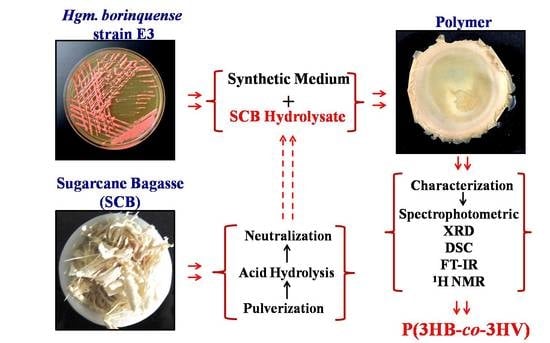
Utilization of Sugarcane Bagasse by Halogeometricum borinquense Strain E3 for Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. HalophilicArchaeal Strains and Media Used
2.2. Procurement, Processing, and Hydrolysis of Sugarcane Bagasse
2.3. Characterization of the SCB
2.4. Screening of the Halophilic Archaeal Isolates for PHA Accumulation using SCB Hydrolysate
2.5. Selection and Further Study of the Best PHA Producer Strain
2.6. The Growth Kinetics and PHA Quantification
2.7. Extraction of the PHA
2.8. Characterization of the PHA
3. Results and Discussion
3.1. Sugarcane Bagasse (SCB)
3.2. Screening for PHA using SCB Hydrolysate
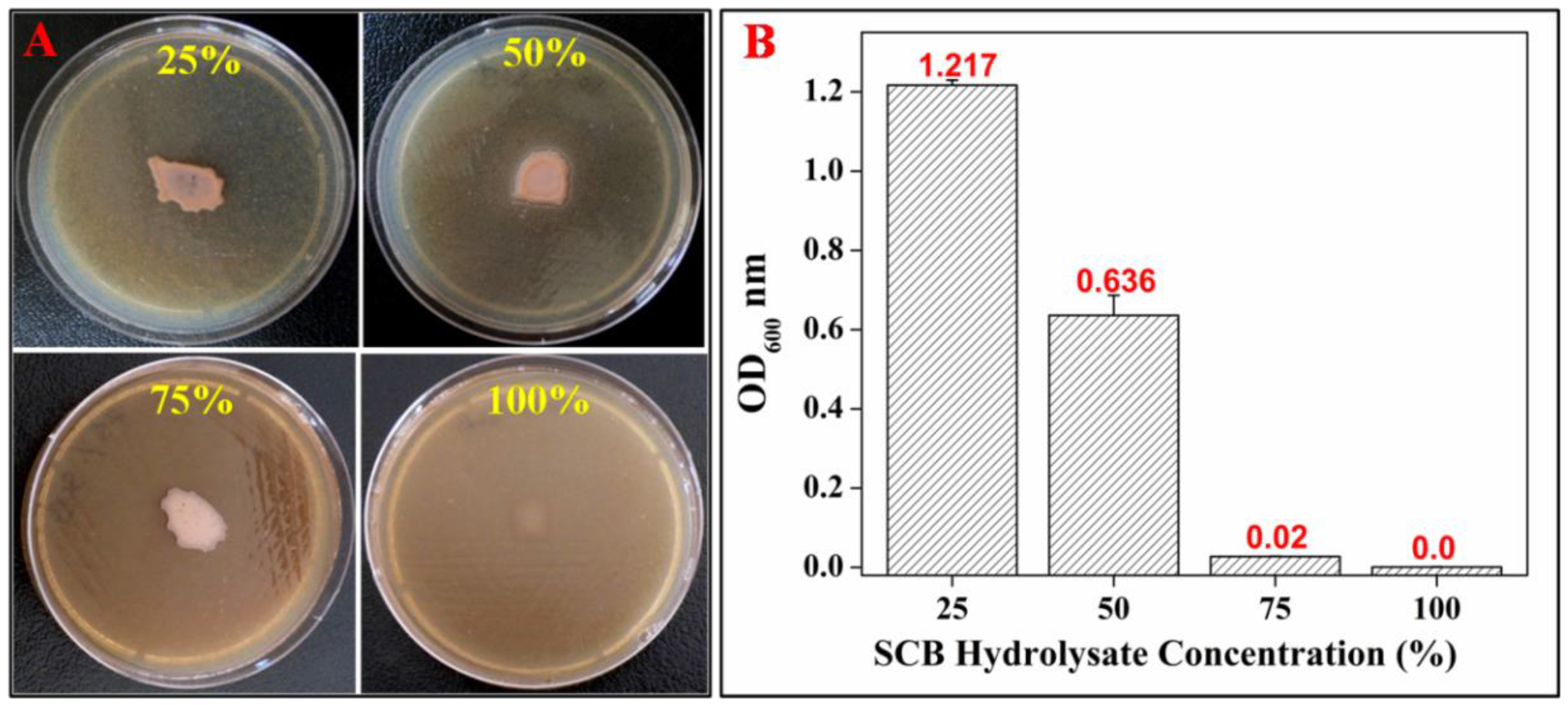
3.3. Optimization of SCB Hydrolysate Concentration
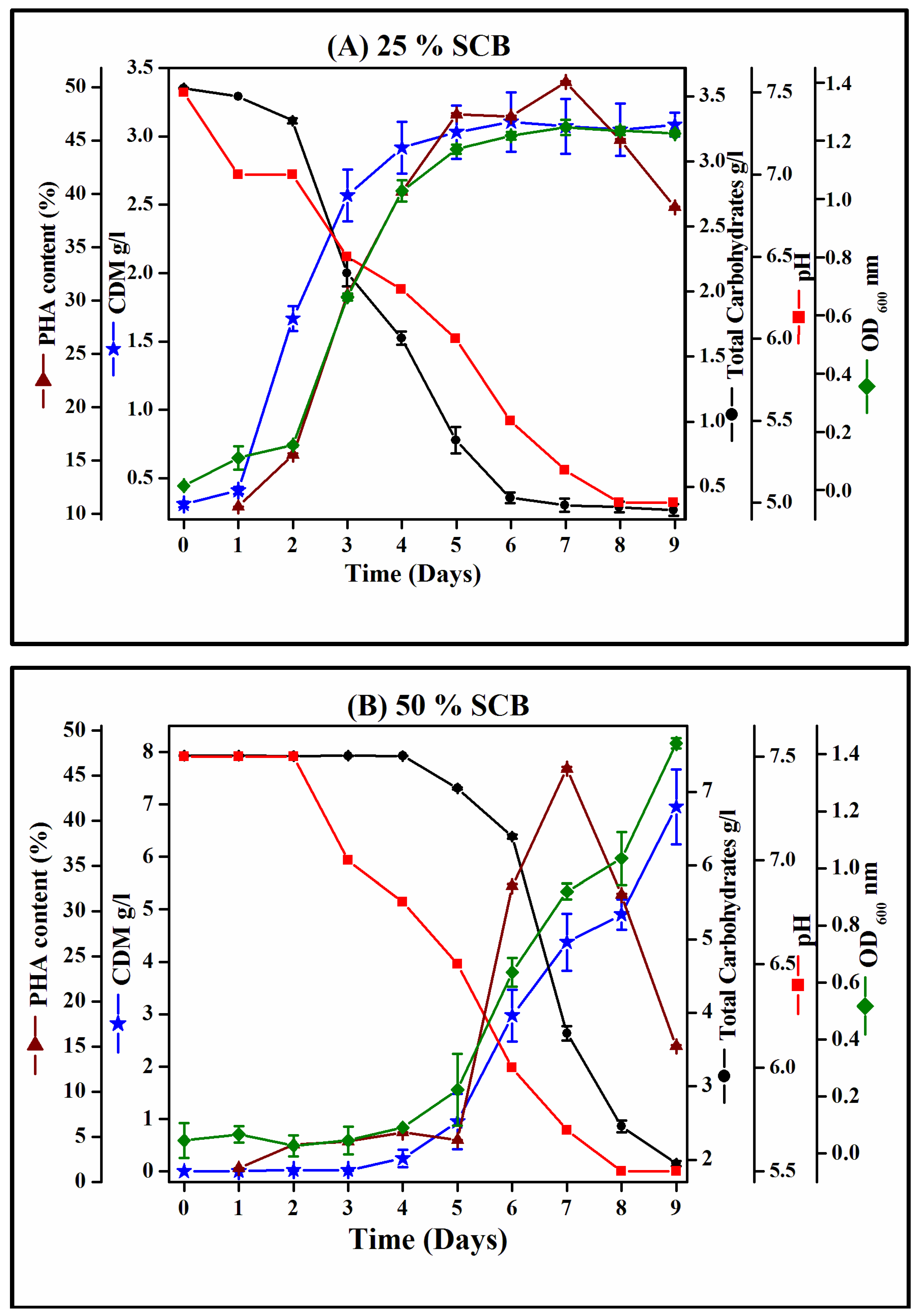
3.4. Growth Profile of Hgm. borinquense Strain E3 and Polymer Quantification Study
3.5. Bench Scale Polymer Production and Extraction by Hgm. borinquense Strain E3
3.6. Polymer Characterization
3.6.1. UV-Visible Spectrophotometric Analysis
3.6.2. XRD Analysis
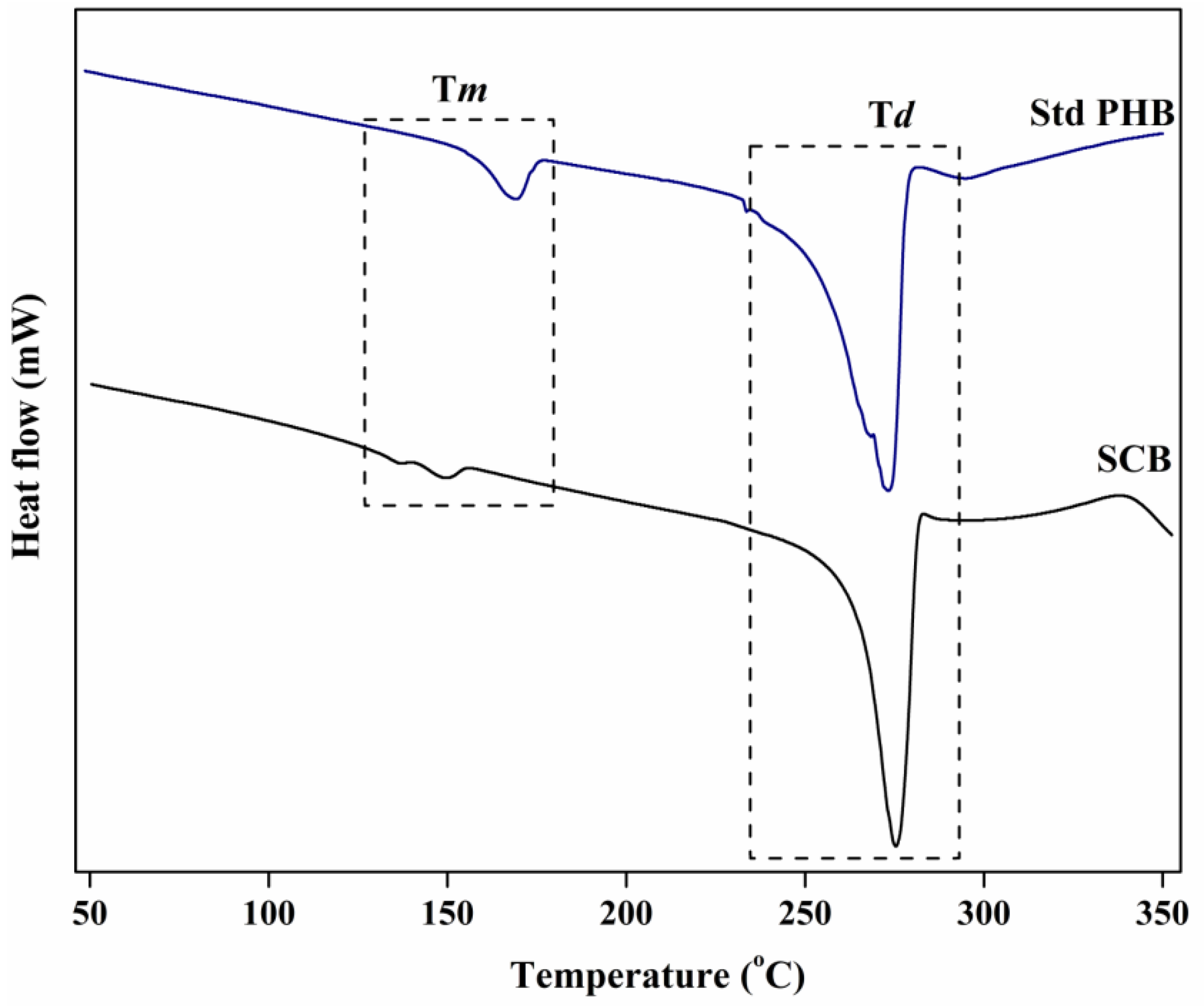
3.6.3. DSC Analysis
3.6.4. FT-IR Analysis
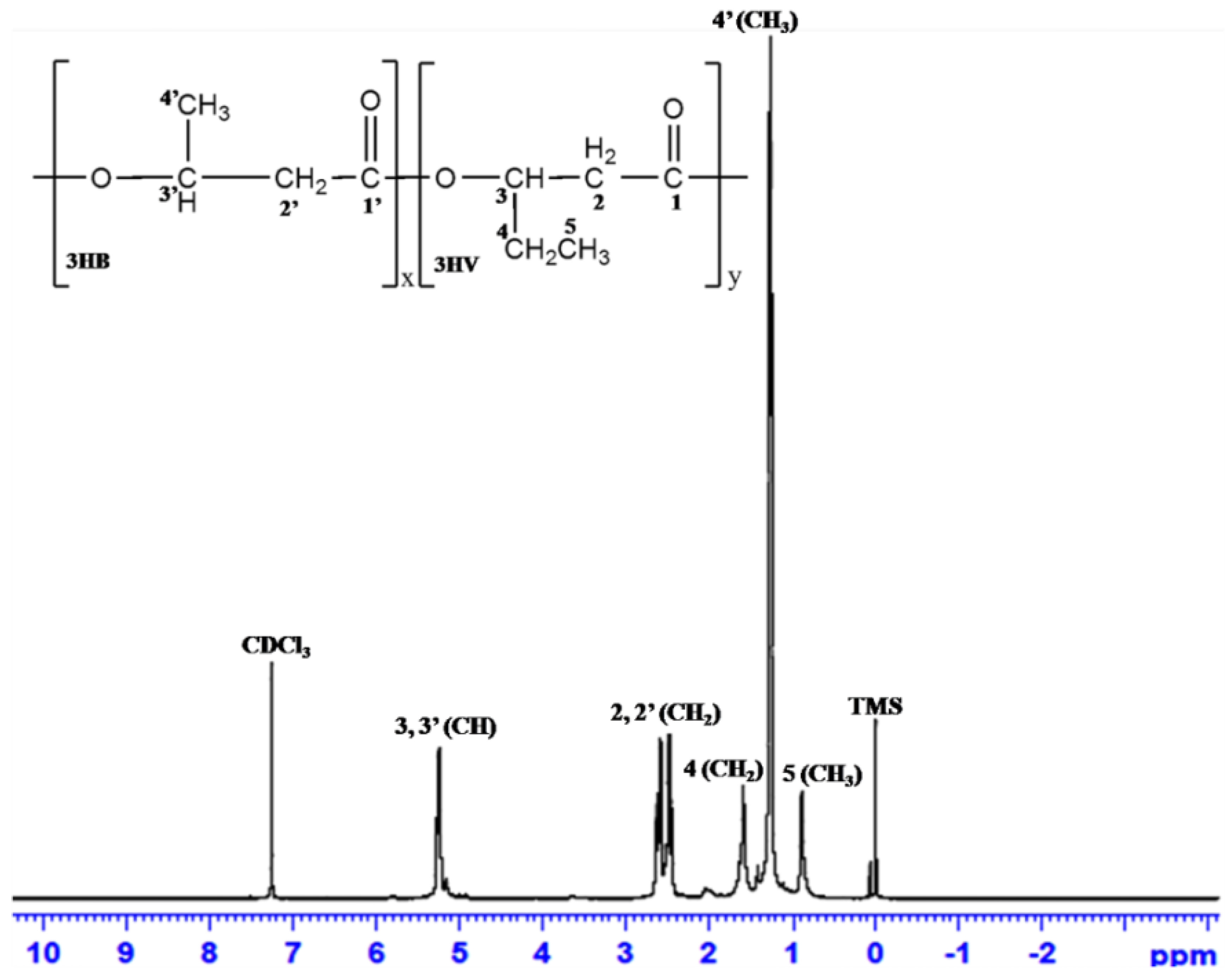
3.6.5. 1H-NMR Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APHA | American Public Health Association |
| CDM | cell dry mass |
| COD | chemical oxygen demand |
| DSC | Differential scanning calorimetry |
| EHM | Extremely Halophilic Medium |
| FT-IR | Fourier transform infrared |
| NMR | nuclear magnetic resonance |
| Har. | Haloarcula |
| Hbf. | Halobiforma |
| Hbt. | Halobacterium |
| Hcc. | Halococcus |
| Hfx. | Haloferax |
| Hgm. | Halogeometricum |
| Hpg. | Halopiger |
| Htg. | Haloterrigena |
| Nnm. | Natrinema |
| NSM | NaCl synthetic medium |
| NTYE | NaCl Tryptone Yeast extract |
| P(3HB-co-3HV) | poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| P3HB | Poly-3-hydroxybutyrate |
| PHA | Polyhydroxyalkanoates |
| SCB | sugarcane bagasse |
| TKN | Total Kjeldahl nitrogen |
| TS | total solids |
| VS | volatile solids |
| XRD | X-ray diffraction |
References
