Response of Fibroblasts MRC-5 to Flufenamic Acid-Grafted MCM-41 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
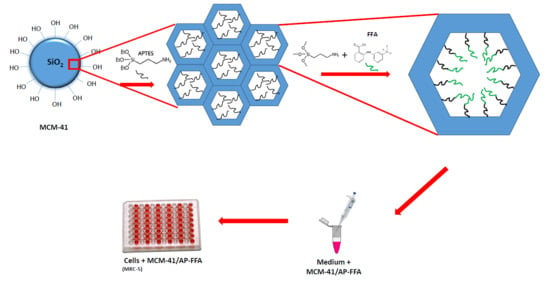
2.2. Synthesis of MCM-41
2.3. Functionalization of MCM-41 with APTES: MCM-41/AP
2.4. Functionalization of MCM-41/AP with FFA: MCM-41/AP-FFA
2.5. Physicochemical and Morphological Characterization of Free MCM-41 and Functionalized Systems
2.6. Flufenamic Acid Release Test
2.7. Cell Culture
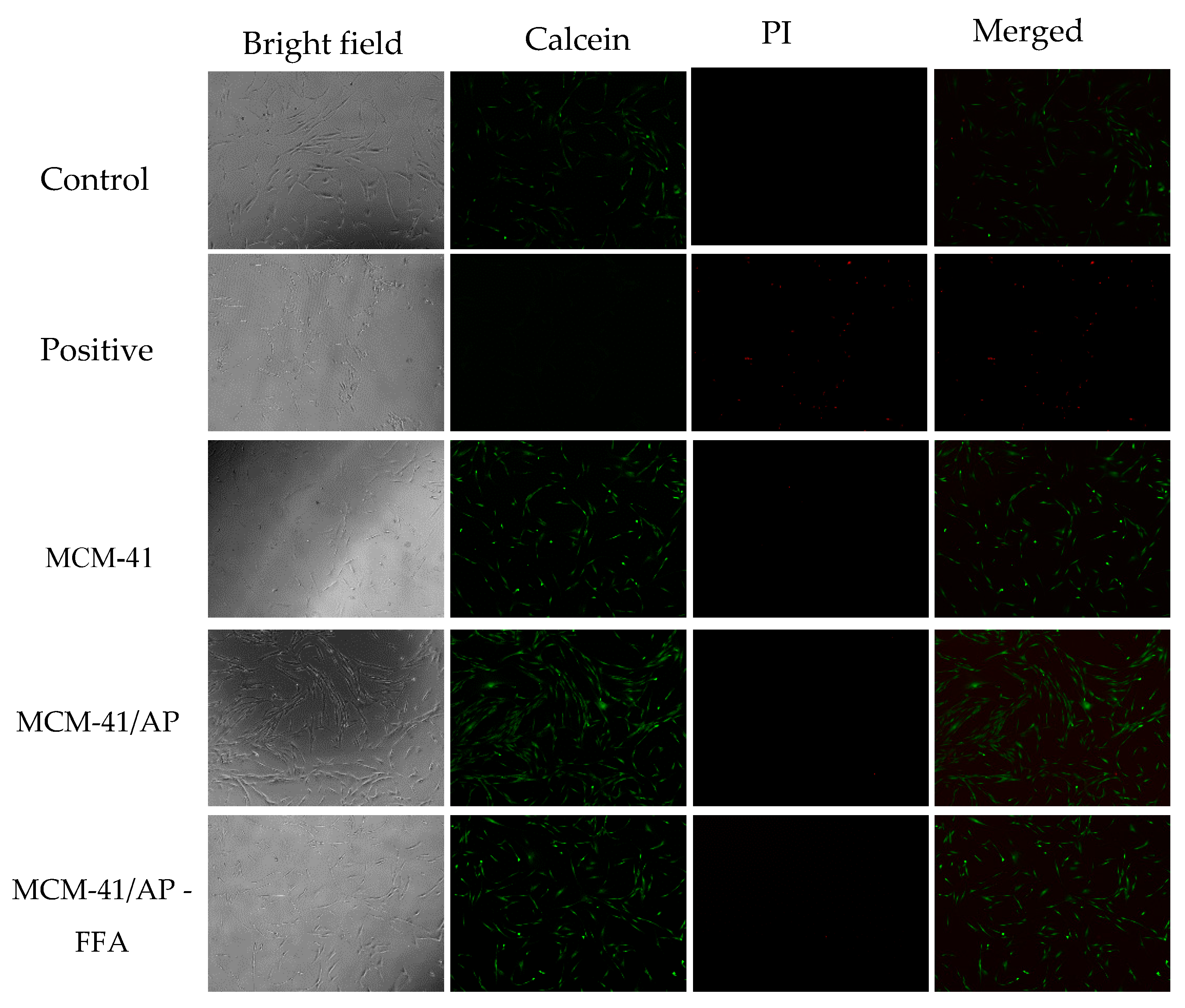
2.8. Biocompatibility Assay
3. Results and Discussion
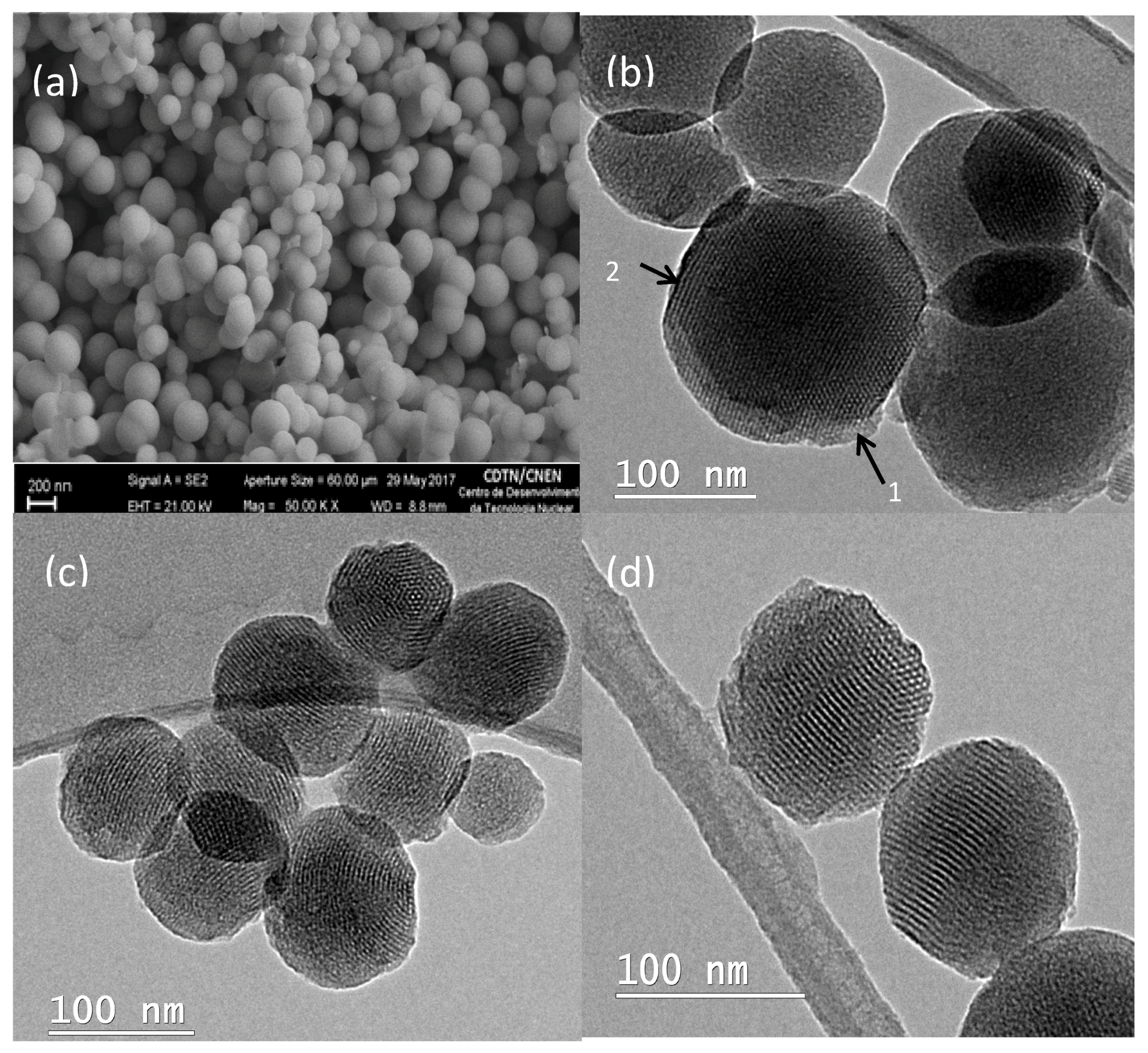
3.1.Scanning Electron Microscopy (SEM) and Transmition Electron Microscopy (TEM) Results
3.2. Nitrogen Adsorption Analysis
3.3. Small-Angle X-ray Scattering (SAXS)
