Safety Aspects of Bio-Based Nanomaterials
Abstract
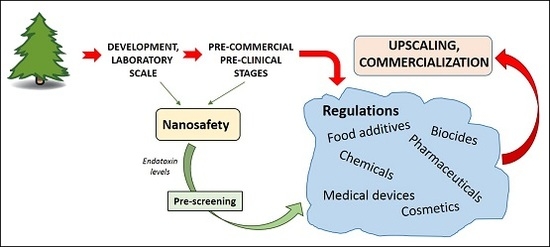
:1. Introduction
2. Toxicity of Bio-Based Nanomaterials
3. Regulatory Requirements and Testing Strategies
4. Environmental Issues
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ronzon, T.; Santini, F.; M’Barek, R. The Bioeconomy in the European Union in Numbers. Facts and Figures on Biomass, Turnover and Employment; European Commission, Joint Research Centre, Institute for Prospective Technological Studies: Seville, Spain, 2015; p. 4. [Google Scholar]
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics—A review. J. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Bell, J.; Paula, L.; Dodd, T.; Németh, S.; Nanou, C.; Mega, V.; Campos, P. EU ambition to build the world’s leading bioeconomy-Uncertain times demand innovative and sustainable solutions. New Biotechnol. 2018, 40, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Shatkin, J.A.; Ong, K.J.; Ede, J.D.; Wegner, T.H.; Goergen, M. Toward cellulose nanomaterial commercialization: Knowledge gap analysis for safety data sheets according to the globally harmonized system. Tappi J. 2016, 15, 425–437. [Google Scholar]
- Broeren, M. Production of Bio-Ethylene. IEA-ETSAP and IRENA© Technology-Policy Brief 2013, 13. Available online: https://iea-etsap.org/E-TechDS/PDF/I13IR_Bioethy_MB_Jan2013_final_GSOK (accessed on 28 October 2017).
- Endes, C.; Camarero-Espinosa, S.; Mueller, S.; Foster, E.J.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J. A critical review of the current knowledge regarding the biological impact of nanocellulose. J. Nanobiotechnol. 2016, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.J.; Shatkin, J.A.; Nelson, K.; Ede, J.D.; Retsina, T. Establishing the safety of novel bio-based cellulose nanomaterials for commercialization. NanoImpact 2017, 6, 19–29. [Google Scholar] [CrossRef]
- Donaldson, K.; Poland, C.A. Nanotoxicology: New insights into nanotubes. Nat. Nanotechnol. 2009, 4, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Schwirn, K.; Tietjen, L.; Beer, I. Why are nanomaterials different and how can they be appropriately regulated under REACH? Environ. Sci. Eur. 2014, 26, 4. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Park, M.V.; Gosens, I.; De Jong, W.H.; Cassee, F.R. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part. Fibre Toxicol. 2014, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, J.; Kyriakides, T.R. Nanomaterials, inflammation and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Roman, M. Toxicity of cellulose nanocrystals: A review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Lindberg, H.K.; Catalán, J.; Aimonen, K.J.; Wolff, H.; Wedin, I.; Nuopponen, M.; Savolainen, K.M.; Norppa, H. Evaluation of the genotoxic potential of different types of nanofibrillated celluloses. TechConnect Briefs 2017, 229–232. [Google Scholar]
- Shvedova, A.A.; Kisin, E.R.; Yanamala, N.; Farcas, M.T.; Menas, A.L.; Williams, A.; Fournier, P.M.; Reynolds, J.S.; Gutkin, D.W.; Star, A.; et al. Gender differences in murine pulmonary responses elicited by cellulose nanocrystals. Part. Fibre Toxicol. 2016, 13, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalán, J.; Rydman, E.; Aimonen, K.; Hannukainen, K.S.; Suhonen, S.; Vanhala, E.; Moreno, C.; Meyer, V.; Perez, D.D.; Sneck, A.; et al. Genotoxic and inflammatory effects of nanofibrillated cellulose in murine lungs. Mutagenesis 2017, 32, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, J.; Pohler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K.; et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Lynch, I.; Weiss, C.; Valsami-Jones, E. A strategy for grouping of nanomaterials based on key physico-chemical descriptors as a basis for safer-by-design nanomaterials. Nano Today 2014, 9, 266–270. [Google Scholar] [CrossRef]