- Chen, G.Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2011, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Jendrossek, D.; Pfeiffer, D. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ. Microbiol. 2014, 16, 2357–2373. [Google Scholar] [CrossRef] [PubMed]
- Vadlja, D.; Koller, M.; Novak, M.; Braunegg, G.; Horvat, P. Footprint area analysis of binary imaged Cupriavidus necator cells to study PHB production at balanced, transient, and limited growth conditions in a cascade process. Appl. Microb. Biotechnol. 2016, 100, 10065–10080. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Valappil, S.P.; Boccaccini, A.R.; Bucke, C.; Roy, I. Polyhydroxyalkanoates in Gram-positive bacteria: Insights from the genera Bacillus and Streptomyces. Antonie Leeuwenhoek. 2007, 91, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hou, J.; Liu, H.; Cai, S.; Feng, B.; Zhou, J.; Xiang, H. Wide distribution among halophilic archaea of a novel polyhydroxyalkanoate synthase subtype with homology to bacterial type III synthases. Appl. Environ. Microbiol. 2010, 76, 7811–7819. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.; Taciro, M.K.; Ramos, M.M.; Carter, J.M.; Pradella, J.G.C.; Gomez, J.G.C. Poly-3-hydroxybutyrate (P3HB) production by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J. Ind. Microbiol. Biotechnol. 2004, 31, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, B. Sugarcane bagasse. In Biotechnology for Agro-Industrial Residues Utilisation; Springer: Dordrecht, The Netherlands, 2009; pp. 239–252. [Google Scholar]
- Pippo, W.A.; Luengo, C.A. Sugarcane energy use: Accounting of feedstock energy considering current agro-industrial trends and their feasibility. Int. J. Energy Environ. Eng. 2013, 4. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Marsalek, L.; Marova, I. Use of lignocellulosic materials for PHA production. Chem. Biochem. Eng. Q. 2015, 29, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Lavarack, B.P.; Griffin, G.J.; Rodman, D. The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenergy 2002, 23, 367–380. [Google Scholar] [CrossRef]
- Kirk, R.G.; Ginzburg, M. Ultrastructure of two species of halobacterium. J. Ultrastruct. Res. 1972, 41, 80–94. [Google Scholar] [CrossRef]