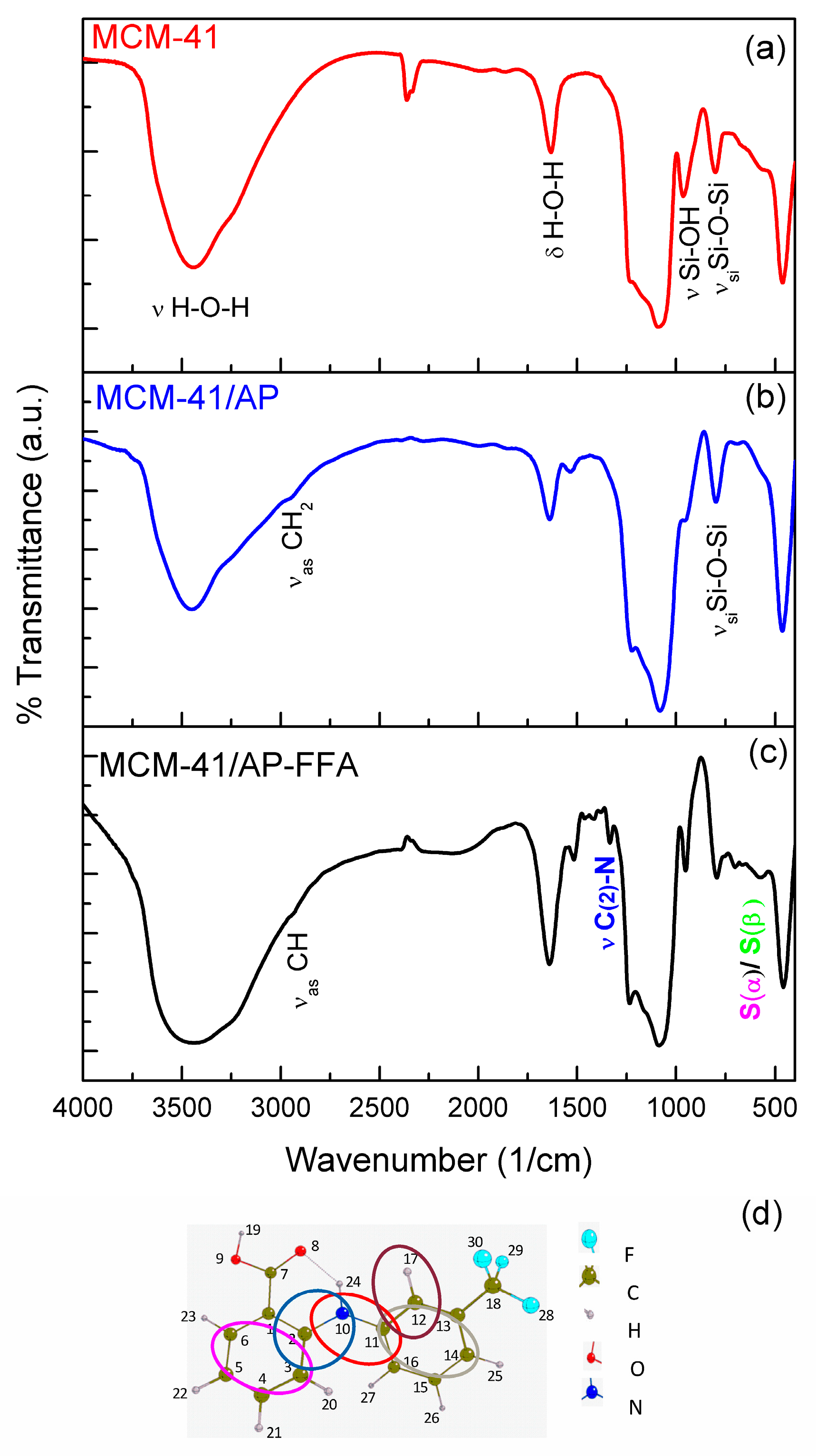
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. Electron Energy Loss Spectroscopy (EELS) and Energy-Filtered Transmission Electron Microscopy (EFTEM)
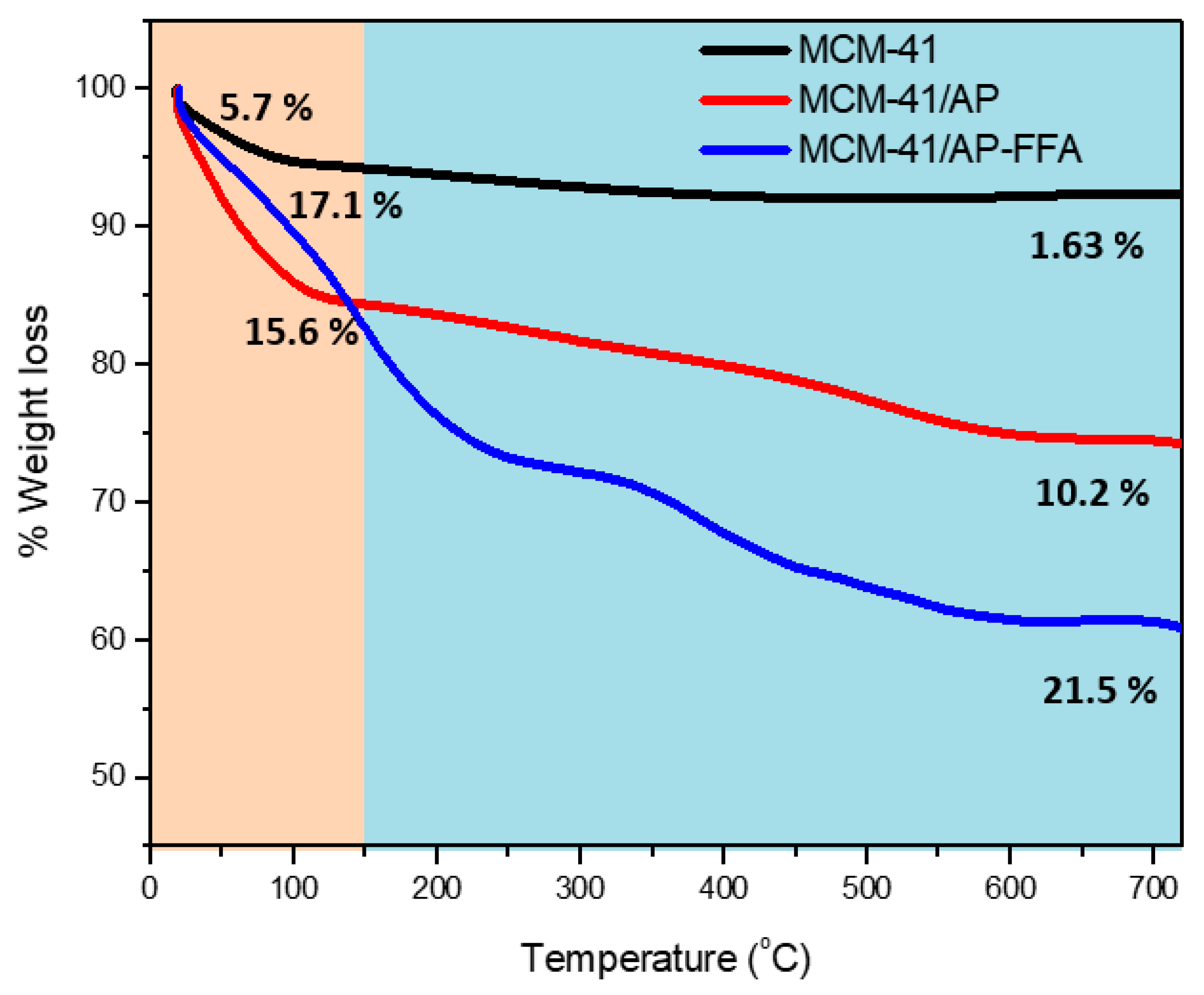
3.6. Thermogravimetric Analysis (TGA-DTG)
3.7. Elemental Analysis
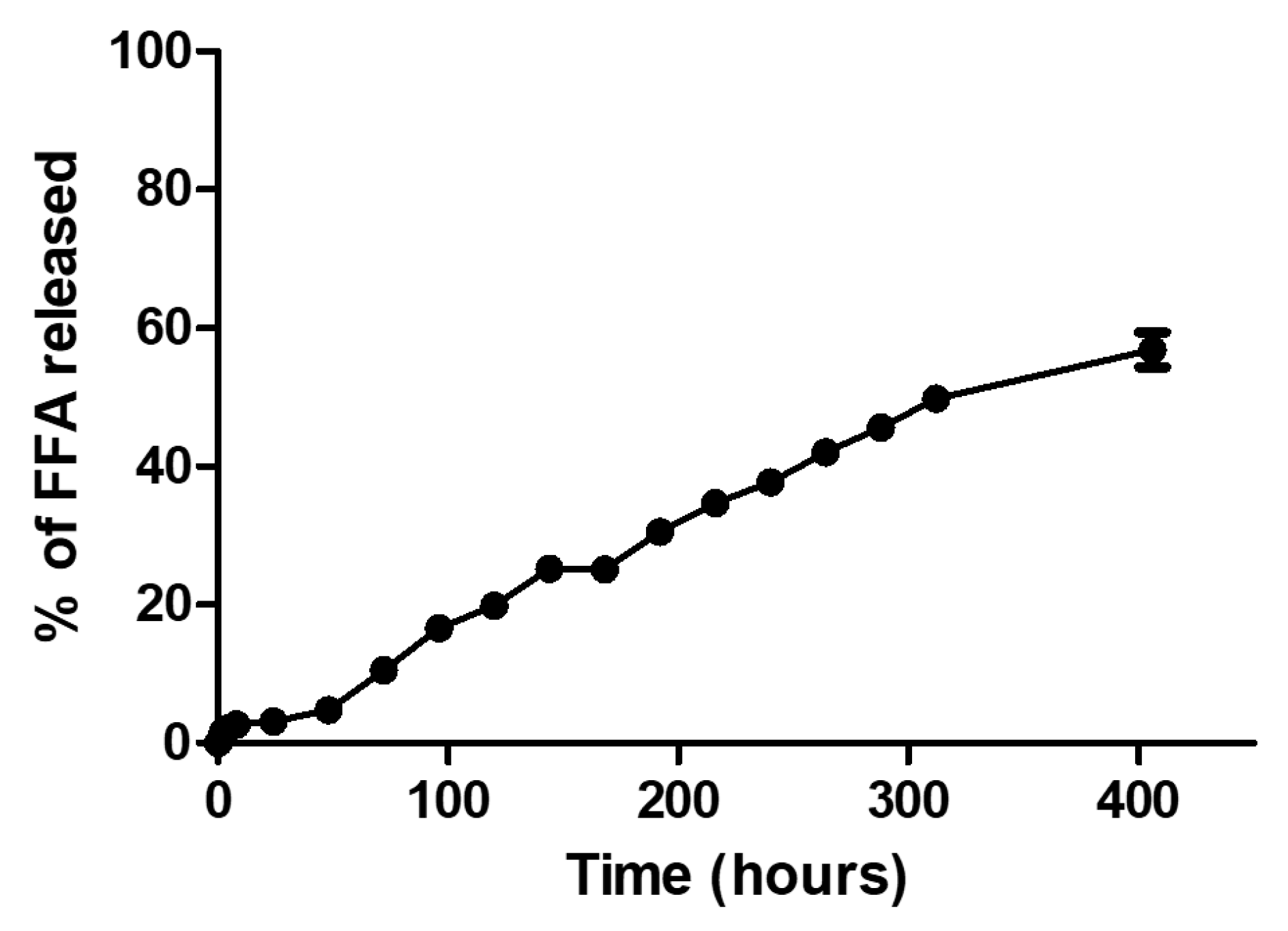
3.8. FFA Release Assay
3.9. Biocompatibilidade Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Untch, M.; Jackisch, C.; Schneeweiss, A.; Conrad, B.; Aktas, B.; Denkert, C.; Eidtmann, H.; Wiebringhaus, H.; Kümmel, S.; Hilfrich, J.; et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): A randomised, phase 3 trial. Lancet Oncol. 2016, 17, 345–356. [Google Scholar] [CrossRef]
