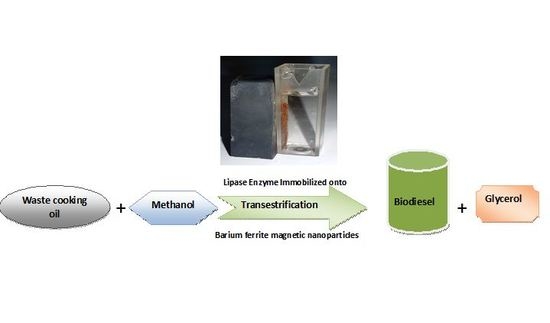
Biodiesel Production by Aspergillus niger Lipase Immobilized on Barium Ferrite Magnetic Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Lipase Production and Purification
2.3. Synthesis of Hexagonal Magnetic Barium Ferrite Nanoparticles (BNF)
2.4. Immobilization of Lipase on BFN Particles
2.5. Assay of Enzyme Activity
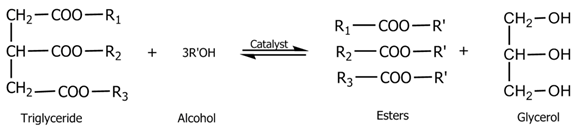
2.6. Biodiesel Production Using the Transestrification Process
2.6.1. Gas Chromatography Analysis of WCO (Waste Cooking Oil)
2.6.2. Fatty Acid Methyl Esters (FAMEs) Analysis (Biodiesel Products)
2.7. Analytical Methods
2.8. Statistical Analysis
3. Results and Discussion
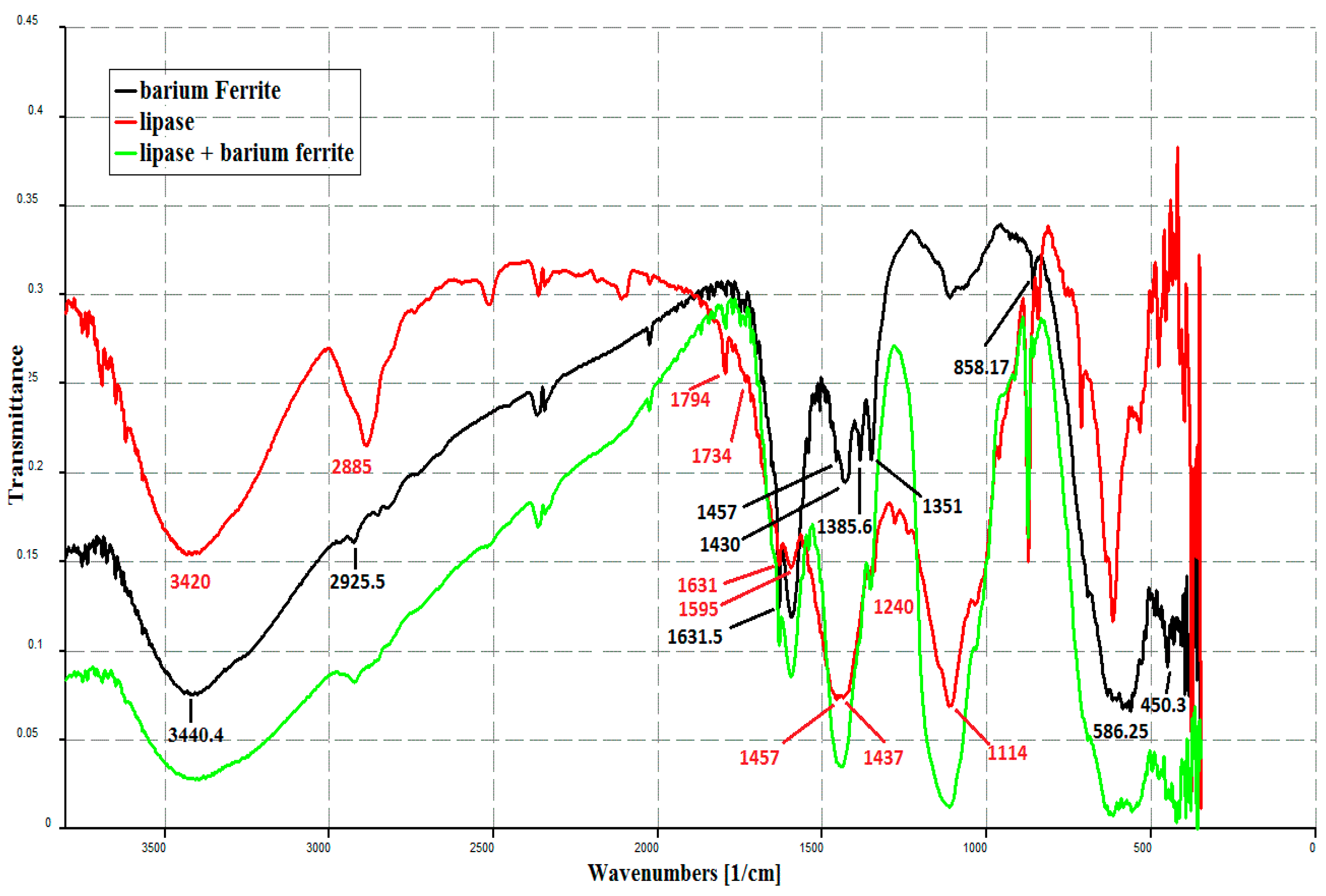
3.1. Characterization of BFN Particles
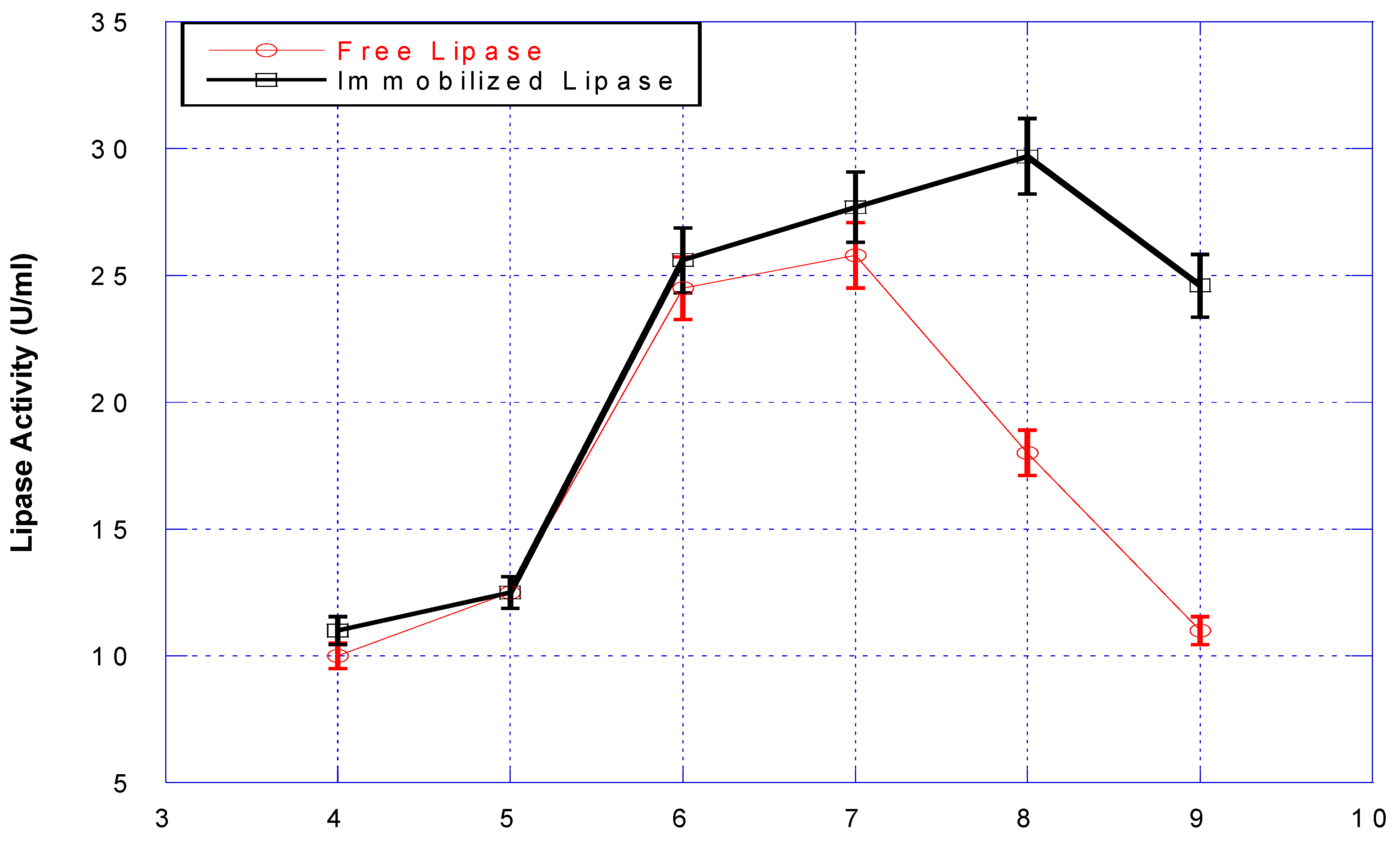
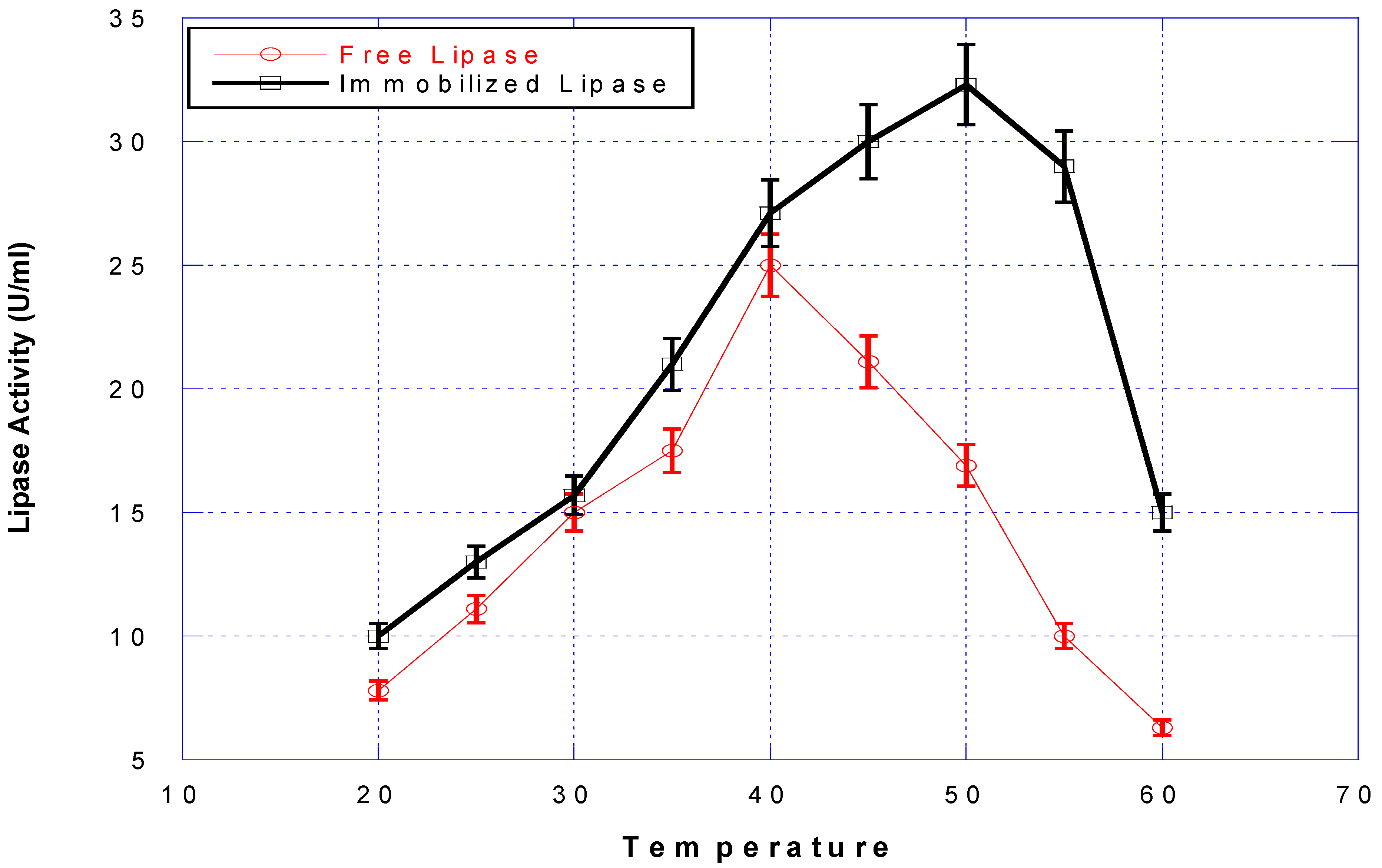
3.2. The Activity of the Lipase Immobilized on BFN Particles
3.3. Recyclability of BFN-Lipase
3.4. Production of Biodiesel Using the Transesterification Process
3.4.1. Effect of Methanol/Waste Cooking Oil Molar Ratio on Biodiesel Production