- Chen, C.W.; Don, T.M.; Yen, H.F. Enzymatic extruded starch as a carbon source for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Haloferax mediterranei. Process Biochem. 2006, 41, 2289–2296. [Google Scholar] [CrossRef]
- Han, J.; Lu, Q.; Zhou, L.; Zhou, J.; Xiang, H. Molecular characterization of the phaECHm genes, required for biosynthesis of poly(3-hydroxybutyrate) in the extremely halophilicarchaeon Haloarculamarismortui. Appl. Environ. Microbiol. 2007, 73, 6058–6065. [Google Scholar] [CrossRef] [PubMed]
- Romano, I.; Poli, A.; Finore, I.; Huertas, F.J.; Gambacorta, A.; Pelliccione, S.; Nicolaus, G.; Lama, L.; Nicolaus, B. Haloterrigena hispanica sp. nov., an extremely halophilicarchaeon from Fuente de Piedra, Southern Spain. Int. J. Syst. Evol. Microbiol. 2007, 57, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Salgaonkar, B.B.; Mani, K.; Bragança, J.M. Accumulation of polyhydroxyalkanoates by halophilic archaea isolated from traditional solar salterns of India. Extremophiles 2013, 17, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Legat, A.; Gruber, C.; Zangger, K.; Wanner, G.; Stan-Lotter, H. Identification of polyhydroxyalkanoates in Halococcus and other haloarchaeal species. Appl. Microbiol. Biotechnol. 2010, 87, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Danis, O.; Ogan, A.; Tatlican, P.; Attar, A.; Cakmakci, E.; Mertoglu, B.; Birbir, M. Preparation of poly(3-hydroxybutyrate-co-hydroxyvalerate) films from halophilic archaea and their potential use in drug delivery. Extremophiles 2015, 19, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Hezayen, F.F.; Rehm, B.H.A.; Eberhardt, R.; Steinbuchel, A. Polymer production by two newly isolated extremely halophilic archaea: Application of a novel corrosion-resistant bioreactor. Appl. Microbiol. Biotechnol. 2000, 54, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Hezayen, F.F.; Gutierrez, M.C.; Steinbuchel, A.; Tindall, B.J.; Rehm, B.H.A. Halopiger aswanensis sp. nov., a polymerproducing and extremely halophilicarchaeon isolated from hypersaline soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, H.; Van-Thuoc, D.; Martín, J.; Hatti-Kaul, R.; Quillaguamán, J. A process for the production of ectoine and poly(3-hydroxybutyrate) by Halomonas boliviensis. Appl. Microbiol. Biotechnol. 