Biophysical Tools to Study Cellular Mechanotransduction
Abstract
:1. Mechanotransduction
1.1. Mechanosensitivity
1.2. Mechanotransduction Related Diseases
2. Tools to Study Cellular Mechanotransduction
2.1. Traction Force Microscopy (TFM)
2.2. Magnetic Twisting/Pulling Cytometry (MTC/MPC)
2.3. Optical Tweezers
2.4. Atomic Force Microscopy (AFM)
2.5. Shear Flow Microfluidic Devices
3. Adhesion Receptors that Transduce Force
3.1. Integrin—The Primary Cell-Matrix Adhesion Molecule
3.1.1. Focal Adhesions
3.1.2. Integrin’s Ability to Transduce Force
3.2. Cadherin—The Primary Calcium Dependent Cell-Cell Adhesion Molecule
3.3. Global Mechanotransduction
3.4. Kinetic Regulation of Mechanotransduction
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S. Mechanotransduction—A field pulling together? J. Cell Sci. 2008, 121, 3285–3292. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Mechanobiology and diseases of mechanotransduction. Ann. Med. 2003, 35, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.A.; DeSimone, D.W. Cell adhesion receptors in mechanotransduction. Curr. Opin. Cell Biol. 2008, 20, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Bai, X.-H.; Lodyga, M.; Xu, J.; Yang, B.B.; Keshavjee, S.; Post, M.; Liu, M. Conversion of mechanical force into biochemical signaling. J. Biol. Chem. 2004, 279, 54793–54801. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Smutny, M.; Yap, A.S. Neighborly relations: Cadherins and mechanotransduction. J. Cell Biol. 2010, 189, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Naruse, K.; Stamenović, D.; Fredberg, J.J.; Mijailovich, S.M.; Tolić-Nørrelykke, I.M.; Polte, T.; Mannix, R.; Ingber, D.E. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. USA 2001, 98, 7765–7770. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F.; Bjerke, M.A.; DeSimone, D.W. Integrins and cadherins join forces to form adhesive networks. J. Cell Sci. 2011, 124, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Schwartz, M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.; Kiseleva, I. Mechanosensitivity in Cells and Tissues. In Academia; Springer: New York, NY, USA, 2005. [Google Scholar]
- Bershadsky, A.D.; Balaban, N.Q.; Geiger, B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003, 19, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Xu, X.Z.S. Mechanosensitive channels: In touch with Piezo. Curr. Biol. 2010, 20, R936–R938. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Ginsberg, M.H.; Shattil, S.J.; Deckmyn, H.; Schwartz, M.A. Matrix-specific suppression of integrin activation in shear stress signaling. Mol. Biol. Cell 2006, 17, 4686–4697. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-J.; Zheng, S.; Sun, J.; Muhamed, I.; Wu, J.; Lei, L.; Kong, X.; Leckband, D.E.; Wang, Y. Dynamic Visualization of α-Catenin Reveals Rapid, Reversible Conformation Switching between Tension States. Curr. Biol. 2014, 25, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, T. Alpha-catenin mechanosensing for adherens junctions. Nat. Cell Biol. 2010, 12, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Qiu, W.; Liu, R.; Efremov, A.K.; Cong, P.; Seddiki, R.; Payre, M.; Lim, C.T.; Ladoux, B.; Mège, R.-M.; et al. Force-dependent conformational switch of α-catenin controls vinculin binding. Nat. Commun. 2014, 5, 4525. [Google Scholar] [CrossRef] [PubMed]
- Riveline, D.; Zamir, E.; Balaban, N.Q.; Schwarz, U.S.; Ishizaki, T.; Narumiya, S.; Kam, Z.; Geiger, B.; Bershadsky, A.D. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 2001, 153, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rico, C.; Pincet, F.; Thiery, J.-P.; Dufour, S. Integrins stimulate E-cadherin-mediated intercellular adhesion by regulating Src-kinase activation and actomyosin contractility. J. Cell Sci. 2010, 123, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Botvinick, E.L.; Zhao, Y.; Berns, M.W.; Usami, S.; Tsien, R.Y.; Chien, S. Visualizing the mechanical activation of Src. Nature 2005, 434, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Le Duc, Q.; Shi, Q.; Blonk, I.; Sonnenberg, A.; Wang, N.; Leckband, D.; de Rooij, J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J. Cell Biol. 2010, 189, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Trichet, L.; Le Digabel, J.; Hawkins, R.J.; Vedula, S.R.K.; Gupta, M.; Ribrault, C.; Hersen, P.; Voituriez, R.; Ladoux, B. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc. Natl. Acad. Sci. USA 2012, 109, 6933–6938. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Tzima, E. Rac[e] to the pole: Setting up polarity in endothelial cells. Small GTPases 2014, 5, e28650. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F.; Bjerke, M.A.; DeSimone, D.W. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev. Cell 2012, 22, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Keely, P.J. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J. Cell Sci. 2011, 124, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Kramer, R.H. Adhesion-mediated squamous cell carcinoma survival through ligand-independent activation of epidermal growth factor receptor. Am. J. Pathol. 2004, 165, 1315–1329. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.W.; Tewari, M.; et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, A.; Gottardi, C.J.; Yap, A.S. Cadherins and cancer: How does cadherin dysfunction promote tumor progression? Oncogene 2008, 27, 6920–6929. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, P.; Helen, W.; Engler, A.J.; Levchenko, A.; Kim, D.-H. Control of stem cell fate and function by engineering physical microenvironments. Integr. Biol. 2012, 4, 1008–1018. [Google Scholar]
- Juliano, R.L. Signal transduction by cell adhesion receptors and the cytoskeleton: Functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 283–323. [Google Scholar] [CrossRef] [PubMed]
- Niessen, C.M.; Leckband, D.; Yap, A.S. Tissue organization by cadherin adhesion molecules: Dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol. Rev. 2011, 91, 691–731. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, T.; Lenne, P.-F.; Munro, E. Force generation, transmission, and integration during cell and tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 2011, 27, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.-S.; McNamara, J.O.; Williams, S.M. Hair Cells and the Mechanoelectrical Transduction of Sound Waves. In Neuroscience, 2nd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Meyer, J.; Preyer, S.; Hofmann, S.I.; Gummer, A.W. Tonic mechanosensitivity of outer hair cells after loss of tip links. Hear. Res. 2005, 202, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Corey, D.P.; Sotomayor, M. Hearing: Tightrope act. Nature 2004, 428, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Brohawn, S.G.; Su, Z.; MacKinnon, R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl. Acad. Sci. USA 2014, 111, 3614–3619. [Google Scholar] [CrossRef] [PubMed]