3.4.2. Effect of Enzyme Concentration Percentage on Biodiesel Production
3.4.3. Effect of Reaction Temperature on Biodiesel Production
3.4.4. Effect of the Reaction Time on Biodiesel Production
3.4.5. Effect of Shaking Speed on Biodiesel Production
3.5. Physicochemical Properties of the Used Cooking Oil and Its Corresponding Production of Biodiesel
3.5.1. Kinematic Viscosity
3.5.2. Density at 15 °C
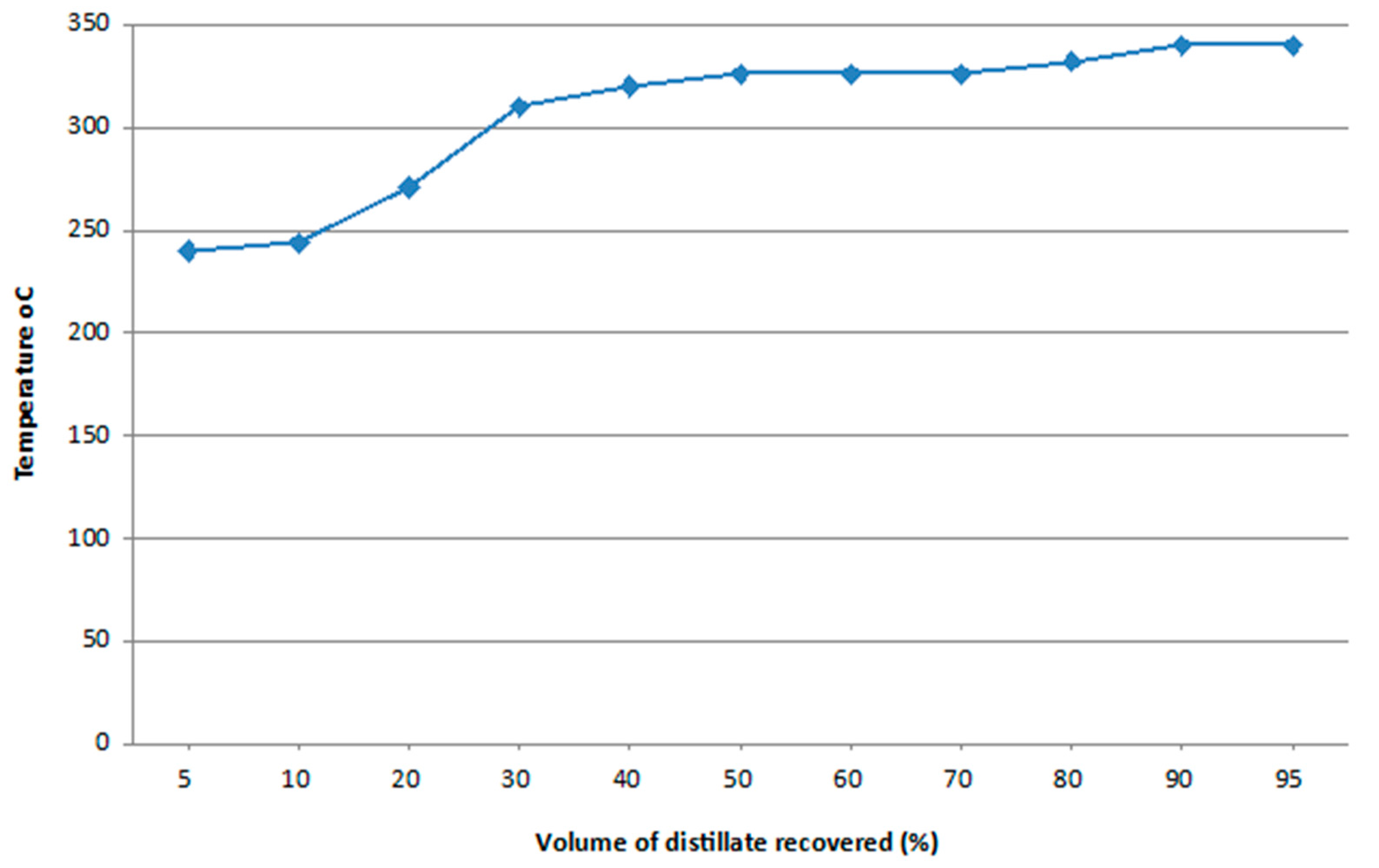
3.5.3. Distillation at Atmospheric Pressure
3.5.4. Flash Point (FP)
3.5.5. Heat of Combustion/Calorific Value
3.5.6. The Cetane Number
3.5.7. Cloud Point (CP) and Pour Point
3.5.8. Water and Sulfur Content
3.5.9. Iodine Number
3.5.10. Acid Number
3.5.11. Saponification Value (mg KOH/g Oil)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, C.Y.; Huang, L.Y.; Kuan, I.; Lee, S.L. Optimized Production of Biodiesel from Waste Cooking Oil by Lipase Immobilized on Magnetic Nanoparticles. Int. J. Mol. Sci. 2013, 14, 24074–24086. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Biodiesel production via non-catalytic SCF method and biodiesel fuel characteristics. Energy Convers. Manag. 2006, 47, 2271–2282. [Google Scholar] [CrossRef]