2009, 84, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Quillaguaman, J.; Hashim, S.; Bento, F.; Mattiasson, B.; Hatti-Kaul, R. Poly(β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1 using starch hydrolysate as substrate. J. Appl. Microbiol. 2005, 99, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Duan, K.J.; Huang, S.Y.; Chen, C.W. Production of polyhydroxyalkanoates from inexpensive extruded rice bran and starch by Haloferax mediterranei. J. Ind. Microbiol. Biotechnol. 2006, 33, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Potential of various archae-and eubacterial strains as industrial polyhydroxyalkanoate producers from whey. Macromol. Biosci. 2007, 7, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Saha, J.; Haldar, S.; Bhowmic, A.; Mukhopadhyay, U.K.; Mukherjee, J. Production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei using rice-based ethanol stillage with simultaneous recovery and re-use of medium salts. Extremophiles 2014, 18, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Pramanik, A.; Maji, S.K.; Haldar, S.; Mukhopadhyay, U.K.; Mukherjee, J. Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Express 2012, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Mitra, A.; Arumugam, M.; Bhattacharyya, A.; Sadhukhan, S.; Ray, A.; Mukherjee, J. Utilization of vinasse for the production of polyhydroxybutyrate by Haloarcula marismortui. Folia Microbiol. 2012, 57, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Taran, M. Utilization of petrochemical wastewater for the production of poly(3-hydroxybutyrate) by Haloarcula sp. IRU1. J. Hazard. Mater. 2011, 188, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Mani, K.; Salgaonkar, B.B.; Bragança, J.M. Culturable halophilic archaea at the initial and final stages of salt production in a natural solar saltern of Goa, India. Aquat. Biosyst. 2012, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Salgaonkar, B.B.; Bragança, J.M. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Halogeometricumborinquense strain E3. Int. J. Biol. Macromol. 2015, 78, 339–346. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association; American Water Works Association. Standard Methods for the Examination of Water and Wastewater: Selected Analytical Methods Approved and Cited by the United States Environmental Protection Agency, 20th ed.; American Public Health Association: Washington, DC, USA, 1981. [Google Scholar]
- Raposo, F.; De la Rubia, M.A.; Borja, R.; Alaiz, M. Assessment of a modified and optimised method for determining chemical oxygen demand of solid substrates and solutions with high suspended solid content. Talanta 2008, 76, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Labconco, C. A Guide to Kjeldahl Nitrogen Determination Methods and Apparatus; Labconco Corporation: Houston, TX, USA, 1998. [Google Scholar]
- Salgaonkar, B.B.; Mani, K.; Braganca, J.M. Characterization of polyhydroxyalkanoates accumulated by a moderately halophilic salt pan isolate Bacillus megaterium strain H16. J. Appl. Microbiol. 2013, 114, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Law, J.H.; Slepecky, R.A. Assay of poly-β-hydroxybutyric acid. J. Bacteriol. 1961, 82, 33–36. [Google Scholar] [PubMed]
- Sánchez, R.J.; Schripsema, J.; da Silva, L.F.; Taciro, M.K.; Pradella, J.G.; Gomez, J.G.C. Medium-chain-length polyhydroxyalkanoic acids (PHA mcl) produced by Pseudomonas putida IPT 046 from renewable sources. Eur. Polym. J. 2003, 39, 1385–1394. [Google Scholar] [CrossRef]
- Follonier, S.; Panke, S.; Zinn, M. A reduction in growth rate of Pseudomonas putida KT2442 counteracts productivity advances in medium-chain-length polyhydroxyalkanoate production from gluconate. Microb. Cell Factories 2011, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Castilho, L.R.; Mitchell, D.A.; Freire, D.M. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour. Technol. 2009, 100, 5996–6009. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Pinto, C.E.; Arizaga, G.G.C.; Wypych, F.; Ramos, L.P.; Satyanarayana, K.G. Studies of the effect of molding pressure and incorporation of sugarcane bagasse fibers on the structure and properties of poly (hydroxy butyrate). Compos. Part A Appl. Sci. Manuf. 2009, 40, 573–582. [Google Scholar] [CrossRef]
