1. Introduction
Chronic wounds are one of the major clinical challenges in the world. It is estimated that the annual cost on the treatment of chronic wounds is over
$25 billion [
1]. Due to aging population and increased number of people with diabetes and obesity worldwide, the financial burden of treating chronic wounds is increasing. Current treatment options of chronic wounds are less effective. Thus, there is an urgent need for the development of novel wound healing therapeutics. Herbal medicines have been used over 5000 years and their enduring popularity may be attributed to the perception that they cause minimal unwanted side effects. Discovering novel therapeutics from herbal medicines remains a promising approach for drug development [
2].
Cardiotonic steroids (CTSs) are a class of natural products that are commonly found in many herbal plants. CTSs have been used for the treatment of cardiac diseases since Hippocrates and the mechanisms of action are associated with Na/K-ATPase [
3]. Recently, researchers found that a signal cascade involving EGFR and PLC could be initiated by CTSs via their binding to Na/K-ATPase, resulting in an increase in collagen expression [
4,
5,
6]. These findings suggest that CTSs may be potential wound healing therapeutics by stimulating collagen synthesis, which is one of the most important mechanisms in wound healing [
7]. A previous study revealed that digoxin significantly promoted wound healing by stimulating collagen synthesis and could be a potential wound healing therapeutics [
4]. After application of digoxin in olive oil on the excisional wounds in rats, wound closure was significantly accelerated and a greater amount of dermal collagen was detected in the wound area as compared with the vehicle control group. This study also demonstrated that olive oil was necessary for the wound healing effect of digoxin.
CTSs can be divided into two distinct groups: cardiac glycosides (e.g., digoxin, ouabain, and digitoxin) and cardiac aglycones (e.g., digoxingenin, ouabagenin, and digitoxigenin). Digitoxigenin is a cardiac aglycone and it has more favorable physicochemical properties for drug delivery than digoxin, such as smaller molecular weight and greater lipophilicity. Particularly, our pervious in vitro Na/K-ATPase assay study showed that digitoxigenin inhibited the Na/K-ATPase with an inhibitory minimum concentration (IC
50) of 0.22 μM, which was lower than the IC
50 for digoxin of 1.76 μM (data not shown). This implies that digitoxigenin showed more affinity with Na/K-ATPase and may be a better candidate for wound healing than digoxin. Despite the stimulated collagen synthesis and wound healing effect of digoxin [
4], it is not known if digitoxigenin exhibits the similar wound healing effect as cardiac glycosides, like digoxin. Therefore, it is necessary to prove that digitoxigenin can stimulate collagen synthesis and accelerate the wound closure.
Olive oil was found as a carrier necessary for the delivery of digoxin due to its lipophilicity [
4]. As digitoxigenin is relatively hydrophobic with a measured olive oil/water partition coefficient of 17.75 [
8], olive oil is expected to be an important carrier for the wound healing effect of digitoxigenin. However, the drug in olive oil solution is inconvenient for topical application. Therefore, we developed a topical formulation capable of loading both digitoxigenin and olive oil. Alginate has been used in wound healing due to its unique gelling property in contact with polyvalent cations, such as calcium [
9]. Calcium alginate-based wound dressings are commercially available. After application of the calcium alginate-based wound dressings on wounds, sodium from the wound’s exudates and calcium from alginate dressings will undergo ion exchange, forming a soluble sodium alginate gel. Alginate was thus selected to formulate the digitoxigenin formulation and olive oil was a vehicle to dissolve digitoxigenin.
The objective of this study was to evaluate the possibility of digitoxigenin and its topical formulation for wound healing effect. We first examined if digitoxigenin could stimulate collagen synthesis in human dermal fibroblasts and exert the wound healing activity in a rat excisional wound model. Next, the microspheres containing both digitoxigenin and olive oil were prepared using alginate and the microsphere-dispersed gel formulation was investigated for in vitro drug release and in vivo wound healing effect.