- Tomić, S.; Kokol, V.; Mihajlović, D.; Mirčić, A.; Čolić, M. Native cellulose nanofibrills induce immune tolerance in vitro by acting on dendritic cells. Sci. Rep. 2016, 6, 31618. [Google Scholar] [CrossRef] [PubMed]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical and oxidative methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.; Miller, M.R.; Clift, M.J.D.; Elder, A.; Mills, N.L.; Møller, P.; Schins, R.P.F.; Vogel, U.; Kreyling, W.G.; Jensen, K.A.; et al. Nanomaterials versus ambient ultrafine particles: An opportunity to exchange toxicology knowledge. Environ. Health Perspect. 2017, 125, 106002. [Google Scholar] [CrossRef] [PubMed]
- Shatkin, J.A.; Kim, B. Cellulose nanomaterials: Life cycle risk assessment, and environmental health and safety roadmap. Environ. Sci. Nano 2015, 2, 477. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; Seehra, M.S.; Fix, N.R.; Leonard, S.S. Lung biodurability and free radical production of cellulose nanomaterials. Inhal. Toxicol. 2014, 26, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Schinwald, A.; Murphy, F.; Cho, W.S.; Duffin, R.; Tran, L.; Poland, C. The biologically effective dose in inhalation nanotoxicology. Acc. Chem. Res. 2013, 46, 723–732. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.; Berry, R.; Goguen, R. Commercialization of Cellulose Nanocrystal (NCC™) Production: A Business Case Focusing on the Importance of Proactive EHS Management. In Nanotechnology Environmental Health and Safety, 2nd ed.; Hull, M., Bowman, D., Eds.; Elsevier Inc.: Oxford, UK, 2014; Chapter 10; pp. 225–246. ISBN 978-1-4557-3188-6. [Google Scholar]
- Stockmann-Juvala, H.; Taxell, P.; Santonen, T. Formulating Occupational Exposure Limits Values (OELs) (Inhalation & Dermal); Finnish Institute of Occupational Health: Helsinki, Finland, 2014; Available online: http://scaffold.eu-vri.eu/filehandler.ashx?file=13717 (accessed on 28 October 2017).
- Van Norman, G.A. Drugs and devices. Comparison of European and U.S. approval processes. JACC Basic Transl. Sci. 2016, 1, 399–412. [Google Scholar] [CrossRef]
- Scholz, N. Medicinal products in the European Union. Eur. Parliam. Res. Serv. 2015. Available online: http://www.europarl.europa.eu/RegData/etudes/IDAN/2015/554174/EPRS_IDA(2015)554174_EN (accessed on 28 October 2017). [CrossRef]
- Regulation, Evaluation, Authorization and Restriction of Chemicals (REACH), 2006. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1907&from=EN (accessed on 28 October 2017).
- United Nations (UN). Globally Harmonized System of Classification and Labelling of Chemicals (GHS). 2011. Available online: https://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev04/English/ST-SG-AC10–30-Rev4e (accessed on 28 October 2017).
- Worth, A.; Barroso, J.; Bremer, S.; Burton, J.; Casati, S.; Coecke, S.; Corvi, R.; Desprez, B.; Dumont, C.; Gouliarmou, V.; et al. Alternative Methods for Regulatory Toxicology—A State-Of-The-Art Review; JRC Science and Policy Reports, European Union: Luxemburg, 2014; ISBN 978-92-79-39651-9. [Google Scholar]
- Stone, V.; Pozzi-Mucelli, S.; Tran, L.; Aschberger, K.; Sabella, S.; Vogel, U.; Poland, C.; Balharry, D.; Fernandes, T.; Gottardo, S.; et al. ITS-NANO—Prioritizing nanosafety research to develop a stakeholder driven intelligent testing strategy. Part. Fibre Toxicol. 2014, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, S.; Oomen, A.G.; Bleeker, E.A.; Vandebriel, R.J.; Micheletti, C.; Cabellos, J.; Janer, G.; Fuentes, N.; Vázquez-Campos, S.; Borges, T.; et al. Towards a nanospecific approach for risk assessment. Regul. Toxicol. Pharmacol. 2016, 80, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Dusinska, M.; Boland, S.; Saunders, M.; Juillerat-Jeanneret, L.; Tran, L.; Pojana, G.; Marcomini, A.; Volkovova, K.; Tulinska, J.; Knudsen, L.E.; et al. Towards an alternative testing strategy for nanomaterials used in nanomedicine: Lessons from NanoTEST. Nanotoxicology 2015, 9, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Dusinska, M.; Tulinska, J.; El Yamani, N.; Kuricova, M.; Liskova, A.; Rollerova, E.; Rundén-Pran, E.; Smolkova, B. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing? Food Chem. Toxicol. 2017, 109, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Use of International Standard ISO 10993-1, Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process. 2016. Available online: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm348890 (accessed on 28 October 2017).