- Brohawn, S.G.; Campbell, E.B.; MacKinnon, R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature 2014, 516, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-H.; Ranade, S.; Weyer, A.D.; Dubin, A.E.; Baba, Y.; Qiu, Z.; Petrus, M.; Miyamoto, T.; Reddy, K.; Lumpkin, E.A.; et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014, 509, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Li, W.; Zhao, Q.; Li, N.; Chen, M.; Zhi, P.; Li, R.; Gao, N.; Xiao, B.; Yang, M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 2015, 527, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauver, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016, 17, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Volkers, L.; Mechioukhi, Y.; Coste, B. Piezo channels: From structure to function. Pflüg. Arch. 2015, 467, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, R.; Martins, J.R.; Duprat, F.; Demolombe, S.; Arhatte, M.; Jodar, M.; Tauc, M.; Duranton, C.; Paulais, M.; Teulon, J.; et al. Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO Rep. 2013, 14, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.; Herget, R.; Lapatsina, L.; Ngo, H.-D.; Lewin, G.R. Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat. Commun. 2014, 5, 3520. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Mochizuki, T.; Nakagomi, H.; Kira, S.; Watanabe, M.; Takayama, Y.; Suzuki, Y.; Koizumi, S.; Takeda, M.; Tominaga, M. Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures. J. Biol. Chem. 2014, 289, 16565–16575. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.E.; Breckenridge, M.T.; Hinde, E.; Gratton, E.; Chen, C.S.; Schwartz, M.A. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 2013, 23, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Hove, J.R.; Köster, R.W.; Forouhar, A.S.; Acevedo-Bolton, G.; Fraser, S.E.; Gharib, M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 2003, 421, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod 1994, 64, 175–188. [Google Scholar] [PubMed]
- Burger, E.H.; Klein-Nulend, J. Mechanotransduction in bone—Role of the lacuno-canalicular network. FASEB J. 1999, 13, S101–S112. [Google Scholar] [PubMed]
- Hoffman, B.D.; Grashoff, C.; Schwartz, M.A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 2011, 475, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Polacheck, W.J.; Chen, C.S. Measuring cell-generated forces: A guide to the available tools. Nat. Methods 2016, 13, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Chrétien, M.L.; Zhang, M.; Jackson, M.R.; Kapus, A.; Langille, B.L. Mechanotransduction by endothelial cells is locally generated, direction-dependent, and ligand-specific. J. Cell. Physiol. 2010, 224, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.M.; Münster, S.; Bonakdar, N.; Butler, J.P.; Fabry, B. 3D Traction forces in cancer cell invasion. PLoS ONE 2012, 7, e33476. [Google Scholar] [CrossRef] [PubMed]
- Legant, W.R.; Miller, J.S.; Blakely, B.L.; Cohen, D.M.; Genin, G.M.; Chen, C.S. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat. Methods 2010, 7, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.S.; Long, R.; Feng, X.; Huang, Y.; Hui, C.-Y.; Wu, M. Toward single cell traction microscopy within 3D collagen matrices. Exp. Cell Res. 2013, 319, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Irianto, J.; Inamdar, S.; Pravincumar, P.; Lee, D.A.; Bader, D.L.; Knight, M.M. Cell mechanics, structure, and function are regulated by the stiffness of the three-dimensional microenvironment. Biophys. J. 2012, 103, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, G.; Li, M.; Wang, L.; Elson, E.L.; Lu, T.J.; Xu, F. An approach to quantifying 3D responses of cells to extreme strain. Sci. Rep. 2016, 6, 19550. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tolić-Nørrelykke, I.M.; Chen, J.; Mijailovich, S.M.; Butler, J.P.; Fredberg, J.J.; Stamenović, D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 2002, 282, C606–C616. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in development: A growing role for contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.; Na, S.; Li, D.; Poh, Y.-C.; Tanaka, T.S.; Wang, F.; Wang, N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010, 9, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kanchanawong, P.; Shtengel, G.; Pasapera, A.M.; Ramko, E.B.; Davidson, M.W.; Hess, H.F.; Waterman, C.M. Nanoscale architecture of integrin-based cell adhesions. Nature 2010, 468, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Zaidel-Bar, R.; Itzkovitz, S.; Ma’ayan, A.; Iyengar, R.; Geiger, B. Functional atlas of the integrin adhesome. Nat. Cell Biol. 2007, 9, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.P.; Tolić-Nørrelykke, I.M.; Fabry, B.; Fredberg, J.J. Traction fields, moments, and strain energy that cells exert on their surroundings. Am. J. Physiol. Cell Physiol. 2002, 282, C595–C605. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Tofangchi, A.; Anand, S.V.; Saif, T.A. A Novel Cell Traction Force Microscopy to Study Multi-Cellular System. PLoS Comput. Biol. 2014, 10, e1003631. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, R.; Mihai, C.; Kniss, D.; Ghadiali, S.N. Finite element analysis of traction force microscopy: Influence of cell mechanics, adhesion, and morphology. J. Biomech. Eng. 2013, 135, 71009. [Google Scholar] [CrossRef] [PubMed]
- Style, R.W.; Boltyanskiy, R.; German, G.K.; Hyland, C.; MacMinn, C.W.; Mertz, A.F.; Wilen, L.A.; Xu, Y.; Dufresne, E.R. Traction force microscopy in physics and biology. Soft Matter 2014, 10, 4047–4055. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, T.; Morino, Y.; Urayama, K.; Masuda, T. Poisson’s ratio of polyacrylamide (PAAm) gels. Polym. Gels Netw. 1996, 4, 1–5. [Google Scholar] [CrossRef]
- Boudou, T.; Ohayon, J.; Picart, C.; Tracqui, P. An extended relationship for the characterization of Young’s modulus and Poisson’s ratio of tunable polyacrylamide gels. Biorheology 2006, 43, 721–728. [Google Scholar] [PubMed]
- Pritchard, R.H.; Lava, P.; Debruyne, D.; Terentjev, E.M. Precise determination of the Poisson ratio in soft materials with 2D digital image correlation. Soft Matter 2013, 9, 6037–6045. [Google Scholar] [CrossRef]
- Maruthamuthu, V.; Sabass, B.; Schwarz, U.S.; Gardel, M.L. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc. Natl. Acad. Sci. USA 2011, 108, 4708–4713. [Google Scholar] [CrossRef] [PubMed]