2. Materials and Methods
2.1. Materials
Digitoxigenin and olive oil (highly refined, low acidity) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Alginic acid sodium salt (low viscosity) was from MP Biomedicals (Solon, OH, USA). Tween 85 (Acros Organics, Morris, NJ, USA) and Span 85 (Sigma-Aldrich, St. Louis, MO, USA) were used in the preparation of digitoxigenin formulations. Other reagents used were isooctane (Acros Organics, Morris, NJ, USA) and calcium chloride dehydrate (Fisher Scientific, Springfield, NJ, USA). Phosphate buffered saline (PBS) of pH 7.4 was prepared by dissolving PBS tablets (MP Biomedicals, Solon, OH, USA) in deionized water, and sodium azide (Acros Organics, Morris, NJ, USA) was added at a concentration of 0.02% (w/v) as a bacteriostat. High performance liquid chromatography (HPLC) grade methanol, water, and formic acid were purchased from Fisher Scientific (Pittsburgh, PA, USA).
2.2. Animals
Sprague-Dawley rats (male and female, 240–260 g) were purchased from Hilltop Lab Animals (Scottdale, PA, USA). All of the experiments were conducted under the approval of the Institutional Animal Care and Use Committee at Marshall University.
2.3. Stimulation of Collagen Synthesis in Human Dermal Fibroblast Cells
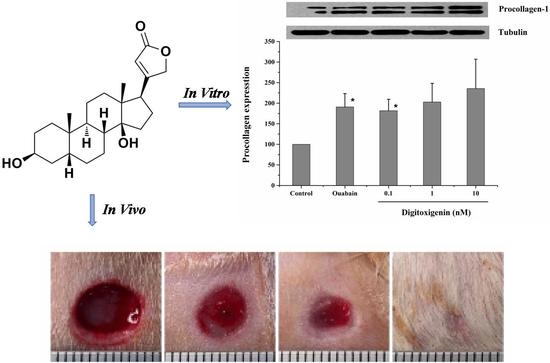
In vitro stimulation of collagen synthesis activity of digitoxigenin was investigated in human dermal fibroblast (HDF) cells (product No. CCD-1072Sk, ATCC No: CRL-2088) supplied from ATCC (Manassas, VA, USA). HDF cells were cultured with ISCOVE’S modified Dulbecco’s medium (Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS, Atlanta Biologicals, Flowery Branch, GA, USA). After the HDF cells grew to confluence, the medium was removed and the cells were incubated with the medium containing 1% FBS for 12 h to avoid any possible interference due to growth factors present in the serum. The cells were then treated with the medium (negative control, containing 10% FBS but no digitoxigenin or ouabain), ouabain (positive control, 1 nM dissolved in the medium containing 10% FBS), or digitoxigenin (0.1, 1 or 10 nM dissolved in the medium containing 10% FBS). After treatment for 24 h, cell lysates were prepared by washing the cells twice with ice-cold PBS, followed by incubating them on ice for 20 min with a lysis buffer containing 50 mM HEPES sodium, 50 mM NaCl, 10% glycerol, 1% Nonidet P-40, 0.25% Na-Dexycholate, and protease and phosphatase inhibitors. Cell lysates were collected and used immediately or stored at −80 °C.
Western blot analysis was performed as described previously [
6]. Briefly, cell lysates were dissolved in loading buffer and proteins (10 μg/lane) were separated by SDS-PAGE using 8% Tris·HCl ready precast gels (Bio-Rad, Hercules, CA, USA). After separation, proteins were transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% non-fat dry milk (Bio-Rad, Hercules, CA, USA) in Tris-buffered saline that was supplemented with 0.05% Tween 20 (TBS-T) at room temperature for 1 h and then incubated with primary antibody (Goat-Anti-Type 1 Collagen-UNLB, Southern Biotech, Birmingham, UK) in blocking buffer at 4 °C overnight. After being washed in TBS-T, the membranes were incubated with secondary antibody in the blocking buffer for 2 h at room temperature. After being washed in TBS, the membranes were developed with ECL (Amersham Biosciences, Piscataway, NJ, USA). For loading controls, tubulin was probed. The images captured on X-ray film were scanned and quantified by using Image J software.
2.4. Preparation of Digitoxigenin Microspheres
Digitoxigenin-loaded alginate microspheres were prepared by an emulsification technique with modifications from the literatures [
10,
11]. Briefly, 250 μL 150 μg/mL digitoxigenin in olive oil were dispersed in 5 g 2%
w/
w alginate solution using a homogenizer (Polytron PT 2100; Kinematica, Lucern, Switzerland) at 11,000 rpm for 1 min. After the addition of 7.5 g isooctane containing 0.254 g Span 85 into the alginate solution followed by homogenization for 1 min, 1 g aqueous solution containing 0.136 g Tween 85 was added and homogenized for 1 min. Two grams of 25% calcium chloride were added and the mixture was homogenized for 10 min to allow for the calcium to react with the sodium alginate. Isooctane was decanted after centrifugation at 4400 rpm for 5 min and microspheres were collected. The microspheres were washed with 2 mL deionized water three times and dried at room temperature for the in vitro and in vivo studies.
