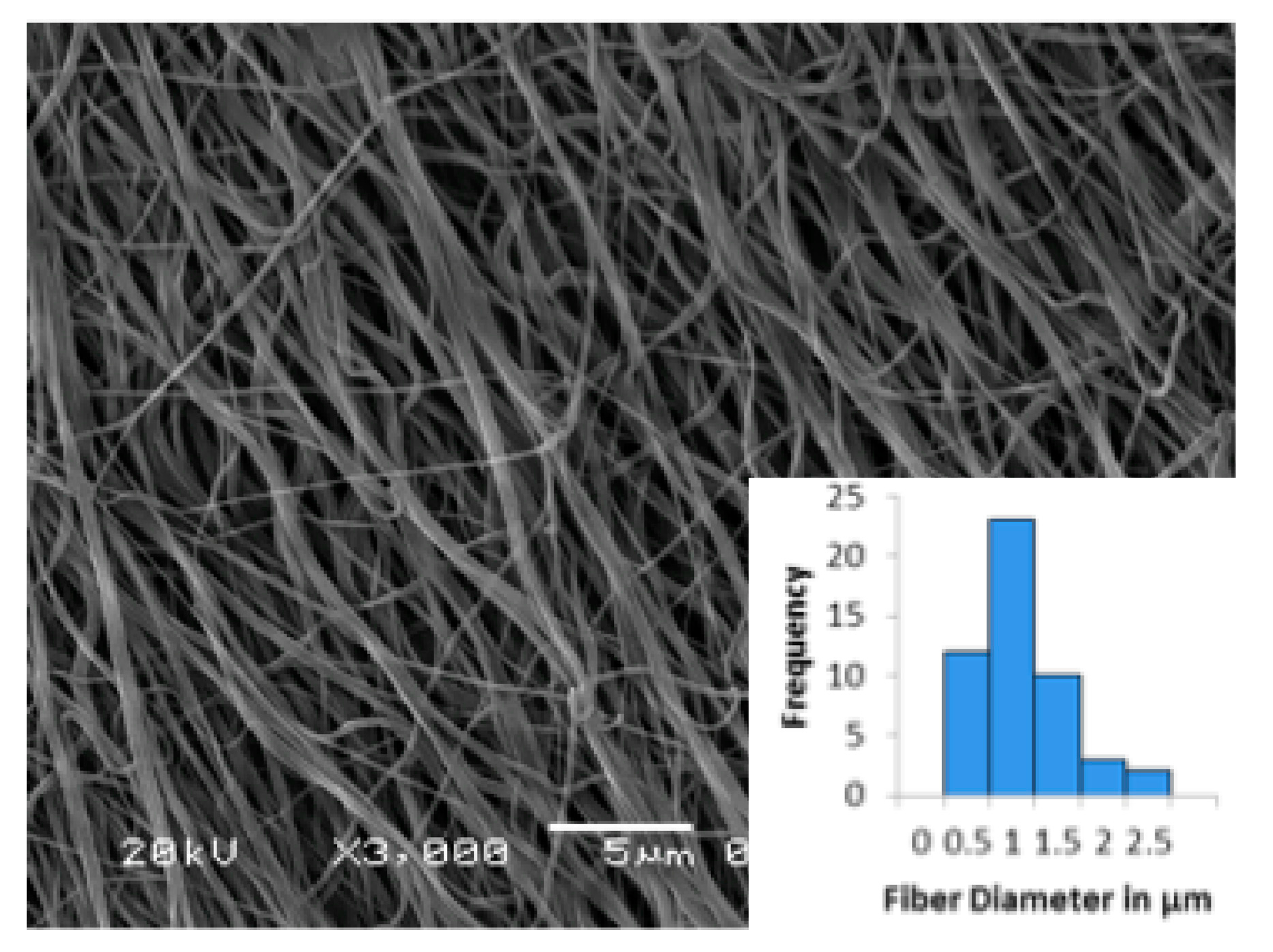
3.3. Electrospun Scaffold Characterization by SEM
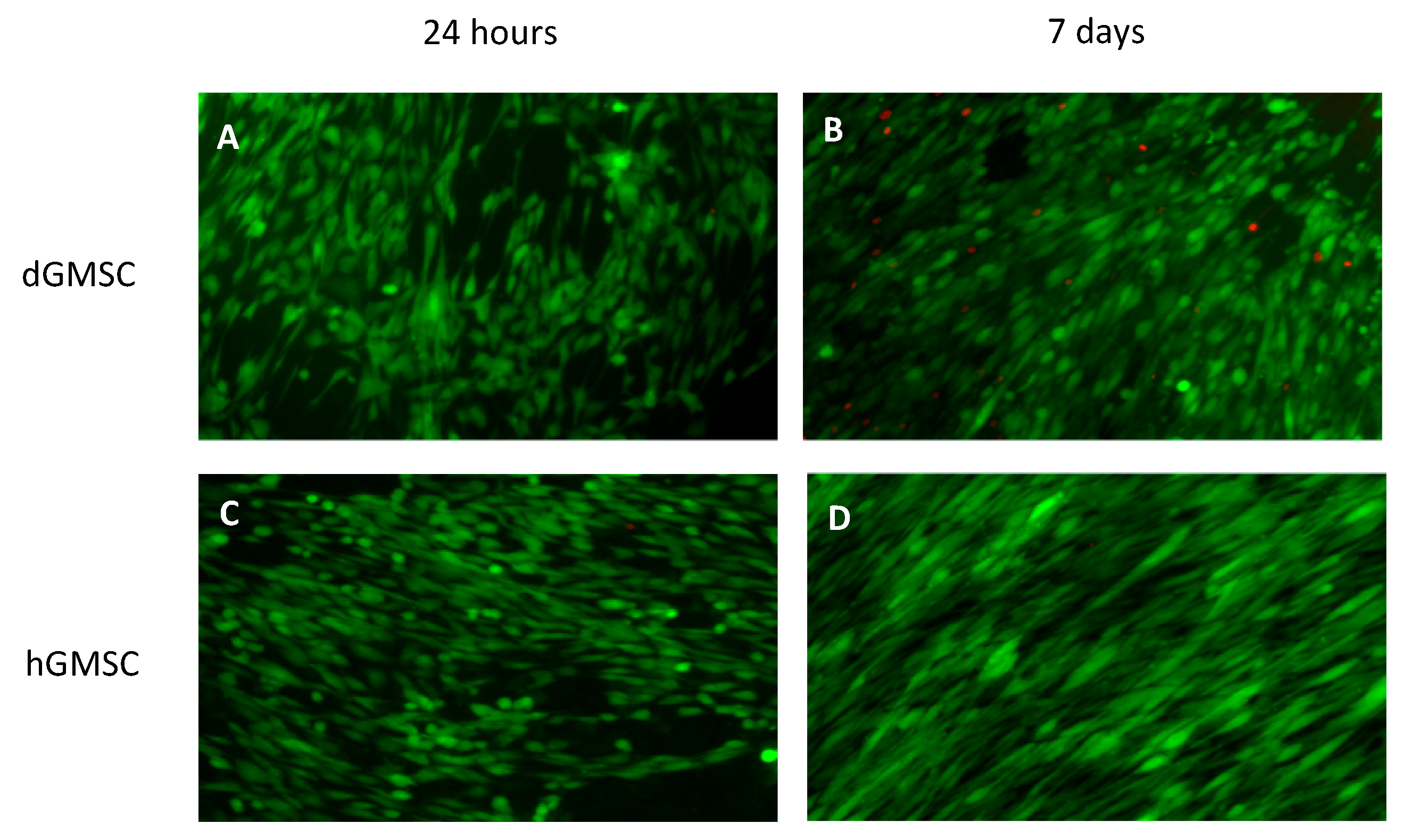
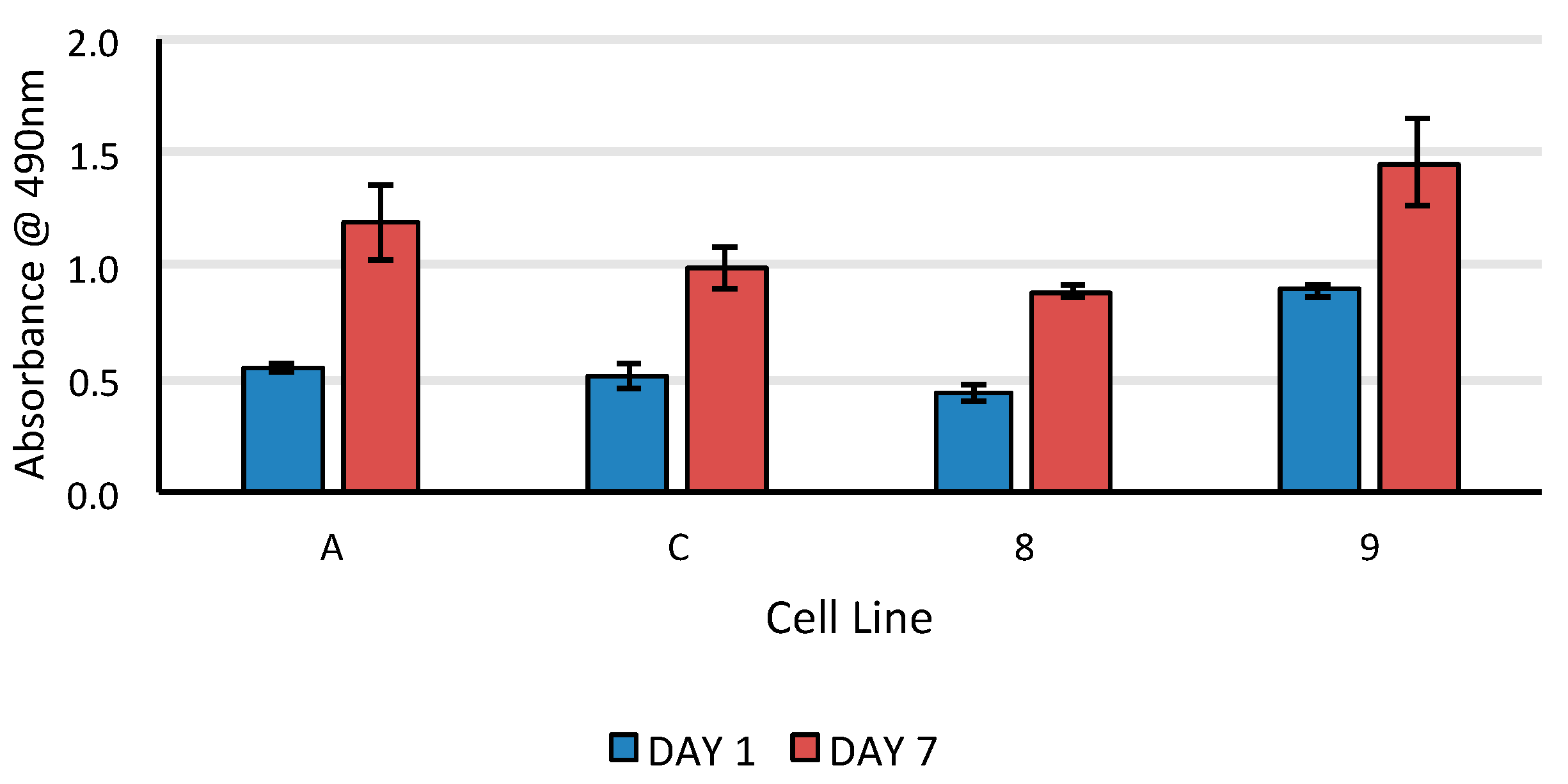
3.4. 24-Hours Cell Survival and 7-Days Proliferation on Electrospun Scaffolds
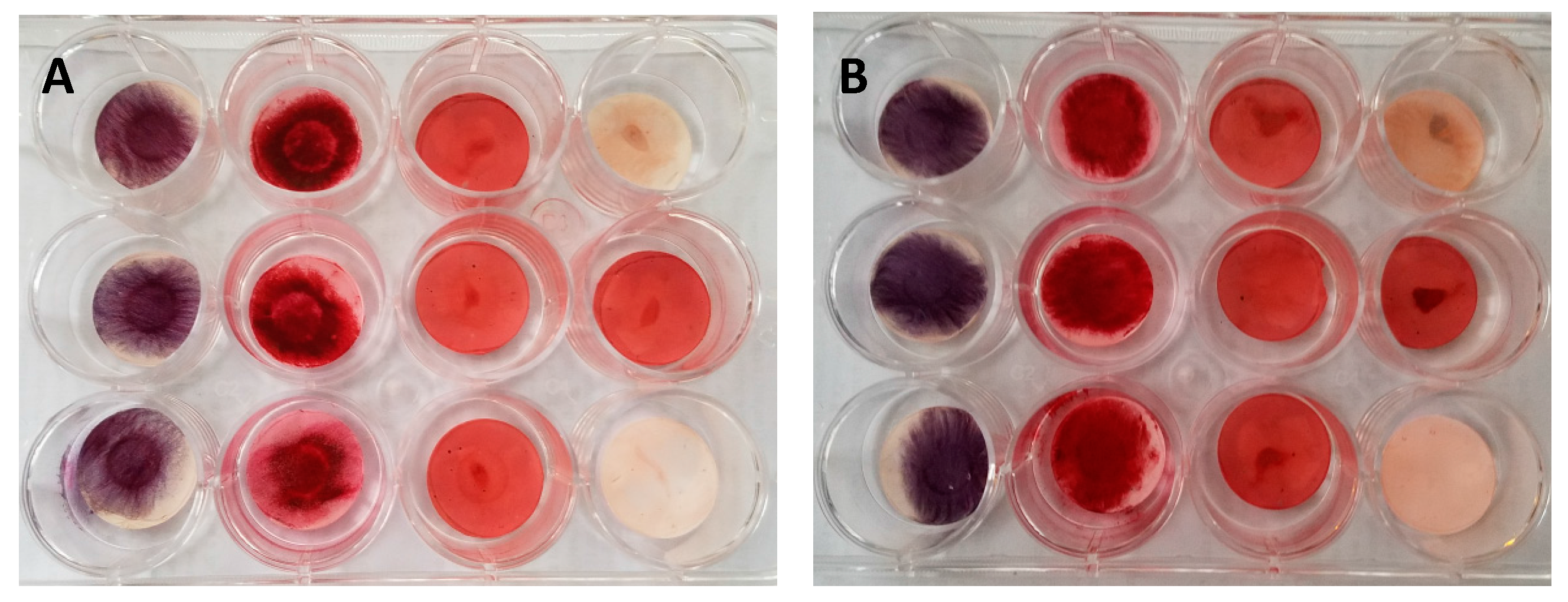
3.5. GMSC Differentiation on Electrospun PCL Scaffolds
4. Discussion
5. Strengths, Limitations, and Future Research Directions
- Generalizability of results: One of the strengths of the study is that the GMSCs evaluated are primary cell sources that are not modified (transformed) in any way. Using finite lines for MSCs is challenging