- Deacon, D.H.; Hogan, K.T.; Swanson, E.M.; Chianese-Bullock, K.A.; Denlinger, C.E.; Czarkowski, A.R.; Schrecengost, R.S.; Patterson, J.W.; Teague, M.W.; Slingluff, C.L., Jr. The use of gamma-irradiation and ultraviolet-irradiation in the preparation of human melanoma cells for use in autologous whole-cell vaccines. BMC Cancer 2008, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Guren, M.G.; Undseth, C.; Rekstad, B.L.; Brændengen, M.; Dueland, S.; Spindler, K.L.; Glynne-Jones, R.; Tveit, K.M. Reirradiation of locally recurrent rectal cancer: A systematic review. Radiother. Oncol. 2014, 113, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Barnett, G.C.; West, C.M.; Dunning, A.M.; Elliott, R.M.; Coles, C.E.; Pharoah, P.D.; Burnet, N.G. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat. Rev. Cancer. 2009, 9, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, M.; Spray, D.C. Closure of gap junction channels by arylaminobenzoates. Mol. Pharmacol. 2003, 63, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Dringen, R. Gap junction hemichannel-mediated release of glutathione from cultured rat astrocytes. Neurosci. Lett. 2007, 415, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.; Uwimpuhwe, H.; Czibere, A.; Sarkar, D.; Libermann, T.A.; Fisher, P.B.; Zerbini, L.F. NSAIDs induce apoptosis in nonproliferating ovarian cancer cells and inhibit tumor growth in vivo. IUBMB Life 2012, 64, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.C.; Hayes, R.B.; Schoen, R.E.; Gunter, M.J.; Huang, W.-Y. Non-Steroidal anti-inflammatory drug use and colorectal polyps in the prostate, lung, colorectal, and ovarian cancer screening trial. Am. J. Gastroenterol. 2010, 105, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Padera, T.P.; Stoll, B.R.; Tooredman, J.B.; Capen, D.; di Tomaso, E.; Jain, R.K. Pathology: Cancer cells compress intratumour vessels. Nature 2004, 427, 695. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Rosenholm, J. Mesoporous Silica: An Alternative Diffusion Controlled Drug Delivery System. 2008. Available online: http://www.oulu.fi/spareparts/ebook_topics_multifunctional/abstracts/andersson.pdf (accessed on 23 November 2017).