- Jasaitis, A.; Estevez, M.; Heysch, J.; Ladoux, B.; Dufour, S. E-cadherin-dependent stimulation of traction force at focal adhesions via the Src and PI3K signaling pathways. Biophys. J. 2012, 103, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, S.V.; Sabass, B.; Schwarz, U.S.; Waterman, C.M. High-resolution traction force microscopy. Methods Cell Biol. 2014, 123, 367–394. [Google Scholar] [PubMed]
- Tambe, D.T.; Corey Hardin, C.; Angelini, T.E.; Rajendran, K.; Park, C.Y.; Serra-Picamal, X.; Zhou, E.H.; Zaman, M.H.; Butle, J.P.; Weitz, D.A.; et al. Collective cell guidance by cooperative intercellular forces. Nat. Mater. 2011, 10, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Bergert, M.; Lendenmann, T.; Zündel, M.; Ehret, A.E.; Panozzo, D.; Richner, P.; Kim, D.K.; Kress, S.J.P.; Norris, D.J.; Sorkine-Hornung, O.; et al. Confocal reference free traction force microscopy. Nat. Commun. 2016, 7, 12814. [Google Scholar] [PubMed]
- Soon, C.F.; Youseffi, M.; Berends, R.F.; Blagden, N.; Denyer, M.C.T. Development of a novel liquid crystal based cell traction force transducer system. Biosens. Bioelectron. 2013, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Schoen, I.; Hu, W.; Klotzsch, E.; Vogel, V. Probing cellular traction forces by micropillar arrays: Contribution of substrate warping to pillar deflection. Nano Lett. 2010, 10, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Du Roure, O.; Saez, A.; Buguin, A.; Austin, R.H.; Chavrier, P.; Silberzan, P.; Ladoux, B. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA 2005, 102, 2390–2395. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; SundarRaj, N.; Wang, J.H.-C. Alpha-smooth muscle actin expression enhances cell traction force. Cell Motil. Cytoskelet. 2007, 64, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Morin, T.R.; Ghassem-Zadeh, S.A.; Lee, J. Traction force microscopy in rapidly moving cells reveals separate roles for ROCK and MLCK in the mechanics of retraction. Exp. Cell Res. 2014, 326, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Munevar, S.; Wang, Y.; Dembo, M. Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys. J. 2001, 80, 1744–1757. [Google Scholar] [CrossRef]
- Li, B.; Li, F.; Puskar, K.M.; Wang, J.H.-C. Spatial patterning of cell proliferation and differentiation depends on mechanical stress magnitude. J. Biomech. 2009, 42, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.J.; Doweidar, M.H. Role of Mechanical Cues in Cell Differentiation and Proliferation: A 3D Numerical Model. PLoS ONE 2015, 10, e0124529. [Google Scholar] [CrossRef] [PubMed]
- Fabry, B.; Maksym, G.N.; Butler, J.P.; Glogauer, M.; Navajas, D.; Fredberg, J.J. Scaling the Microrheology of Living Cells. Phys. Rev. Lett. 2001, 87, 148102. [Google Scholar] [CrossRef] [PubMed]
- Stamenović, D.; Rosenblatt, N.; Montoya-Zavala, M.; Matthews, B.D.; Hu, S.; Suki, B.; Wang, N.; Ingber, D.E. Rheological behavior of living cells is timescale-dependent. Biophys. J. 2007, 93, L39–L41. [Google Scholar] [CrossRef] [PubMed]
- Trepat, X.; Deng, L.; An, S.S.; Navajas, D.; Tschumperlin, D.J.; Gerthoffer, W.T.; Butler, J.P.; Fredberg, J.J. Universal physical responses to stretch in the living cell. Nature 2007, 447, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Collin, O.; Chowdhury, F.; Tay, B.; Ouyang, M.; Wang, Y.; Wang, N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. USA 2008, 105, 6626–6631. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.; Na, S.; Collin, O.; Tay, B.; Li, F.; Tanaka, T.; Leckband, D.E.; Wang, N. Is cell rheology governed by nonequilibrium-to-equilibrium transition of noncovalent bonds? Biophys. J. 2008, 95, 5719–5727. [Google Scholar] [CrossRef] [PubMed]
- Overby, D.R.; Matthews, B.D.; Alsberg, E.; Ingber, D.E. Novel dynamic rheological behavior of individual focal adhesions measured within single cells using electromagnetic pulling cytometry. Acta Biomater. 2005, 1, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.D.; Overby, D.R.; Alenghat, F.J.; Karavitis, J.; Numaguchi, Y.; Allen, P.G.; Ingber, D.E. Mechanical properties of individual focal adhesions probed with a magnetic microneedle. Biochem. Biophys. Res. Commun. 2004, 313, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.D.; Overby, D.R.; Mannix, R.; Ingber, D.E. Cellular adaptation to mechanical stress: Role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J. Cell Sci. 2006, 119, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.D.; Thodeti, C.K.; Tytell, J.D.; Mammoto, A.; Overby, D.R.; Ingber, D.E. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr. Biol. 2010, 2, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Discher, D.E. Cell Mechanics; Elsevier Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Matthews, B.D.; LaVan, D.A.; Overby, D.R.; Karavitis, J.; Ingber, D.E. Electromagnetic needles with submicron pole tip radii for nanomanipulation of biomolecules and living cells. Appl. Phys. Lett. 2004, 85, 2968–2970. [Google Scholar] [CrossRef]
- Barry, A.K.; Tabdili, H.; Muhamed, I.; Wu, J.; Shashikanth, N.; Gomez, G.A.; Yap, A.S.; Gottardi, C.J.; de Rooij, J.; Wang, N.; et al. α-Catenin cytomechanics: Role in cadherin-dependent adhesion and mechanotransduction. J. Cell Sci. 2014, 127, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A. Acceleration and Trapping of Particles by Radiation Pressure. Phys. Rev. Lett. 1970, 24, 156–159. [Google Scholar] [CrossRef]
- Swank, D.M.; Bartoo, M.L.; Knowles, A.F.; Iliffe, C.; Bernstein, S.I.; Molloy, J.E.; Sparrow, J.C. Alternative Exon-encoded Regions of Drosophila Myosin Heavy Chain Modulate ATPase Rates and Actin Sliding Velocity. J. Biol. Chem. 2001, 276, 15117–15124. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.; Park, H.; Safer, D.; Yang, Z.; Chen, L.-Q.; Selvin, P.R.; Sweeney, H.L. Myosin VI steps via a hand-over-hand mechanism with its lever arm undergoing fluctuations when attached to actin. J. Biol. Chem. 2004, 279, 37223–37226. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.; Forkey, J.N.; McKinney, S.A.; Ha, T.; Goldman, Y.E.; Selvin, P.R. Myosin V walks hand-over-hand: Single fluorophore imaging with 1.5-nm localization. Science 2003, 300, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Asbury, C.L.; Fehr, A.N.; Block, S.M. Kinesin Moves by an Asymmetric Hand-Over-Hand Mechanism. Science 2003, 302, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Block, S.M.; Asbury, C.L.; Shaevitz, J.W.; Lang, M.J. Probing the kinesin reaction cycle with a 2D optical force clamp. Proc. Natl. Acad. Sci. USA 2003, 100, 2351–2356. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Carter, B.C.; Lex, S.A.; King, S.J.; Gross, S.P. Cytoplasmic dynein functions as a gear in response to load. Nature 2004, 427, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Reck-Peterson, S.L.; Yildiz, A.; Carter, A.P.; Gennerich, A.; Zhang, N.; Vale, R.D. Single-molecule analysis of dynein processivity and stepping behavior. Cell 2006, 126, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Gennerich, A.; Carter, A.P.; Reck-Peterson, S.L.; Vale, R.D. Force-induced bidirectional stepping of cytoplasmic dynein. Cell 2007, 131, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.M.; Berg, H.C. Absence of a barrier to backwards rotation of the bacterial flagellar motor demonstrated with optical tweezers. Biophysics 1997, 94, 14433–14437. [Google Scholar] [CrossRef]