2.5. Digitoxigenin Content Assay
The amounts of digitoxigenin loaded to the microspheres were determined by extracting digitoxigenin from the microspheres and subsequent assaying by LC-MS/MS. Microspheres of 0.03 g were dispersed in 1 mL PBS and then sonicated in an ultrasonic bath for 1 h. The mixture was centrifuged at 10,000 rpm for 5 min and the supernatant was collected. The residue was subjected to two more extractions and the supernatants were collected. Digitoxigenin was extracted from the supernatant with methanol and analyzed by LC-MS/MS. The amount of digitoxigenin in the microspheres was determined.
2.6. In Vitro Release Study
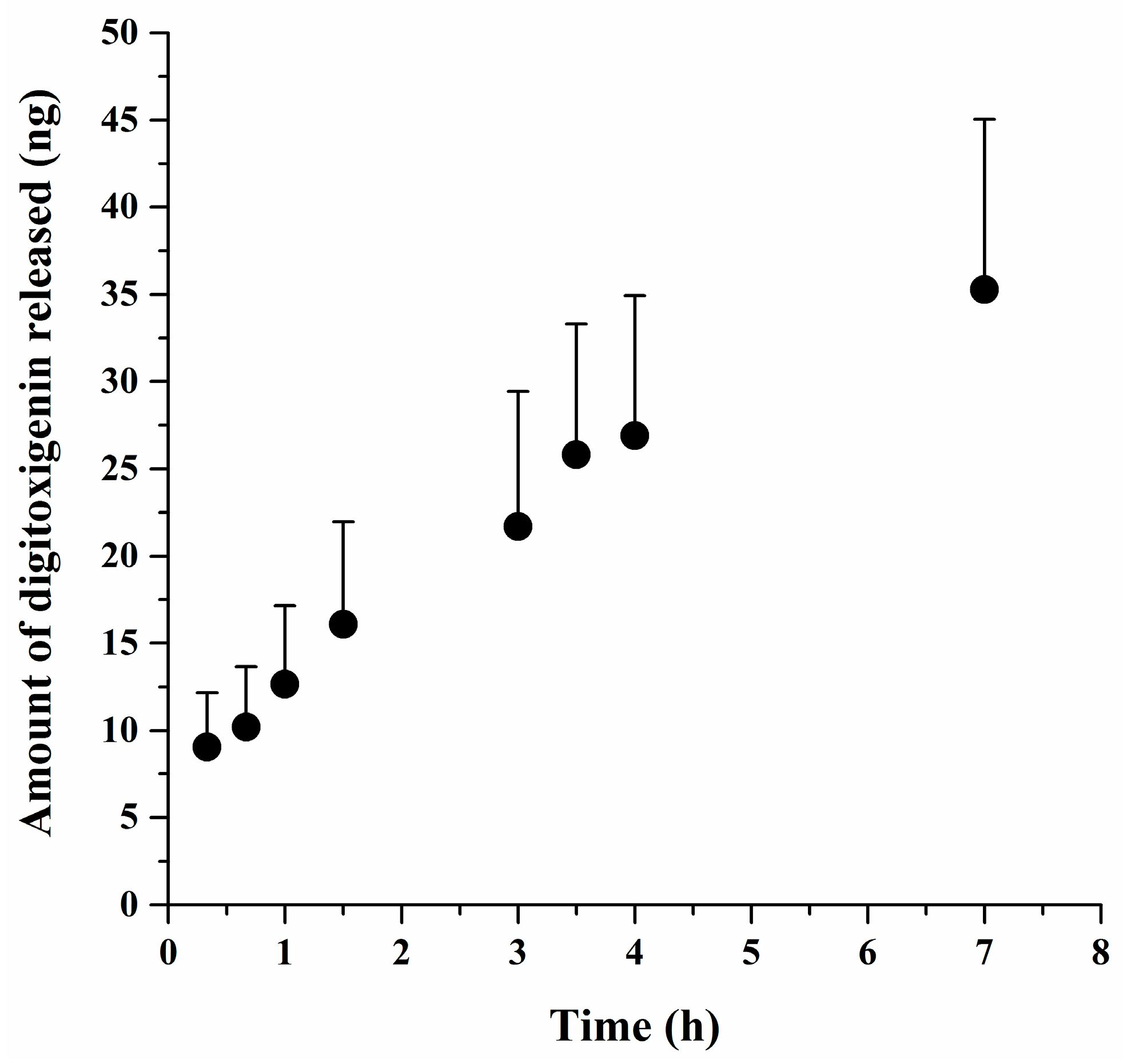
Digitoxigenin released from the microsphere was determined using Franz diffusion cells with an effective diffusion area of 0.64 cm2 and a MF-Millipore membrane (0.45 μm) that was sandwiched between the donor and receptor compartments. The digitoxigenin-loaded microspheres of 0.05 g were dispersed in 600 μL 2% (w/w) alginate solution containing 0.9% (w/v) NaCl and then loaded to the donor chamber. The receptor solution was 5 mL PBS containing 0.02% (w/v) sodium azide and was