- Vidhate, S.; Innocentini-Mei, L.; D’Souza, N.A. Mechanical and electrical multifunctional poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-multiwall carbon nanotube nanocomposites. Polym. Eng. Sci. 2012, 52, 1367–1374. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Araújo, E.S.; Guedes, S.M.L. Gamma irradiation effects on poly(hydroxybutyrate). Polym. Degrad. Stab. 2006, 91, 2157–2162. [Google Scholar] [CrossRef]
- Buzarovska, A.; Grozdanov, A.; Avella, M.; Gentile, G.; Errico, M. Poly(hydroxybutyrate-co-hydroxyvalerate)/titanium dioxide nanocomposites: A degradation study. J. Appl. Polym. Sci. 2009, 114, 3118–3124. [Google Scholar] [CrossRef]
- Sudesh, K. Polyhydroxyalkanoates from Palm Oil: Biodegradable Plastics; Springer Science & Business Media: New York, NY, USA, 2012; Volume 76. [Google Scholar]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal production of polyhydroxyalkanoate (PHA) co-and terpolyesters from biodiesel industry-derived by-products. Archaea 2013, 2013, 129268. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Biosynthesis of high quality polyhydroxyalkanoate co-and terpolyesters for potential medical application by the archaeon Haloferax mediterranei. Macromol. Symp. 2007, 253, 33–39. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Catzin, J.; Vargas, I.; Herrera-Kao, W.; Moguel, F.; Ramirez, E.; Lizama-Uc, G. Biosynthesis and characterization of polyhydroxyalkanoates produced by an extreme halophilic bacterium, Halomonas nitroreducens, isolated from hypersaline ponds. J. Appl. Microbiol. 2014, 117, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Van-Thuoc, D.; Huu-Phong, T.; Thi-Binh, N.; Thi-Tho, N.; Minh-Lam, D.; Quillaguaman, J. Polyester production by halophilic and halotolerant bacterial strains obtained from mangrove soil samples located in Northern Vietnam. Microbiol. Open 2012, 1, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.O.; Kanekar, P.P.; Nilegaonkar, S.S.; Sarnaik, S.S.; Jog, J.P. Production and characterization of a biodegradable poly(hydroxybutyrate-co-hydroxyvalerate) (PHB-co-PHV) copolymer by moderately haloalkalitolerant Halomonas campisalis MCM B-1027 isolated from Lonar Lake, India. Bioresour. Technol. 