because they are slow and hard to establish, impose limits on the passage numbers that can be used for differentiation, and are expensive to maintain (with specialized media). However, these are the biologically more appropriate cells to study because of their relevance in clinical translation. The results of this study should be interpreted with caution because the data is derived from four primary cell sources that have inherent variability. Future efforts could involve establishing a gingival tissue repository and its distribution to laboratories for evaluating the behavior of these cells. As mentioned above, another option would be to study MSCs derived from healthy and diseased gingiva from the same patient.
- Data quality: The current study used qualitative measures (staining for Alizarin red, Oil Red O, Alkaline phosphatase) to study GMSC differentiation. We adopted this approach because staining can provide early proof-of-concept information about the behavior of GSMCs on electrospun scaffolds. Careful experimental setup with proper controls allowed us to visually verify the differentiation of GMSCs. However, lack of quantitative measures is a limitation and future studies with robust quantifiable data will improve the strength of conclusions. More detailed experiments involving RT-PCR and Western blotting can provide insights into the mechanisms controlled the fate of MSCs.
- A minor limitation of this study could be not validating the GMSCs for their chondrogenic lineage. Detailed characterization of GMSCs by previous research groups has established that GMSCs do possess multi-lineage potential and, hence, these tests were not repeated in our study. Since our primary goal was to evaluate effectiveness of GMSCs in bone engineering, we felt that doing chondrogenic assays would not add significantly to the scientific merit of the study.