- Björk, E.M.; Söderlind, F.; Odén, M. Tuning the shape of mesoporous silica particles by alterations in parameter space: From rods to platelets. Langmuir 2013, 29, 13551–13561. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.M. Controlling the Pore Size and Morphology of Mesoporous Silica. 2010. Available online: https://www.diva-portal.org/smash/get/diva2:439011/FULLTEXT01.pdf (accessed on 23 November 2017).
- Ellahioui, Y.; Prashar, S.; Gómez-Ruiz, S. A short overview on the biomedical applications of silica, alumina and calcium phosphate-based nanostructured materials. Curr. Med. Chem. 2016, 23, 4450–4467. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Viviane, A.; Gomide, S.; Goes, M. An in situ synthesis of mesoporous SBA-16 / hydroxyapatite for ciprofloxacin release : In vitro stability and cytocompatibility studies. J. Mater. Sci. Mater. Med. 2014, 25, 2527–2540. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Nourbakhsh, M.S.; Khodaverdi, E.; Hadizadeh, F.; Omid Malayeri, S. Enhanced Loading and Release of Non-Steroidal Anti-Inflammatory Drugs from Silica-Based Nanoparticle Carriers. Chem. Biol. Drug Des. 2016, 88, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Popova, M.; Szegedi, A.; Mihaly, J.; Tzankov, B.; Lambov, N.; Konstantinov, S.; Tzankova, V.; Pessina, F.; Valoti, M. Functionalized mesoporous silica nanoparticles for oral delivery of budesonide. J. Solid State Chem. 2014, 211, 154–161. [Google Scholar] [CrossRef]
- De Oliveira Freitas, L.B.; de Melo Corgosinho, L.; Faria, J.A.Q.A.; dos Santos, V.M.; Resende, J.M.; Leal, A.S.; Gomes, D.A.; de Sousa, E.M.B. Multifunctional mesoporous silica nanoparticles for cancer-targeted, controlled drug delivery and imaging. Microporous Mesoporous Mater. 2017, 242, 271–283. [Google Scholar] [CrossRef]
- De Oliveira Freitas, L.B.; Bravo, I.J.G.; de Almeida Macedo, W.A.; de Sousa, E.M.B. Mesoporous silica materials functionalized with folic acid: Preparation, characterization and release profile study with methotrexate. J. Sol-Gel Sci. Technol. 2016, 77, 186–204. [Google Scholar] [CrossRef]
- Khosravian, P.; Ardestani, M.S.; Khoobi, M.; Ostad, S.N.; Dorkoosh, F.A.; Javar, H.A.; Amanlou, M. Mesoporous silica nanoparticles functionalized with folic acid/methionine for active targeted delivery of docetaxel. Onco Targets Ther. 2016, 9, 7315–7330. [Google Scholar] [CrossRef] [PubMed]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.-T.; Lin, V.S.-Y. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol–gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-N.; Zhang, C.-Q.; Wang, W.; Wang, P.C.; Zhou, J.-P.; Liang, X.-J. pH-responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment. Cancer Biol. Med. 2014, 11, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, S.; Gößl, D.; Schmidt, A.; Niedermayer, S.; Argyo, C.; Endres, S.; Bein, T.; Bourquin, C. Immune response to functionalized mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kempen, P.J.; Greasley, S.; Parker, K.A.; Campbell, J.L.; Chang, H.Y.; Jones, J.R.; Sinclair, R.; Gambhir, S.S.; Jokerst, J.V. Theranostic Mesoporous Silica Nanoparticles Biodegrade after Pro-Survival Drug Delivery and Ultrasound/Magnetic Resonance Imaging of Stem Cells. Theranostics 2015, 5, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Talavera-Pech, W.A.; Esparza-Ruiz, A.; Quintana-Owen, P.; Vilchis-Nestor, A.R.; Carrera-Figueiras, C.; Ávila-Ortega, A. Effects of different amounts of APTES on physicochemical and structural properties of amino-functionalized MCM-41-MSNs. J. Sol-Gel Sci. Technol. 2016, 80, 697–708. [Google Scholar] [CrossRef]
- Ghasemnejad, M.; Ahmadi, E.; Mohamadnia, Z.; Doustgani, A.; Hashemikia, S. Functionalized silica nanoparticles as a carrier for Betamethasone Sodium Phosphate: Drug release study and statistical optimization of drug loading by response surface method. Mater. Sci. Eng. C 2015, 56, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.C.M. Quantikov Image Analyser. 1996. Available online: http://www.ipen.br/biblioteca/teses/21165.pdf (accessed on 23 November 2017).