- Block, S.M.; Blair, D.F.; Berg, H.C. Compliance of bacterial flagella measured with optical tweezers. Nature 1989, 338, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, M.D.; Svoboda, K.; Landick, R.; Block, S.M.; Gelles, J. Transcription against an applied force. Science 1995, 270, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Wuite, G.J.L.; Smith, S.B.; Young, M.; Keller, D.; Bustamante, C. Single-molecule studies of the effect of template tension on T7 DNA polymerase activity. Nature 2000, 404, 103–106. [Google Scholar] [PubMed]
- Liphardt, J.; Onoa, B.; Smith, S.B.; Tinoco, I.; Bustamante, C. Reversible Unfolding of Single RNA Molecules by Mechanical Force. Science 2001, 292, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Allemand, J.-F.; Bensimon, D.; Croquette, V. Stretching DNA and RNA to probe their interactions with proteins. Curr. Opin. Struct. Biol. 2003, 13, 266–274. [Google Scholar] [CrossRef]
- Abbondanzieri, E.A.; Greenleaf, W.J.; Shaevitz, J.W.; Landick, R.; Block, S.M. Direct observation of base-pair stepping by RNA polymerase. Nature 2005, 438, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Myong, S.; Bruno, M.M.; Pyle, A.M.; Ha, T. Spring-Loaded Mechanism of DNA Unwinding by Hepatitis C Virus NS3 Helicase. Science 2007, 317, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Balci, H.; Jia, H.; Lohman, T.M.; Ha, T. Direct imaging of single UvrD helicase dynamics on long single-stranded DNA. Nat. Commun. 2013, 4, 1878. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.T.M.; Zhang, Q.; Zhou, R.; Yodh, J.G.; Ha, T. Asymmetric Unwrapping of Nucleosomes under Tension Directed by DNA Local Flexibility. Cell 2015, 160, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Choquet, D.; Felsenfeld, D.P.; Sheetz, M.P. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 1997, 88, 39–48. [Google Scholar] [CrossRef]
- Raucher, D.; Stauffer, T.; Chen, W.; Shen, K.; Guo, S.; York, J.D.; Sheetz, M.P.; Meyer, T. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell 2000, 100, 221–228. [Google Scholar] [CrossRef]
- Suresh, S.; Spatz, J.; Mills, J.P.; Micoulet, A.; Dao, M.; Lim, C.T.; Beil, M.; Seufferlein, T. Connections between single-cell biomechanics and human disease states: Gastrointestinal cancer and malaria. Acta Biomater. 2005, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Grashoff, C.; Hoffman, B.D.; Brenner, M.D.; Zhou, R.; Parsons, M.; Yang, M.T.; McLean, M.A.; Sligar, S.G.; Chen, C.S.; Ha, T.; et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 2010, 466, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Blakely, B.L.; Dumelin, C.E.; Trappmann, B.; McGregor, L.M.; Choi, C.K.; Anthony, P.C.; Duesterberg, V.K.; Baker, B.M.; Block, S.M.; Liu, D.R.; et al. A DNA-based molecular probe for optically reporting cellular traction forces. Nat. Methods 2014, 11, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.; Li, I.T.S.; Ngo, T.T.M.; Leslie, B.J.; Kim, B.C.; Sokoloski, J.E.; Weiland, E.; Wang, X.; Chemla, Y.R.; Lohman, T.M.; et al. Defining Single Molecular Forces Required for Notch Activation Using Nano Yoyo. Nano Lett. 2016, 16, 3892–3897. [Google Scholar] [CrossRef] [PubMed]
- Chu, S. Nobel Lecture: The manipulation of neutral particles. Rev. Mod. Phys. 1998, 70, 685–706. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.-K. Optical tweezers for single cells. J. R. Soc. Interface 2008, 5, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Ramser, K.; Hanstorp, D. Optical manipulation for single-cell studies. J. Biophotonics 2010, 3, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Neuman, K.C.; Nagy, A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, J.R.; Chemla, Y.R.; Izhaky, D.; Bustamante, C. Differential detection of dual traps improves the spatial resolution of optical tweezers. Proc. Natl. Acad. Sci. USA 2006, 103, 9006–9011. [Google Scholar] [CrossRef] [PubMed]
- Gaczynska, M.; Osmulski, P.A. AFM of biological complexes: What can we learn? Curr. Opin. Colloid Interface Sci. 2008, 13, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Haase, K.; Pelling, A.E. Investigating cell mechanics with atomic force microscopy. J. R. Soc. Interface 2015, 12, 20140970. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.B.; Truskey, G.A.; Reichert, W.M. Atomic force and total internal reflection fluorescence microscopy for the study of force transmission in endothelial cells. Biophys. J. 2000, 78, 1725–1735. [Google Scholar] [CrossRef]
- Akhremitchev, B.B.; Walker, G.C. Finite sample thickness effects on elasticity determination using atomic force microscopy. Langmuir 1999, 15, 5630–5634. [Google Scholar] [CrossRef]
- Maki, K.; Han, S.-W.; Hirano, Y.; Yonemura, S.; Hakoshima, T.; Adachi, T. Mechano-adaptive sensory mechanism of α-catenin under tension. Sci. Rep. 2016, 6, 24878. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Trigueros, S.; Cartagena, A.; Stevenson, A.P.Z.; Susilo, M.; Nauman, E.; Contera, S.A. Mapping nanomechanical properties of live cells using multi-harmonic atomic force microscopy. Nat. Nanotechnol. 2011, 6, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Sivasankar, S.; Brieher, W.; Lavrik, N.; Gumbiner, B.; Leckband, D. Direct molecular force measurements of multiple adhesive interactions between cadherin ectodomains. Proc. Natl. Acad. Sci. USA 1999, 96, 11820–11824. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chien, Y.-H.; Leckband, D. Biophysical properties of cadherin bonds do not predict cell sorting. J. Biol. Chem. 2008, 283, 28454–28463. [Google Scholar] [CrossRef] [PubMed]
- Dragovich, M.A.; Chester, D.; Fu, B.M.; Wu, C.; Xu, Y.; Goligorsky, M.S.; Zhang, X.F. Mechanotransduction of the endothelial glycocalyx mediates nitric oxide production through activation of TRP channels. Am. J. Physiol. Cell Physiol. 2016, 311, C846–C853. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.W.; Mulet, X.; Klug, D.R. Absolute Measurement of Phosphorylation Levels in a Biological Membrane Using Atomic Force Microscopy: The Creation of Phosphorylation Maps. Biochemisty 2002, 41, 8535–8539. [Google Scholar] [CrossRef]
- Philippsen, A.; Im, W.; Engel, A.; Schirmer, T.; Roux, B.; Müller, D.J. Imaging the Electrostatic Potential of Transmembrane Channels: Atomic Probe Microscopy of OmpF Porin. Biophys. J. 2002, 82, 1667–1676. [Google Scholar] [CrossRef]