- Bala, B.K. Studies on biodiesels from transformation of vegetable oils for diesel engines. Energy Educ. Sci. Technol. 2005, 15, 1–45. [Google Scholar]
- Pighinelli, A.L.; Ferrari, R.A.; Miguel, A.M.; Park, K.J. High oleic sunflower biodiesel: Quality control and different purification methods. Grasas Aceites 2011, 62, 171–180. [Google Scholar]
- Pogaku, R.; Raman, J.K.; Ravikumar, G. Evaluation of Activation Energy and Thermodynamic Properties of Enzyme-Catalysed Transesterification Reactions. Adv. Chem. Eng. Sci. 2012, 2, 150–154. [Google Scholar] [CrossRef]
- Ahmia, A.; Danane, F.; Bessah, R.; Boumesbah, I. Raw material for biodiesel production. Valorization of used edible oil. Rev. Energies Renouv. 2014, 17, 335–343. [Google Scholar]
- Gupta, R.; Kumari, A.; Syal, P.; Singh, Y. Molecular and functional diversity of yeast and fungal lipases: Their role in biotechnology and cellular physiology. Prog. Lipid Res. 2015, 57, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Karimi, A.; Hejazi, M.A. Use of mesoporous MnO2 as a support for immobilization of lipase from Candida rugosa. Chem. Ind. Chem. Eng. Q. 2014, 20, 371–378. [Google Scholar] [CrossRef]