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Logeart-Avramoglou, D.; Anagnostou, F.; Bizios, R.; Petite, H. Engineering bone: Challenges and obstacles. J. Cell. Mol. Med. 2005, 9, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.; Khan, Y.; El-Amin, S.F. Bone graft substitutes. Expert Rev. Med. Dev. 2006, 3, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of stem cells in bone tissue engineering: A review. Stem Cells Int. 2016, 2016, 6180487. [Google Scholar] [CrossRef] [PubMed]
- Madurantakam, P.A.; Cost, C.P.; Simpson, D.G.; Bowlin, G.L. Science of nanofibrous scaffold fabrication: Strategies for next generation tissue-engineering scaffolds. Nanomedicine 2009, 4, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.; Barnes, C.; Smith, M.; McClure, M.; Madurantakam, P.; Grant, J.; McManus, M.; Bowlin, G. Extracellular matrix regenerated: Tissue engineering via electrospun biomimetic nanofibers. Polym. Int. 2007, 56, 1349–1360. [Google Scholar] [CrossRef]
- Mitrano, T.I.; Grob, M.S.; Carrión, F.; Nova-Lamperti, E.; Luz, P.A.; Fierro, F.S.; Quintero, A.; Chaparro, A.; Sanz, A. Culture and characterization of mesenchymal stem cells from human gingival tissue. J. Periodontol. 2010, 81, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Elahi, K.C.; Klein, G.; Avci-Adali, M.; Sievert, K.D.; Macneil, S.; Aicher, W.K. Human mesenchymal stromal cells from different sources diverge in their expression of cell surface proteins and display distinct differentiation patterns. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Trento, C.; Dazzi, F. Mesenchymal stem cells and innate tolerance: Biology and clinical applications. Swiss Med. Wkly. 2010, 140, w13121. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, M.; Yan, X.; Wen, Y.; Zeng, Q.; Yue, W.; Yang, P.; Pei, X. Gingiva-Derived Mesenchymal Stem Cell-Mediated Therapeutic Approach for Bone Tissue Regeneration. Stem Cells Dev. 2011, 20, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Mrozik, K.M.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Isolation and characterization of mesenchymal stem cell-like cells from healthy and inflamed gingival tissue: Potential use for clinical therapy. Regen. Med. 2012, 7, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Su, W.R.; Shi, S.H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B.P.J.; Larjava, H.; Häkkinen, L. Gingiva as a source of stem cells with therapeutic potential. Stem Cells Dev. 2013, 22, 3157–3177. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Lemmouchi, Y.; Schacht, E.; Kageruka, P.; De Deken, R.; Diarra, B.; Diall, O.; Geerts, S. Biodegradable polyesters for controlled release of trypanocidal drugs: In vitro and in vivo studies. Biomaterials 1998, 19, 1827–1837. [Google Scholar] [CrossRef]
- Zhang, S.; Uludaǧ, H. Nanoparticulate systems for growth factor delivery. Pharm. Res. 2009, 26, 1561–1580. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2011, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Coss, D.; Yang, L.; Kuo, C.B.; Xu, X.; Luben, R.A.; Walker, A.M. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1216–E1225. [Google Scholar] [CrossRef] [PubMed]
- Reger, R.; Tucker, A.; Wolfe, M. Mesenchymal Stem Cells; Prockop, D.J., Phinney, D.G., Bunnell, B.A., Eds.; Humana Press: New York, NY, USA, 2008; pp. 93–109. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Stem cells, tissue engineering and periodontal regeneration. Aust. Dent. J. 2014, 59 (Suppl. S1), 117–130. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.W.; Mao, J.J. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006, 420, 339–361. [Google Scholar] [PubMed]