- Meléndez-Ortiz, H.I.; Mercado-Silva, A.; García-Cerda, L.A.; Castruita, G.; Perera-Mercado, Y.A. Hydrothermal synthesis of mesoporous silica MCM-41 using commercial sodium silicate. J. Mex. Chem. Soc. 2013, 57, 73–79. [Google Scholar]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chemie Ingenieur Technik 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Kaneda, M.; Terasaki, O.; Zhao, D.Y.; Kim, J.M.; Stucky, G.; Shin, H.J.; Ryoo, R. Direct imaging of the pores and cages of three-dimensional mesoporous materials. Nature 2000, 408, 449–453. [Google Scholar] [PubMed]
- Choi, M.; Kleitz, F.; Liu, D.; Hee, Y.L.; Ahn, W.S.; Ryoo, R. Controlled polymerization in mesoporous silica toward the design of organic-inorganic composite nanoporous materials. J. Am. Chem. Soc. 2005, 127, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Pasternack, R.M.; Amy, S.R.; Chabal, Y.J. Attachment of 3-(Aminopropyl)triethoxysilane on silicon oxide surfaces: Dependence on solution temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef] [PubMed]
- Kiwilsza, A.; Milanowski, B.; Drużbicki, K.; Coy, L.E.; Grzeszkowiak, M.; Jarek, M.; Mielcarek, J.; Lulek, J.; Pajzderska, A.; Wąsicki, J. Mesoporous drug carrier systems for enhanced delivery rate of poorly water-soluble drug: Nimodipine. J. Porous Mater. 2015, 22, 817–829. [Google Scholar] [CrossRef]
- Gilpin, R.K.; Zhou, W. Infrared studies of the polymorphic states of the fenamates. J. Pharm. Biomed. Anal. 2005, 37, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Vunain, E.; Opembe, N.N.; Jalamba, K.; Mishra, A.K.; Meijboom, R. Thermal stability of amine-functionalized MCM-41 in different atmospheres. J. Therm. Anal. Calorim. 2014, 115, 1487–1496. [Google Scholar] [CrossRef]
- Kishor, R.; Ghoshal, A.K. APTES grafted ordered mesoporous silica KIT-6 for CO2 adsorption. Chem. Eng. J. 2015, 262, 882–890. [Google Scholar] [CrossRef]
- Andrade, G.F.; Soares, D.C.F.; dos Santos, R.G.; Sousa, E.M.B. Mesoporous silica SBA-16 nanoparticles: Synthesis, physicochemical characterization, release profile, and in vitro cytocompatibility studies. Microporous Mesoporous Mater. 2013, 168, 102–110. [Google Scholar] [CrossRef]
- Dell, H.D.; Jacobi, H.; Kamp, R.; Kolle, J. Studies on metabolism and elimination of etofenamate by dogs. Arzneimittelforschung 1981, 31, 21–26. [Google Scholar] [PubMed]
- Chaves, N.L.; Estrela-Lopis, I.; Böttner, J.; Lopes, C.A.; Guido, B.C.; de Sousa, A.R.; Báo, S.N. Exploring cellular uptake of iron oxide nanoparticles associated with rhodium citrate in breast cancer cells. Int. J. Nanomed. 2017, 12, 5511–5523. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Hodali, H.A. Use of mesoporous silicate nanoparticles as drug carrier for mefenamic acid. IOP Conf. Ser. Mater. Sci. Eng. 2015, 92, 1–6. [Google Scholar] [CrossRef]