- Farcal, L.; Torres Andón, F.; Di Cristo, L.; Rotoli, B.M.; Bussolati, O.; Bergamaschi, E.; Mech, A.; Hartmann, N.B.; Rasmussen, K.; Riego-Sintes, J.; et al. Comprehensive in vitro toxicity testing of a panel of representative oxide nanomaterials: First steps towards an intelligent testing strategy. PLoS ONE 2015, 10, e0127174. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Hamilton, R.F.; Bonner, J.C.; Crandall, E.D.; Elder, A.; Fazlollahi, F.; Girtsman, T.A.; Kim, K.; Mitra, S.; Ntim, S.A.; et al. Interlaboratory evaluation of in vitro cytotoxicity and inflammatory responses to engineered nanomaterials: The NIEHS Nano GO Consortium. Environ. Health Perspect. 2013, 121, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.; Stockmann-Juvala, H.; Norppa, H. A theoretical approach for a weighted assessment of the mutagenic potential of nanomaterials. Nanotoxicology 2017, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Alenius, H.; Catalán, J.; Lindberg, H.; Norppa, H.; Palomäki, J.; Savolainen, K. Nanomaterials and human health. In Handbook of Nanosafety—Measurement, Exposure and Toxicology; Vogel, U., Savolainen, K., Wu, Q., van Tongeren, M., Brouwer, D., Berges, M., Eds.; Elsevier Inc.: Oxford, UK, 2014; Chapter 3; pp. 59–133. ISBN 978-0-12-416604-2. [Google Scholar]
- Doak, S.H.; Manshian, B.; Jenkins, G.J.; Singh, N. In vitro genotoxicity testing strategy for nanomaterials and the adaptation of current OECD guidelines. Mutat Res. 2012, 745, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Giannakou, C.; Park, M.V.; de Jong, W.H.; van Loveren, H.; Vandebriel, R.J.; Geertsma, R.E. A comparison of immunotoxic effects of nanomedicinal products with regulatory immunotoxicity testing requirements. Int. J. Nanomed. 2016, 11, 2935–2952. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). 2006—Committee for Medicinal Products for Human Use (CHMP). ICH Topic S8 Immunotoxicity Studies for Human Pharmaceuticals. London CHMP/167235/2004. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002851 (accessed on 28 October 2017).
- Donaldson, K.; Poland, C.A. Inhaled nanoparticles and lung cancer—What we can learn from conventional particle toxicology. Swiss Med. Wkly. 2012, 142, w13547. [Google Scholar] [CrossRef] [PubMed]
- Endes, C.; Schmid, O.; Kinnear, C.; Mueller, S.; Camarero-Espinosa, S.; Vanhecke, D.; Foster, E.J.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; et al. An in vitro testing strategy towards mimicking the inhalation of high aspect ratio nanoparticles. Part. Fibre Toxicol. 2014, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Nordli, H.R.; Chinga-Carrasco, G.; Rokstad, A.M.; Pukstad, B. Producing ultrapure wood cellulose nanofibrils and evaluating the cytotoxicity using human skin cells. Carbohydr. Polym. 2016, 150, 65–73. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency (ECHA). Guidance on Information Requirements and Chemical Safety Assessment. Appendix R7-1 Recommendations for Nanomaterials Applicable to Chapter R7a Endpoint Specific Guidance, Version 2.0, May 2017. Available online: https://echa.europa.eu/documents/10162/13632/appendix_r7a_nanomaterials_en.pdf/1bef8a8a-6ffa-406a-88cd-fd800ab163ae (accessed on 28 October 2017).
- Giannakou, C.; Geertsma, R.E.; de Jong, W.H.; van Loveren, H.; Vandebriel, R.J.; Park, M.V.D.Z. Immunotoxicity testing of nanomedicinal products: Possible pitfalls in endotoxin determination. Curr. Bionanotechnol. 2016, 2, 95–102. [Google Scholar] [CrossRef]
- Evans, S.J.; Clift, M.J.; Singh, N.; de Oliveira Mallia, J.; Burgum, M.; Wills, J.W.; Wilkinson, T.S.; Jenkins, G.J.; Doak, S.H. Critical review of the current and future challenges associated with advanced in vitro systems towards the study of nanoparticle (secondary) genotoxicity. Mutagenesis 2017, 32, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Horikoshi, Y. Bio-based polymers. Fujitsu Sci. Tech. J. 2005, 41, 173–180. [Google Scholar]
- Narayan, R. Biobased and biodegradable plastics: Rationale, drivers, and technology exemplars. In Degradable Polymers and Materials: Principles and Practice, 2nd ed.; Khemani, K., Scholz, C., Eds.; American Chemical Society: Washington, DC, USA, 2012; Chapter 2; pp. 13–31. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalán, J.; Norppa, H. Safety Aspects of Bio-Based Nanomaterials. Bioengineering 2017, 4, 94. https://doi.org/10.3390/bioengineering4040094
Catalán J, Norppa H. Safety Aspects of Bio-Based Nanomaterials. Bioengineering. 2017; 4(4):94. https://doi.org/10.3390/bioengineering4040094
Chicago/Turabian StyleCatalán, Julia, and Hannu Norppa. 2017. "Safety Aspects of Bio-Based Nanomaterials" Bioengineering 4, no. 4: 94. https://doi.org/10.3390/bioengineering4040094
APA StyleCatalán, J., & Norppa, H. (2017). Safety Aspects of Bio-Based Nanomaterials. Bioengineering, 4(4), 94. https://doi.org/10.3390/bioengineering4040094