kept at 37 °C ± 1 °C. One milliliter of the receptor solution was collected at 20 and 40 min as well as at 1, 1.5, 3, 3.5, 4, and 7 h, with replacement of a fresh receptor solution to maintain the volume of the receptor solution constant. The samples were assayed for digitoxigenin by LC–MS/MS. The amounts of digitoxigenin released from the microspheres were determined and plotted versus the time.
2.7. Wound Healing Study
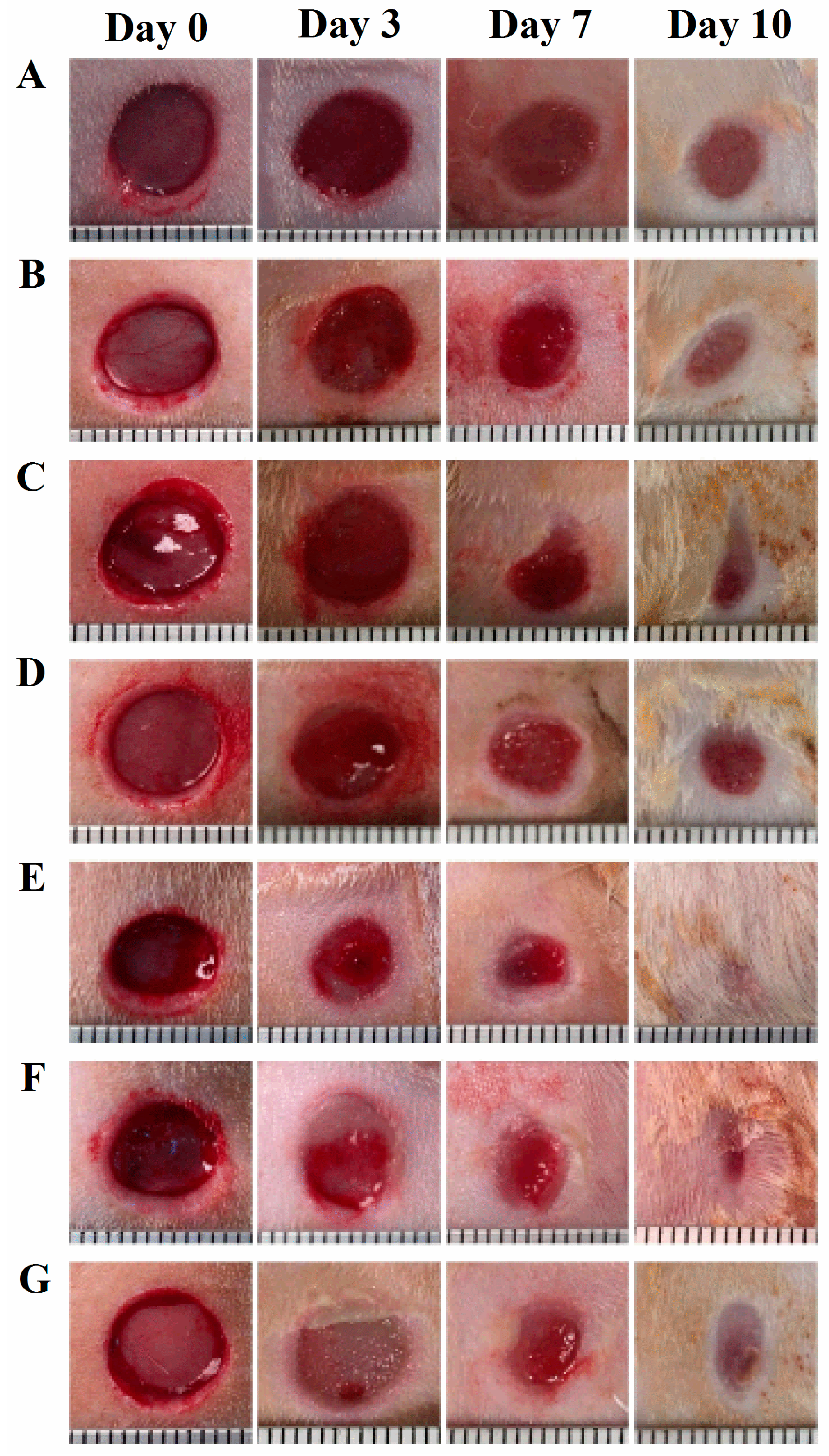
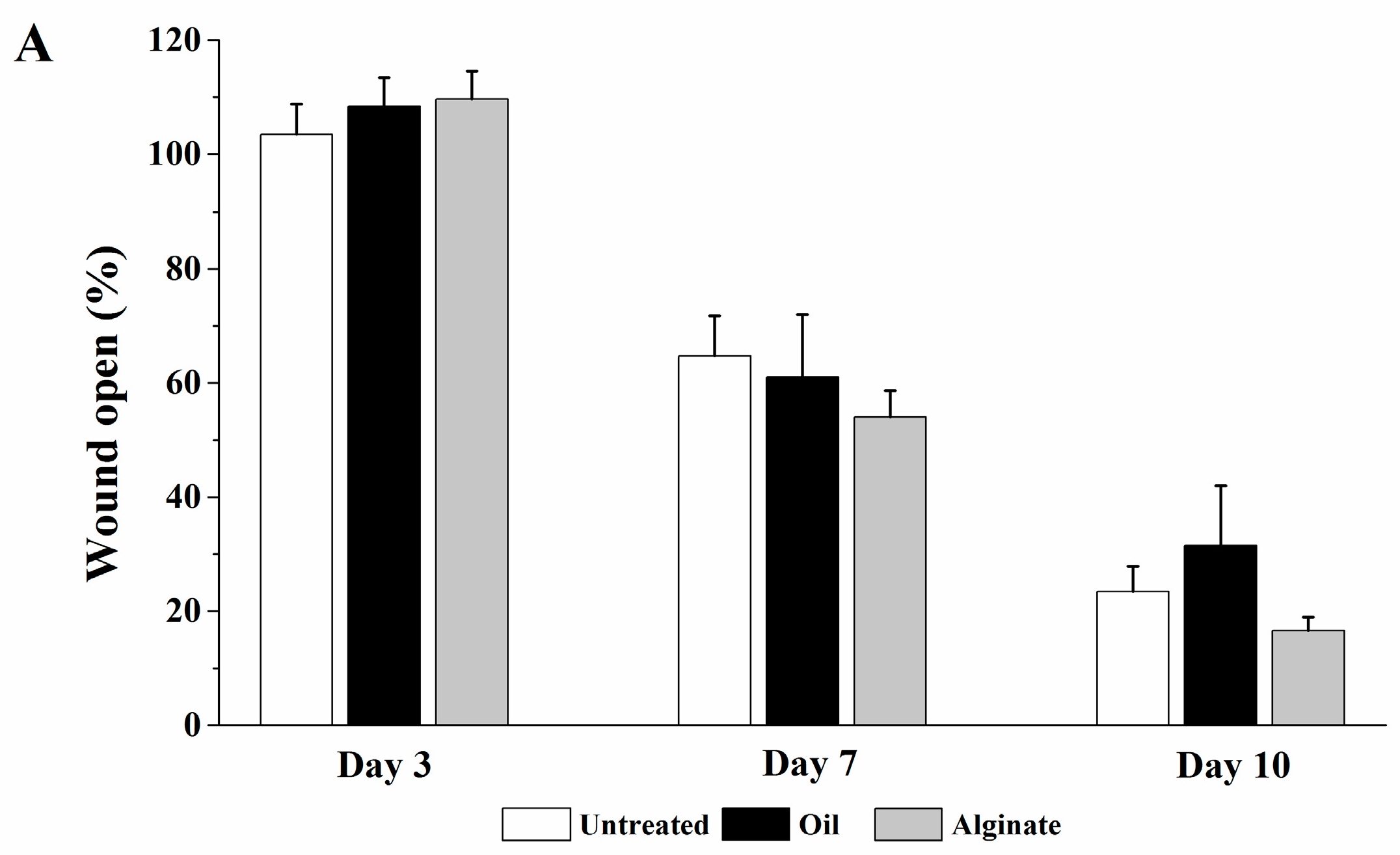
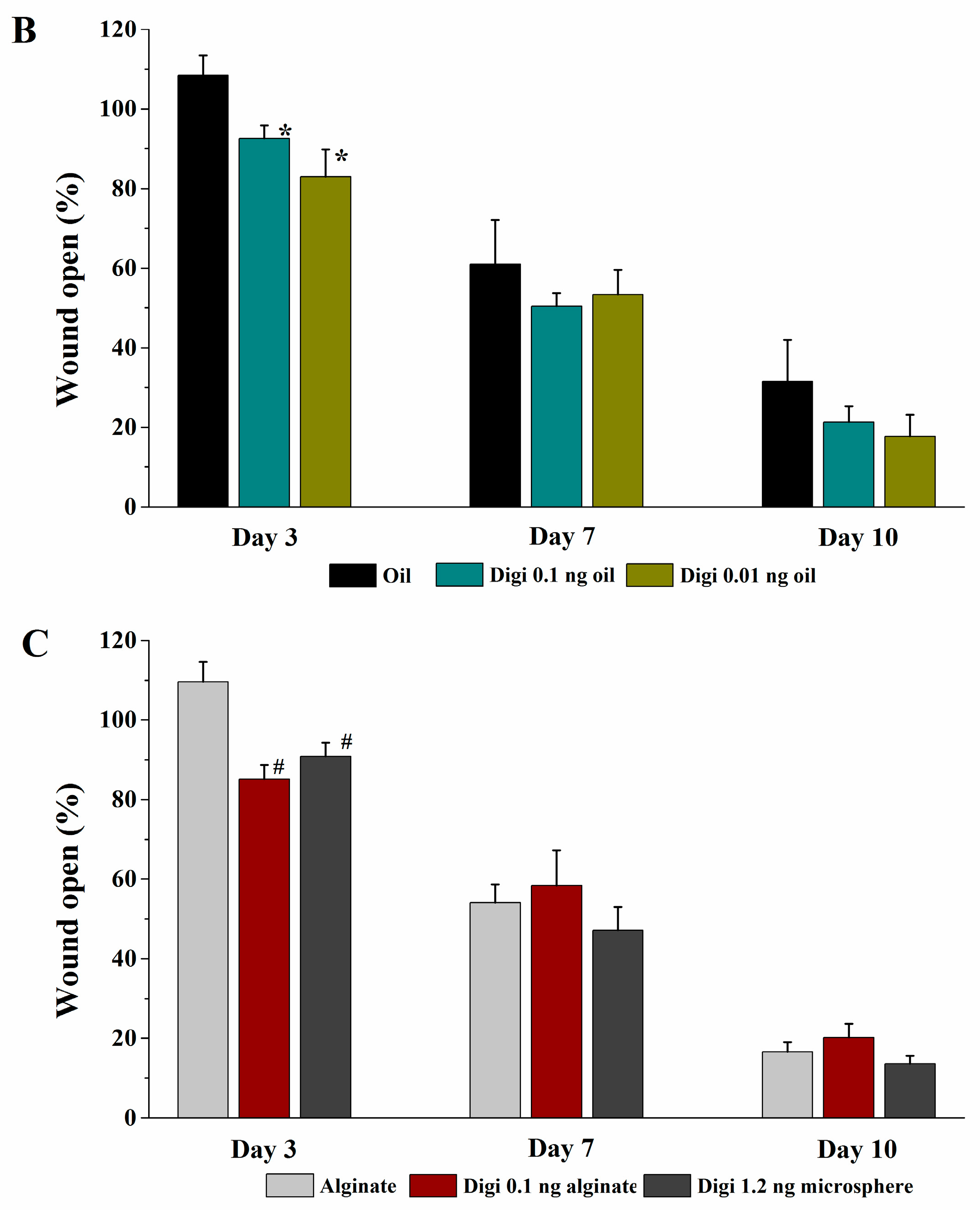
The rats were randomly divided into seven groups and seven rats for each group. The rats were anesthetized with intraperitoneal injection of ketamine/xylazine (30–50 mg/kg & 5–10 mg/kg, respectively). Prior to wound creation, buprenorphine was administered intraperitoneally (0.01–0.05 mg/kg). The hair in an area of about 500 mm2 in the dorsal skin of the rat was shaved and the hair-removed area was wiped with alcohol swab (Becton-Dickinson, Franklin Lakes, NJ, USA). Full-thickness skin biopsy was performed with an 8.0-mm sterile punch biopsy (Acu-punch, Acuderm, Fort Lauderdale, FL, USA). Two symmetric lesions with