- Tao, Z.; Toms, B.B.; Goodisman, J.; Asefa, T. Mesoporosity and functional group dependent endocytosis and cytotoxicity of silica nanomaterials. Chem. Res. Toxicol. 2009, 22, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Halamoda-Kenzaoui, B.; Ceridono, M.; Colpo, P.; Valsesia, A.; Urbán, P.; Ojea-Jiménez, I.; Gioria, S.; Gilliland, D.; Rossi, F.; Kinsner-Ovaskainen, A. Dispersion behaviour of silica nanoparticles in biological media and its influence on cellular uptake. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pisani, C.; Rascol, E.; Dorandeu, C.; Gaillard, J.C.; Charnay, C.; Guari, Y.; Chopineau, J.; Armengaud, J.; Devoisselle, J.M.; Prat, O. The species origin of the serum in the culture medium influences the in vitro toxicity of silica nanoparticles to HepG2 cells. PLoS ONE 2017, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Choi, E.J.; Webster, T.J.; Kim, S.H.; Khang, D. Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int. J. Nanomed. 2014, 10, 97–113. [Google Scholar] [CrossRef]






| Sample | Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cc/g) |
|---|---|---|---|
| MCM-41 | 1145 | 3.2 | 0.87 |
| MCM-41/AP | 409 | 3.0 | 0.33 |
| MCM-41/AP–FFA | 272 | 3.0 | 0.24 |
| Sample | 2θd100 | d100 (nm) | a100 (nm) | h (nm) |
|---|---|---|---|---|
| MCM-41 | 2.46 | 3.58 | 4.13 | 0.93 |
| MCM-41/AP | 2.64 | 3.34 | 3.85 | 0.85 |
| MCM-41/AP-FFA | 2.64 | 3.34 | 3.85 | 0.85 |
| Sample | 25–150 °C | 150–700 °C |
|---|---|---|
| MCM-41 | 5.7% | 1.63% |
| MCM-41/AP | 15.6% | 10.0% |
| MCM-41/AP-FFA | 17.1% | 21.5% |
| Sample | Carbon (C) | Hydrogen (H) | Nitrogen (N) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % | mmol·g−1 | ∆ C | % | mmol·g−1 | ∆ H | % | mmol·g−1 | ∆ N | |
| MCM-41 | 0.1 | 0.2 | |||||||
| MCM-41/AP | 4.7 | 3.9 | 4.6 | 2.7 | 27.0 | 2.5 | 1.5 | 1.0 | 1.6 |
| MCM-41/AP-AFF | 12.5 | 10.4 | 7.9 | 2.5 | 25.0 | 1.6 | 1.1 | 0.1 | |
| Sample | Zeta Potential | Hydrodynamic | Polydispersion Index (PDI) |
|---|---|---|---|
| MCM-41 | −23.2 meV | 130 nm | 0.4 |
| MCM-41/AP | +39 meV | 242 nm | 0.2 |
| MCM-41/AP-AFF | +36 meV | 210 nm | 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara, G.G.; Cipreste, M.F.; Andrade, G.F.; Silva, W.M.d.; Sousa, E.M.B.d. Response of Fibroblasts MRC-5 to Flufenamic Acid-Grafted MCM-41 Nanoparticles. Bioengineering 2018, 5, 4. https://doi.org/10.3390/bioengineering5010004
Lara GG, Cipreste MF, Andrade GF, Silva WMd, Sousa EMBd. Response of Fibroblasts MRC-5 to Flufenamic Acid-Grafted MCM-41 Nanoparticles. Bioengineering. 2018; 5(1):4. https://doi.org/10.3390/bioengineering5010004
Chicago/Turabian StyleLara, Giovanna Gomes, Marcelo Fernandes Cipreste, Gracielle Ferreira Andrade, Wellington Marcos da Silva, and Edésia Martins Barros de Sousa. 2018. "Response of Fibroblasts MRC-5 to Flufenamic Acid-Grafted MCM-41 Nanoparticles" Bioengineering 5, no. 1: 4. https://doi.org/10.3390/bioengineering5010004
APA StyleLara, G. G., Cipreste, M. F., Andrade, G. F., Silva, W. M. d., & Sousa, E. M. B. d. (2018). Response of Fibroblasts MRC-5 to Flufenamic Acid-Grafted MCM-41 Nanoparticles. Bioengineering, 5(1), 4. https://doi.org/10.3390/bioengineering5010004