- Tzima, E.; del Pozo, M.A.; Shattil, S.J.; Chien, S.; Schwartz, M.A. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001, 20, 4639–4647. [Google Scholar] [CrossRef] [PubMed]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; DeLisser, H.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, A.; Engler, A.J. Acute shear stress direction dictates adherent cell remodeling and verifies shear profile of spinning disk assays. Phys. Biol. 2015, 12, 16011. [Google Scholar] [CrossRef] [PubMed]
- Bacabac, R.G.; Smit, T.H.; Cowin, S.C.; Van Loon, J.J.W.A.; Nieuwstadt, F.T.M.; Heethaar, R.; Klein-Nulend, J. Dynamic shear stress in parallel-plate flow chambers. J. Biomech. 2005, 38, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lane, W.O.; Jantzen, A.E.; Carlon, T.A.; Jamiolkowski, R.M.; Grenet, J.E.; Ley, M.M.; Haseltine, J.M.; Galinat, L.J.; Lin, F.H.; Allen, J.D.; et al. Parallel-plate flow chamber and continuous flow circuit to evaluate endothelial progenitor cells under laminar flow shear stress. J. Vis. Exp. 2012, 59. [Google Scholar] [CrossRef] [PubMed]
- Steward, R.; Tambe, D.; Hardin, C.C.; Krishnan, R.; Fredberg, J.J.; Fredberg, J.J. Fluid shear, intercellular stress, and endothelial cell alignment. Am. J. Physiol. Cell Physiol. 2015, 308, C657–C664. [Google Scholar] [CrossRef] [PubMed]
- Polacheck, W.J.; Li, R.; Uzel, S.G.M.; Kamm, R.D. Microfluidic platforms for mechanobiology. Lab Chip 2013, 13, 2252–2267. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.; Reinhart-King, C.A.; Erickson, D. Elucidating mechanical transition effects of invading cancer cells with a subnucleus-scaled microfluidic serial dimensional modulation device. Lab Chip 2013, 13, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Vickerman, V.; Blundo, J.; Chung, S.; Kamm, R. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip 2008, 8, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Malboubi, M.; Jayo, A.; Parsons, M.; Charras, G. An open access microfluidic device for the study of the physical limits of cancer cell deformation during migration in confined environments. Microelectron. Eng. 2015, 144, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Gu, W.; Futai, N.; Warner, K.A.; Nor, J.E.; Takayama, S. Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal. Chem. 2005, 77, 3993–3999. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Tatsumi, H.; Sokabe, M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J. Cell Sci. 2008, 121, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; Jensen, C.C.; Chaturvedi, A.; Yoshigi, M.; Beckerle, M.C. Stretch-induced actin remodeling requires targeting of zyxin to stress fibers and recruitment of actin regulators. Mol. Biol. Cell 2012, 23, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Dugan, J.M.; Cartmell, S.H.; Gough, J.E. Uniaxial cyclic strain of human adipose-derived mesenchymal stem cells and C2C12 myoblasts in coculture. J. Tissue Eng. 2014, 5, 2041731414530138. [Google Scholar] [CrossRef] [PubMed]
- Upton, M.L.; Hennerbichler, A.; Fermor, B.; Guilak, F.; Weinberg, J.B.; Setton, L.A. Biaxial strain effects on cells from the inner and outer regions of the meniscus. Connect. Tissue Res. 2006, 47, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ursekar, C.P.; Teo, S.-K.; Hirata, H.; Harada, I.; Chiam, K.-H.; Sawada, Y. Design and construction of an equibiaxial cell stretching system that is improved for biochemical analysis. PLoS ONE 2014, 9, e90665. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, M.; Jalali, S.; Usami, S.; Shyy, J.Y.; Chien, S. A strain device imposing dynamic and uniform equi-biaxial strain to cultured cells. Ann. Biomed. Eng. 1998, 26, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.; Berk, A.; Matsudaira, P.; Kaiser, C.A.; Krieger, M.; Scott, M.P.; Bretscher, A.; Ploegh, H. Molecular Cell Biology; W.H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Collins, C.; Guilluy, C.; Welch, C.; O’Brien, E.T.; Hahn, K.; Superfine, R.; Burridge, K.; Tzima, E. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr. Biol. 2012, 22, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.; Tajik, A.; Sun, J.; Guan, J.; Humphries, M.J.; Craig, S.E.; Shekaran, A.; García, A.J.; Lu, S.; Lin, M.Z.; et al. Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 19372–19377. [Google Scholar] [CrossRef] [PubMed]
- Leckband, D.; Shi, Q.; Langer, M.; Tabdili, H.; Wang, N.; Poh, Y.-C. Cadherin-dependent mechanotransduction depends on ligand identity but not affinity. J. Cell Sci. 2012, 125, 4362–4371. [Google Scholar]
- Li, J.; Han, D.; Zhao, Y.-P. Kinetic behaviour of the cells touching substrate: The interfacial stiffness guides cell spreading. Sci. Rep. 2014, 4, 3910. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lou, J.; Evans, E.A.; Zhu, C. Observing force-regulated conformational changes and ligand dissociation from a single integrin on cells. J. Cell Biol. 2012, 199, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, E.S.; Izard, T. The cytoskeletal protein α-catenin unfurls upon binding to vinculin. J. Biol. Chem. 2012, 287, 18492–18499. [Google Scholar] [CrossRef] [PubMed]
- Bakolitsa, C.; Cohen, D.M.; Bankston, L.A.; Bobkov, A.A.; Cadwell, G.W.; Jennings, L.; Critchley, D.R.; Craig, S.W.; Liddington, R.C. Structural basis for vinculin activation at sites of cell adhesion. Nature 2004, 430, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Choudhury, D.M.; Craig, S.W. Coincidence of actin filaments and talin is required to activate vinculin. J. Biol. Chem. 2006, 281, 40389–40398. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Pokutta, S.; Cadwell, G.W.; Bobkov, A.A.; Bankston, L.A.; Liddington, R.C.; Weis, W.I. αE-catenin is an autoinhibited molecule that coactivates vinculin. Proc. Natl. Acad. Sci. USA 2012, 109, 8576–8581. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Maiers, J.L.; Choudhury, D.; Craig, S.W.; DeMali, K.A. α-Catenin uses a novel mechanism to activate vinculin. J. Biol. Chem. 2012, 287, 7728–7737. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Michael, K.E.; Dumbauld, D.W.; Burns, K.L.; Hanks, S.K.; García, A.J. Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol. Biol. Cell 2009, 20, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, W.S.; Saif, T.A.; Wang, Y. Calcium signaling in live cells on elastic gels under mechanical vibration at subcellular levels. PLoS ONE 2011, 6, e26181. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Cary, L.A.; Jamieson, J.S.; Cooper, J.A.; Turner, C.E. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol. Biol. Cell 2005, 16, 4316–4328. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, M.J.; Johnson, K.R. Cadherin-mediated cellular signaling. Curr. Opin. Cell Biol. 2003, 15, 509–514. [Google Scholar] [CrossRef]