at least 15 mm apart were made in each rat and received two different treatments immediately after wound creation; one wound was treated with a vehicle and the other with a digitoxigenin-loaded formulation. The seven treatment groups were (1) untreated (no vehicle or digitoxigenin-loaded formulation applied), (2) olive oil, (3) 2% w/w alginate solution containing 0.9% sodium chloride, (4) digitoxigenin in olive oil (1 ng/mL), (5) digitoxigenin in olive oil (0.1 ng/mL), (6) digitoxigenin in 2% alginate solution (1 ng/mL), and (7) digitoxigenin-loaded microspheres in 2% alginate solution containing 0.9% sodium chloride. The digitoxigenin microsphere gels were freshly prepared by dispersing 10 mg microspheres in 1 mL 2% w/w alginate solution containing 0.9% NaCl.
The vehicle or the digitoxigenin-loaded formulations of 100 µL was first applied to a Curad clear water proof bandage (Medline Industries, Mundelein, IL, USA), which was cut into two rectangle pieces (1.25 × 0.75 cm). The wounds were immediately covered with the Curad bandages after wound creation and an adhesive porous tape (Kendall, Covidien, Mansfield, MA, USA) was applied to hold the Curad bandage in place. To ensure the bandages were not removed by rats and wound contraction was inhibited in the wound healing process, additional self-adhesive wrap (Coban, 3M Health Care, St. Paul, MN, USA) and adhesive tapes were applied. At 3, 7 and 10 days following the wounding, the bandages were gently removed and digital photographs were taken immediately. A new bandage was applied to the wounds, but no vehicle or digitoxigenin was added. Photographs were analyzed with Image J and the percentage of wound open was calculated as the remaining wound area with respect to the initial wound area.
2.8. LC–MS/MS Assay
The LC–MS/MS assay method was developed and modified from the literature [
8]. The LC–MS/MS system consisted of an Agilent 1290 series UHPLC system and an Agilent 6490 Triple Quadrupole (QqQ) mass spectrometer equipped with Agilent Jet-Stream ESI interface. Chromatographic separation was carried out on an Agilent Poroshell 120 EC-C18 column (2.1 × 50 mm I.D., 2.7 μm) with an Agilent UHPLC guard cartridge (2.1 × 5 mm I.D., 2.7 μm) at room temperature with a flow rate of 200 μL/min. The mobile phase consisted of water (0.1% formic acid)–methanol (0.1% formic acid) (40:60,
v/
v), and the total run time was 4.0 min. ESI source was operated in the positive ionization mode. The autosampler temperature was set at 10 °C. The general source settings were as follows: gas temperature 220 °C; gas flow 12 L/min; nebulizer 22 psi; sheath gas temperature 250 °C; sheath gas flow 12 L/min; capillary voltage 3500 V; and, nozzle voltage 1500 V. The fragmentor voltage was 380 V for all mass transitions, and both scanning quadrupoles (Q1 and Q3) were set to unit resolution. Selective reaction monitoring (SRM) for digitoxigenin was conducted by monitoring the precursor-product ion transition of
m/
z 375.5 → 339 and the collision energy was 30 eV. LC–MS/MS data acquisition and processing were performed using Agilent’s MassHunter Quantitative and Qualitative Analysis software (version B.06.00). The calibration curves were linear over the concentration range of 0.1–20 ng/mL.
2.9. Statistical Analysis
All of the data are presented as mean ± standard error (SE). Statistical differences of means were determined using one-way analysis of variance (ANOVA) and are considered to be significant at a level of p < 0.05.