2010, 101, 9765–9771. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.; Mishra, S.K.; Shethia, B.; Pancha, I.; Jain, D.; Mishra, S. Isolation of promising bacterial strains from soil and marine environment for polyhydroxyalkanoates (PHAs) production utilizing Jatropha biodiesel byproduct. Int. J. Biol. Macromol. 2010, 47, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Stahl, H. Microbial utilization and biopolyester synthesis of bagasse hydrolysates. Bioresour. Technol. 2008, 99, 8042–8048. [Google Scholar] [CrossRef] [PubMed]


| Ingredients (g/L) | Maintenance Media | Production Medium | ||
|---|---|---|---|---|
| NTYE | NT | EHM | NSM | |
| NaCl | 250.0 | 250.0 | 250.0 | 200.0 |
| MgSO4·7H2O | 20.0 | 20.0 | 20.0 | - |
| MgCl2·6H2O | - | - | - | 13.0 |
| KCl | 5.0 | 2.0 | 2.0 | 4.0 |
| Tryptone | 5.0 | - | - | - |
| Yeast Extract | 3.0 | 10.0 | 10.0 | 1.0 |
| Tri-Sodium citrate | - | 3.0 | - | - |
| CaCl2·2H2O | - | - | 0.36 | 1.0 |
| NaBr | 0.23 | - | ||
| NaHCO3 | - | - | 0.06 | 0.2 |
| NH4Cl | - | - | - | 0.2 |
| KH2PO4 | - | - | - | 0.5 |
| Peptone | - | - | 5.0 | - |
| FeCl3·6H2O | - | - | Trace | 0.005 |
| HalophilicArchaeal Strain | Production Medium | Lag (h) | CDM (g/L) | PHA (g/L) | PHA Content (%) | µmax (1/h) | qp a (mg/g/h) | YP/S b | Vol. Productivity c (g/L/h) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Hgm. borinquense strain E3 | NSM25% SCB | 48 | 3.17 ± 0.19 | 1.6 ± 0.09 | 50.4 ± 0.1 | 0.017 | 3.0 | 0.448 | 0.0095 | Present study |
| NSM50% SCB | 96 | 4.15 ± 0.7 | 1.9 ± 0.3 | 45.7 ± 0.19 | 0.023 | 2.7 | 0.253 | 0.0113 | ||
| NGSM2% Glucose | - | 5.78 ± 0.4 | 4.25 ± 0.045 | 73.5 ± 0.045 | ND | 4.3 | 0.212 | 0.0252 | [30] * | |
| Har. marismortui MTCC 1596 | NDM10% Raw vinasse | 96 ± 12 | 12.0 ± 0.20 | 2.8 ± 0.2 | 23 ± 1.0 | 0.086 | 1.21 | 2.17 | 0.015 | [27] |