- Leckband, D.E.; de Rooij, J. Cadherin adhesion and mechanotransduction. Annu. Rev. Cell Dev. Biol. 2014, 30, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.W.; Harley, B.A.C. Mechanobiology of Cell-Cell and Cell-Matrix Interactions; Springer: Berlin, Germnay, 2011; pp. 11–23. [Google Scholar]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Orgovan, N.; Peter, B.; Bősze, S.; Ramsden, J.J.; Szabó, B.; Horvath, R. Dependence of cancer cell adhesion kinetics on integrin ligand surface density measured by a high-throughput label-free resonant waveguide grating biosensor. Sci. Rep. 2014, 4, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Askari, J.A.; Buckley, P.A.; Mould, A.P.; Humphries, M.J. Linking integrin conformation to function. J. Cell Sci. 2008, 122, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Mecham, R.P. Overview of Extracellular Matrix. In Current Protocols in Cell Biology; Hoboken, N.J., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Villard, V.; Kalyuzhniy, O.; Riccio, O.; Potekhin, S.; Melnik, T.N.; Kajava, A.V.; Rüegg, C.; Corradin, G. Synthetic RGD-containing alpha-helical coiled coil peptides promote integrin-dependent cell adhesion. J. Pept. Sci. 2006, 12, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef] [PubMed]
- Borghi, N.; Lowndes, M.; Maruthamuthu, V.; Gardel, M.L.; Nelson, W.J. Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proc. Natl. Acad. Sci. USA 2010, 107, 13324–13329. [Google Scholar] [CrossRef] [PubMed]
- Chigaev, A.; Buranda, T.; Dwyer, D.C.; Prossnitz, E.R.; Sklar, L.A. FRET Detection of Cellular α4-Integrin Conformational Activation. Biophys. J. 2003, 85, 3951–3962. [Google Scholar] [CrossRef]
- Ye, F.; Liu, J.; Winkler, H.; Taylor, K.A. Integrin αIIbβ3 in a Membrane Environment Remains the Same Height after Mn2+ Activation when Observed by Cryoelectron Tomography. J. Mol. Biol. 2008, 378, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Takagi, J.; Petre, B.M.; Walz, T.; Springer, T.A. Global Conformational Rearrangements in Integrin Extracellular Domains in Outside-In and Inside-Out Signaling. Cell 2002, 110, 599–611. [Google Scholar] [CrossRef]
- Gallant, N.D.; Michael, K.E.; García, A.J. Cell adhesion strengthening: Contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol. Biol. Cell 2005, 16, 4329–4340. [Google Scholar] [CrossRef] [PubMed]
- Zaidel-Bar, R.; Cohen, M.; Addadi, L.; Geiger, B. Hierarchical assembly of cell–matrix adhesion complexes. Biochem. Soc. Trans. 2004, 32, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zimerman, B.; Volberg, T.; Geiger, B. Early molecular events in the assembly of the focal adhesion-stress fiber complex during fibroblast spreading. Cell Motil. Cytoskelet. 2004, 58, 143–159. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, J.; Kerstens, A.; Danuser, G.; Schwartz, M.A.; Waterman-Storer, C.M. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J. Cell Biol. 2005, 171, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Danuser, G.; Waterman-Storer, C.M. Quantitative fluorescent speckle microscopy of cytoskeleton dynamics. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 361–387. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.K.; Vicente-Manzanares, M.; Zareno, J.; Whitmore, L.A.; Mogilner, A.; Horwitz, A.R. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008, 10, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Birukova, A.A.; Fu, P.; Xing, J.; Yakubov, B.; Cokic, I.; Birukov, K.G. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L837–L848. [Google Scholar] [CrossRef] [PubMed]
- Totsukawa, G.; Yamakita, Y.; Yamashiro, S.; Hartshorne, D.J.; Sasaki, Y.; Matsumura, F. Distinct Roles of Rock (Rho-Kinase) and Mlck in Spatial Regulation of Mlc Phosphorylation for Assembly of Stress Fibers and Focal Adhesions in 3t3 Fibroblasts. J. Cell Biol. 2000, 150, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ito, M.; Amano, M.; Chihara, K.; Fukata, Y.; Nakafuku, M.; Okawa, K.; Iwamatsu, A.; Kaibuchi, K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996, 273, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M.A.; Modzelewska, K.; Kwong, L.; Keely, P.J. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1692, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Oakes, P.W.; Beckham, Y.; Stricker, J.; Gardel, M.L. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J. Cell Biol. 2012, 196, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K. Role of stress fibers and focal adhesions as a mediator for mechano-signal transduction in endothelial cells in situ. Vasc. Health Risk Manag. 2008, 4, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, D.A.; Campbell, I.D.; Critchley, D.R. Talins and kindlins: Partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013, 14, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Dumbauld, D.W.; Lee, T.T.; Singh, A.; Scrimgeour, J.; Gersbach, C.A.; Zamir, E.A.; Fu, J.; Chen, C.S.; Curtis, J.E.; Craig, S.W.; et al. How vinculin regulates force transmission. Proc. Natl. Acad. Sci. USA 2013, 110, 9788–9793. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J.; Xu, Q.; Chowdhury, F.; Roein-Peikar, M.; Wang, Y.; Ha, T. Integrin Molecular Tension within Motile Focal Adhesions. Biophys. J. 2015, 109, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov S, V.; Pasapera, A.M.; Sabass, B.; Waterman, C.M. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 2012, 151, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X. Integrins outside focal adhesions transmit tensions during stable cell adhesion. Sci. Rep. 2016, 6, 36959. [Google Scholar] [CrossRef] [PubMed]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Calderwood, D.A.; Ginsberg, M.H. Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 2000, 113, 3563–3571. [Google Scholar] [PubMed]
- Herzog, F.A.; Vogel, V. Multiple steps to activate FAK’s kinase domain: Adaptation to confined environments? Biophys. J. 2013, 104, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Lietha, D.; Cai, X.; Ceccarelli, D.F.J.; Li, Y.; Schaller, M.D.; Eck, M.J. Structural basis for the autoinhibition of focal adhesion kinase. Cell 2007, 129, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Sun, J.; Chien, S.; Wang, Y. Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc. Natl. Acad. Sci. USA 2008, 105, 14353–14358. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.; Lu, S.; Wang, Y. Live Cell Imaging of Src/FAK Signaling by FRET. Cell. Mol. Bioeng. 2011, 2, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Lee, S.; Briscoe, C.; Ellsworth, C.; Firtel, R.A. Role of Rac in controlling the actin cytoskeleton and chemotaxis in motile cells. Proc. Natl. Acad. Sci. USA 2000, 97, 5225–5230. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K.; Seto, M.; Noma, K. Rho kinase (ROCK) inhibitors. J. Cardiovasc. Pharmacol. 2007, 50, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tapon, N.; Hall, A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr. Opin. Cell Biol. 1997, 9, 86–92. [Google Scholar] [CrossRef]