- Tuan, R.S.; Boland, G.; Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2003, 5, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Tae, S.-K.; Lee, S.-H.; Park, J.-S.; Im, G.-I. Mesenchymal stem cells for tissue engineering and regenerative medicine. Biomed. Mater. 2006, 1, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Parekkadan, B.; Milwid, J.M. Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 2010, 12, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, R.; Trygg, C.; Patel, B.; Kriedt, C.; Dufour, J.; Gimble, J.M.; Bunnell, B.A. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J. Cell. Biochem. 2006, 99, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Requicha, J.F.; Viegas, C.A.; Albuquerque, C.M.; Azevedo, J.M.; Reis, R.L.; Gomes, M.E. Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev. Rep. 2012, 8, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.C.; Lacey, M.R.; Gilliam, J.K.; Tucker, H.A.; Phinney, D.G.; O’Connor, K.C. Clonal analysis of the proliferation potential of human bone marrow mesenchymal stem cells as a function of potency. Biotechnol. Bioeng. 2011, 108, 2716–2726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef] [PubMed]
- Tomar, G.B.; Srivastava, R.K.; Gupta, N.; Barhanpurkar, A.P.; Pote, S.T.; Jhaveri, H.M.; Mishra, G.C.; Wani, M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010, 393, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Castilho, R.M.; Giannobile, W.V. Epigenetics and Its Role in Periodontal Diseases: A State-of-the-Art Review. J. Periodontol. 2015, 86, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Lavu, V.; Venkatesan, V.; Rao, S.R. The epigenetic paradigm in periodontitis pathogenesis. J. Indian Soc. Periodontol. 2015, 19, 142–149. [Google Scholar] [PubMed]
- Cho, Y.-D.; Kim, P.-J.; Kim, H.-G.; Seol, Y.-J.; Lee, Y.-M.; Ku, Y.; Rhyu, I.-C.; Ryoo, H.-M. Transcriptomics and methylomics in chronic periodontitis with tobacco use: A pilot study. Clin. Epigenet. 2017, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wang, B.; et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.J.; Yamaza, T.; Song, Y.; Fouad, A.F.; Romberg, E.E.; Shi, S.; Tuan, R.S.; Huang, G.T.-J. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen. Med. 2010, 5, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Xu, X.; Chen, C.; Ansari, S.; Zadeh, H.H.; Snead, M.L.; Shi, S. Application of stem cells derived from the periodontal ligament or gingival tissue sources for tendon tissue regeneration. Biomaterials 2014, 35, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Chen, C.; Xu, X.; Akiyama, K.; Ansari, S.; Zadeh, H.H.; Shi, S. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in rgd-modified alginate scaffold. Tissue Eng. Part A 2013, 20, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Chen, C.; Xu, X.; Annabi, N.; Zadeh, H.H.; Wu, B.M.; Khademhosseini, A.; Shi, S.; Moshaverinia, A. Muscle tissue engineering using gingival mesenchymal stem cells encapsulated in alginate hydrogels containing multiple growth factors. Ann. Biomed. Eng. 2016, 44, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jauregui, C.; Yoganarasimha, S.; Madurantakam, P. Mesenchymal Stem Cells Derived from Healthy and Diseased Human Gingiva Support Osteogenesis on Electrospun Polycaprolactone Scaffolds. Bioengineering 2018, 5, 8. https://doi.org/10.3390/bioengineering5010008
Jauregui C, Yoganarasimha S, Madurantakam P. Mesenchymal Stem Cells Derived from Healthy and Diseased Human Gingiva Support Osteogenesis on Electrospun Polycaprolactone Scaffolds. Bioengineering. 2018; 5(1):8. https://doi.org/10.3390/bioengineering5010008
Chicago/Turabian StyleJauregui, Catherine, Suyog Yoganarasimha, and Parthasarathy Madurantakam. 2018. "Mesenchymal Stem Cells Derived from Healthy and Diseased Human Gingiva Support Osteogenesis on Electrospun Polycaprolactone Scaffolds" Bioengineering 5, no. 1: 8. https://doi.org/10.3390/bioengineering5010008
APA StyleJauregui, C., Yoganarasimha, S., & Madurantakam, P. (2018). Mesenchymal Stem Cells Derived from Healthy and Diseased Human Gingiva Support Osteogenesis on Electrospun Polycaprolactone Scaffolds. Bioengineering, 5(1), 8. https://doi.org/10.3390/bioengineering5010008