| NDM100% treated vinasse | 144 ± 12 | 15.0 ± 0.35 | 4.5 ± 0.2 | 30 ± 0.3 | 0.128 | 1.39 | 0.77 | 0.020 |
| PHA from Various Substrates | Haloarchaeal Isolate | DSC Characterization (°C) | Reference | ||
|---|---|---|---|---|---|
| Tm1 | Tm2 | Td | |||
| SCB | Hgm. borinquense strain E3 | 136.59 | 149.4 | 275.4 | Present study |
| Glucose | 138.15 | 154.74 | 231.08 | [30] | |
| Cornstarch | Hfx. mediterraneic ATCC 33500 | 129.1 | 144.0 | NR | [13] |
| Whey | Hfx. mediterranei DSM 1411 | 150.8 | 158.9 | 241 | [46] |
| PHA from Various Substrates | Haloarchaeal Isolate | Relative Chemical Structure | Reference | ||||
|---|---|---|---|---|---|---|---|
| CH3 (3HB) | CH2 (3HV/3HB) | CH (3HV/3HB) | CH3 (3HV) | CH2 (3HV) | |||
| Chemical Shifts of Each Peak (ppm) | |||||||
| SCB | Hgm. borinquense strain E3 | 1.26–1.27 | 2.44–2.63 | 5.22–5.27 | 0.889 | 1.618–1.635 | Present study |
| Glucose | 1.26–1.28 | 2.44–2.63 | 5.26 | 0.85–0.91 | 1.6 | [30] | |
| Cornstarch | Hfx. mediterranei ATCC 33500 | 1.2 | 2.5 | 5.2 | 0.9 | 1.6 | [13] |
| Vinasse | Hfx. mediterranei DSM 1411 | 1.26–1.28 | 2.43-2.645 | 5.22-5.28 | 0.86–0.95 | 1.586 | [26] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgaonkar, B.B.; Bragança, J.M. Utilization of Sugarcane Bagasse by Halogeometricum borinquense Strain E3 for Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering 2017, 4, 50. https://doi.org/10.3390/bioengineering4020050
Salgaonkar BB, Bragança JM. Utilization of Sugarcane Bagasse by Halogeometricum borinquense Strain E3 for Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering. 2017; 4(2):50. https://doi.org/10.3390/bioengineering4020050
Chicago/Turabian StyleSalgaonkar, Bhakti B., and Judith M. Bragança. 2017. "Utilization of Sugarcane Bagasse by Halogeometricum borinquense Strain E3 for Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)" Bioengineering 4, no. 2: 50. https://doi.org/10.3390/bioengineering4020050
APA StyleSalgaonkar, B. B., & Bragança, J. M. (2017). Utilization of Sugarcane Bagasse by Halogeometricum borinquense Strain E3 for Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering, 4(2), 50. https://doi.org/10.3390/bioengineering4020050