- Spiering, D.; Hodgson, L. Dynamics of the rho-family small GTPases in actin regulation and motility. Cell Adhes. Migr. 2011, 5, 170–180. [Google Scholar] [CrossRef]
- Lawrence, D.S.; Wang, Q. Seeing is believing: Peptide-based fluorescent sensors of protein tyrosine kinase activity. ChemBioChem 2007, 8, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.D.; Ehrhardt, A.G.; Cellurale, C.; Zhong, H.; Yasuda, R.; Davis, R.J.; Svoboda, K. A genetically encoded fluorescent sensor of ERK activity. Proc. Natl. Acad. Sci. USA 2008, 105, 19264–19269. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B.E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef]
- Bazzoni, G.; Dejana, E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.G. Gap junctions and connexin-interacting proteins. Cardiovasc. Res. 2004, 62, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Vendome, J.; Posy, S.; Jin, X.; Bahna, F.; Ahlsen, G.; Shapiro, L.; Honig, B. Molecular design principles underlying β-strand swapping in the adhesive dimerization of cadherins. Nat. Struct. Mol. Biol. 2011, 18, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.H.; Stewart, D.B.; Laurents, D.V.; Nelson, W.J.; Weis, W.I. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J. Biol. Chem. 2001, 276, 12301–12309. [Google Scholar] [CrossRef] [PubMed]
- Pokutta, S.; Weis, W.I. Structure of the dimerization and beta-catenin-binding region of alpha-catenin. Mol. Cell 2000, 5, 533–543. [Google Scholar] [CrossRef]
- Hirano, S.; Suzuki, S.T.; Redies, C. The cadherin superfamily in neural development: Diversity, function and interaction with other molecules. Front. Biosci. 2003, 8, d306–d355. [Google Scholar] [CrossRef] [PubMed]
- Nollet, F.; Kools, P.; van Roy, F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J. Mol. Biol. 2000, 299, 551–572. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Tai, C.-Y.; Mok, L.-P.; Mosser, E.A.; Schuman, E.M. Calcium-dependent dynamics of cadherin interactions at cell-cell junctions. Proc. Natl. Acad. Sci. USA 2011, 108, 9857–9862. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M.; Nakagawa, S. Cadherin-dependent cell-cell adhesion. In Current Protocols in Cell Biology; Wiley Online Library: Hoboken, NJ, USA, 2001. [Google Scholar]
- Davis, M.A.; Ireton, R.C.; Reynolds, A.B. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 2003, 163, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Pokutta, S.; Drees, F.; Yamada, S.; Nelson, W.J.; Weis, W.I. Biochemical and structural analysis of alpha-catenin in cell-cell contacts. Biochem. Soc. Trans. 2008, 36, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Nagafuchi, A.; Ishihara, S.; Tsukita, S. The roles of catenins in the cadherin-mediated cell adhesion: Functional analysis of E-cadherin-alpha catenin fusion molecules. J. Cell Biol. 1994, 127, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S.; Wada, Y.; Watanabe, T.; Nagafuchi, A.; Shibata, M. Alpha-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 2010, 12, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Tan, J.; Anderson, K.L.; Hanein, D.; Volkmann, N.; Weis, W.I.; Nelson, W.J.; Dunn, A.R. The minimal cadherin-catenin complex binds to actin filaments under force. Science 2014, 346, 1254211. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Mushika, Y.; Ichii, T.; Takeichi, M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell 2008, 135, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dokurno, P.; Tonks, N.K.; Barford, D. Crystal structure of the M-fragment of alpha-catenin: Implications for modulation of cell adhesion. EMBO J. 2001, 20, 3645–3656. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Sarpal, R.; Ishiyama, N.; Pellikka, M.; Ikura, M.; Tepass, U. Monomeric α-catenin links cadherin to the actin cytoskeleton. Nat. Cell Biol. 2013, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Kobielak, A.; Fuchs, E. Alpha-catenin: At the junction of intercellular adhesion and actin dynamics. Nat. Rev. Mol. Cell Biol. 2004, 5, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, E.S.; Izard, T. Dimer asymmetry defines α-catenin interactions. Nat. Struct. Mol. Biol. 2013, 20, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Escobar, D.J.; Desai, R.; Ishiyama, N.; Folmsbee, S.S.; Novak, M.N.; Flozak, A.S.; Daugherty, R.L.; Mo, R.; Nanavati, D.; Sarpal, R.; et al. α-Catenin phosphorylation promotes intercellular adhesion through a dual-kinase mechanism. J. Cell Sci. 2015, 128, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Maiden, S.L.; Hardin, J. The secret life of alpha-catenin: Moonlighting in morphogenesis. J Cell Biol. 2011, 195, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Drees, F.; Pokutta, S.; Yamada, S.; Nelson, W.J.; Weis, W.I. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005, 123, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Giannini, A.L.; Vivanco, M.D.; Kypta, R.M. α-catenin inhibits β-catenin signaling by preventing formation of a β-catenin T-cell factor DNA complex. J. Biol. Chem. 2000, 275, 21883–21888. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.M.; Kwiatkowski, A.V.; Yang, C.; Korobova, F.; Pokutta, S.; Svitkina, T.; Weis, W.I.; Nelson, W.J. αE-catenin regulates actin dynamics independently of cadherin-mediated cell-cell adhesion. J. Cell Biol. 2010, 189, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.A.; Yap, A.S. Cinderella no longer: α-catenin steps out of cadherin’s shadow. J. Cell Sci. 2006, 119, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Nelson, W.J. Re-solving the cadherin-catenin-actin conundrum. J. Biol. Chem. 2006, 281, 35593–35597. [Google Scholar] [CrossRef] [PubMed]
- Izard, T.; Evans, G.; Borgon, R.A.; Rush, C.L.; Bricogne, G.; Bois, P.R.J. Vinculin activation by talin through helical bundle conversion. Nature 2004, 427, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Borghi, N.; Sorokina, M.; Shcherbakova, O.G.; Weis, W.I.; Pruitt, B.L.; Nelson, W.J.; Dunn, A.R. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc. Natl. Acad. Sci. USA 2012, 109, 12568–12573. [Google Scholar] [CrossRef] [PubMed]
- Tabdili, H.; Barry, A.K.; Langer, M.D.; Chien, Y.-H.; Shi, Q.; Lee, K.J.; Lu, S.; Leckband, D.E. Cadherin point mutations alter cell sorting and modulate GTPase signaling. J. Cell Sci. 2012, 125, 3299–3309. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tan, J.L.; Cohen, D.M.; Yang, M.T.; Sniadecki, N.J.; Ruiz, S.A.; Nelson, C.M.; Chen, C.S. Mechanical tugging force regulates the size of cell-cell junctions. Proc. Natl. Acad. Sci. USA 2010, 107, 9944–9949. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; Pirone, D.M.; Tan, J.L.; Chen, C.S. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol. Biol. Cell 2004, 15, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Leckband, D.E.; le Duc, Q.; Wang, N.; de Rooij, J. Mechanotransduction at cadherin-mediated adhesions. Curr. Opin. Cell Biol. 2011, 23, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Muhamed, I.; Wu, J.; Sehgal, P.; Kong, X.; Tajik, A.; Wang, N.; Leckband, D.E. E-Cadherin-mediated force transduction signals regulate global cell mechanics. J. Cell Sci. 2016, 129, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Perez, T.D.; Tamada, M.; Sheetz, M.P.; Nelson, W.J. Immediate-early signaling induced by E-cadherin engagement and adhesion. J. Biol. Chem. 2008, 283, 5014–5022. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Marshall, C.J. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat. Cell Biol. 2002, 4, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.B.; Roczniak-Ferguson, A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene 2004, 23, 7947–7956. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, J. The Nonreceptor Protein Tyrosine Phosphatase PTP1B Binds to the Cytoplasmic Domain of N-Cadherin and Regulates the Cadherin-Actin Linkage. J. Cell Biol. 1998, 143, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Brady-Kalnay, S.M.; Rimm, D.L.; Tonks, N.K. Receptor protein tyrosine phosphatase PTPmu associates with cadherins and catenins in vivo. J. Cell Biol. 1995, 130, 977–986. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, R.W.; Yap, A.S. Protein tyrosine phosphatase activity is necessary for E-cadherin-activated Src signaling. Cytoskeleton 2011, 68, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-S.; Huang, C.-C.; Liang, Y.-C.; Wu, H.-M.; Chen, Y.-L.; Lo, S.-W.; Ho, W.C. Alterations in the balance of protein kinase and phosphatase activities and age-related impairments of synaptic transmission and long-term potentiation. Hippocampus 2002, 12, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, A.; Choi, H.; Sharom, J.R.; Boucher, L.; Neduva, V.; Larsen, B.; Lin, Z.Y.; Breitkreutz, B.J.; Stark, C.; Liu, G.; et al. A global protein kinase and phosphatase interaction network in yeast. Science 2010, 328, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Resink, T.J.; Philippova, M.; Joshi, M.B.; Kyriakakis, E.; Erne, P. Cadherins and cardiovascular disease. Swiss Med. Wkly. 2009, 139, 122–134. [Google Scholar] [PubMed]
- Schulte, D.; Küppers, V.; Dartsch, N.; Broermann, A.; Li, H.; Zarbock, A.; Kamenyeva, O.; Kiefer, F.; Khandoga, A.; Massberg, S.; et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011, 30, 4157–4170. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Lu, S.; Kim, T.; Chen, C.-E.; Seong, J.; Leckband, D.E.; Wang, F.; Reynolds, A.B.; Schwartz, M.A.; Wang, Y. N-cadherin regulates spatially polarized signals through distinct p120ctn and β-catenin-dependent signalling pathways. Nat. Commun. 2013, 4, 1589. [Google Scholar] [CrossRef] [PubMed]
- Alenghat, F.J.; Nauli, S.M.; Kolb, R.; Zhou, J.; Ingber, D.E. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp. Cell Res. 2004, 301, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Tzima, E. RhoA goes GLOBAL. Small GTPases. 2013, 4, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E.; Tensegrity, I. Cell structure and hierarchical systems biology. J. Cell Sci. 2003, 116, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chen, J.; Fabry, B.; Numaguchi, Y.; Gouldstone, A.; Ingber, D.E.; Fredberg, J.J.; Butler, J.P.; Wang, N. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am. J. Physiol. Cell Physiol. 2003, 285, C1082–C1090. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of Mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ladoux, B.; Nicolas, A. Physically based principles of cell adhesion mechanosensitivity in tissues. Rep. Prog. Phys. 2012, 75, 116601. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; McCulloch, C.A. Cell mechanics: Integrating cell responses to mechanical stimuli. Annu. Rev. Biomed. Eng. 2007, 9, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Beningo, K.A.; Lo, C.-M.; Wang, Y.-L. Flexible polyacrylamide substrata for the analysis of mechanical interactions at cell-substratum adhesions. Methods Cell Biol. 2002, 69, 325–339. [Google Scholar] [PubMed]
- Deng, L.; Fairbank, N.J.; Fabry, B.; Smith, P.G.; Maksym, G.N. Localized mechanical stress induces time-dependent actin cytoskeletal remodeling and stiffening in cultured airway smooth muscle cells. Am. J. Physiol. Cell Physiol. 2004, 287, C440–C448. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Yamada, K.M. Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 2011, 3, a005033. [Google Scholar] [CrossRef] [PubMed]

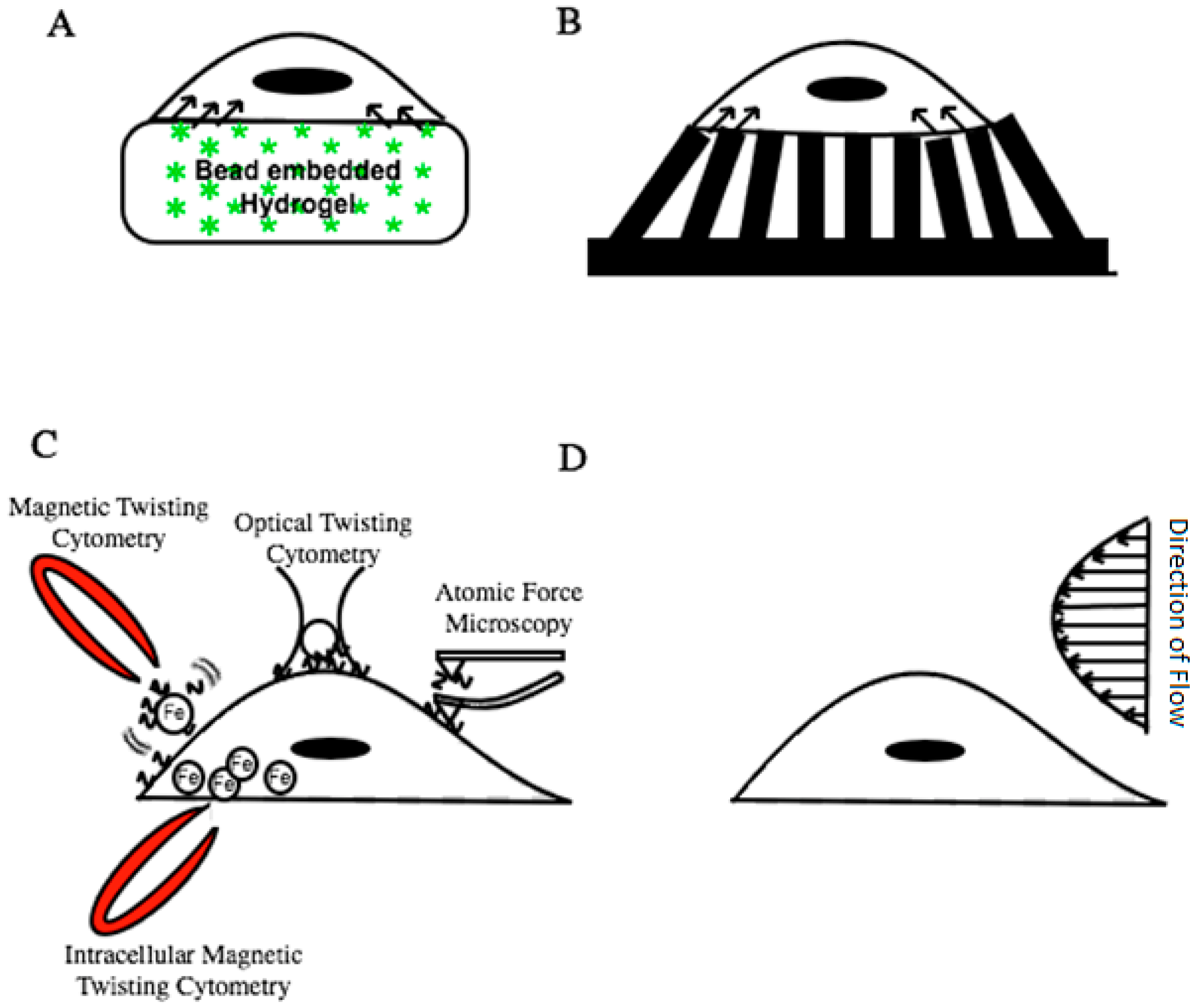
| Experimental Method/Aspect of Mechanotransduction | Representative References | Schematics/Tables That Explain Concepts/Theory |
|---|---|---|
| Mechanotransduction | [272,273] | Figure 1 from [9] and Figure 6 from [274] |
| Mechanotransduction diseases | [3] | Not applicable |
| Cytoskeletal mechanotransduction | [64,275] | Figure 4 from [203] |
| Traction Force/Stress Microscopy | [68,276] | Figure 4 from [63] |
| Magnetic Twisting Cytometry | [277] | Figure 1 from [277] |
| Microfluidic Shear | [149] | Figure 2 from [149] |
| Comparison of force application tools | [55] | Table 1 from [55,128] |
| Integrin mechanotransduction | [278] | Figure 4 from [5] |
| Cadherin mechanotransduction | [177] | Figure 1 from [9] & Figure 4 from [177] |
| Mechanotransduction signals | [28,34,176] | Figures 2–7 from [34] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhamed, I.; Chowdhury, F.; Maruthamuthu, V. Biophysical Tools to Study Cellular Mechanotransduction. Bioengineering 2017, 4, 12. https://doi.org/10.3390/bioengineering4010012
Muhamed I, Chowdhury F, Maruthamuthu V. Biophysical Tools to Study Cellular Mechanotransduction. Bioengineering. 2017; 4(1):12. https://doi.org/10.3390/bioengineering4010012
Chicago/Turabian StyleMuhamed, Ismaeel, Farhan Chowdhury, and Venkat Maruthamuthu. 2017. "Biophysical Tools to Study Cellular Mechanotransduction" Bioengineering 4, no. 1: 12. https://doi.org/10.3390/bioengineering4010012
APA StyleMuhamed, I., Chowdhury, F., & Maruthamuthu, V. (2017). Biophysical Tools to Study Cellular Mechanotransduction. Bioengineering, 4(1), 12. https://doi.org/10.3390/bioengineering4010012