- Villeneuve, P.; Muderhwa, J.M.; Graille, J.; Haas, M.J. Customizing lipases for biocatalysis: A survey of chemical, physical and molecular biological approaches. J. Mol. Catal. B Enzym. 2000, 9, 113–148. [Google Scholar] [CrossRef]
- Metin, A.Ü. Immobilization studies and biochemical properties of free and immobilized Candida rugosa lipase onto hydrophobic group carrying polymeric support. Macromol. Res. 2013, 21, 176–183. [Google Scholar] [CrossRef]
- Mendes, A.A.; Castro, H.F.D.; Pereira, E.B.; Furigo Júnio, F. Application of lipases for wastewater treatment containing high levels of lipids. Quím. Nova 2005, 28, 296–305. [Google Scholar] [CrossRef]
- Turinayo, Y.K.; Kalanzi, F.; Mudoma, J.M.; Kiwuso, P.; Asiimwe, G.M.; Esegu, J.P.O.; Balitta, P.; Mwanja, C. Physicochemical Characterization of Jatropha curcas Linn Oil for Biodiesel Production in Nebbi and Mokono Districts in Uganda. J. Sustain. Bioenergy Syst. 2015, 5, 104–113. [Google Scholar] [CrossRef]
- Cesarini, S.; Infanzón, B.; Pastor, F.I.; Diza, P. Fast and economic immobilization methods described for non-commercial Pseudomonas lipases. BMC Biotechnol. 2014, 14, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- El-Hadi, A.A.; Saleh, H.I.; Moustafa, S.A.; Ahmed, H.M. Immobilization of Mucor racemosus NRRL 3631 lipase and characterization of silica-coated magnetite (Fe3O4) nanoparticles. Egypt. Pharm. J. 2013, 12, 28–35. [Google Scholar]
- El-Batal, A.I.; Farrag Ahmed, A.; Elsayed, M.A.; Soltan, A.M.; El-Khawaga, A.M. Enhancement of Lipase Biosynthesis by Aspergillus nigerADM110 using Gamma Radiation. Egypt. J. Med. Microbiol. 2015, 24, 87–94. [Google Scholar]
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of iron magnetic nanoparticles in protein immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, D.; Rajakumar, S.; Kumar, A. Influence of fuel ratios on auto combustion synthesis of barium ferrite nano particles. J. Chem. Sci. 2006, 118, 15–21. [Google Scholar] [CrossRef]
- Abedini Khorrami, S.; Islampour, R.; Bakhtiari, H.; Sadr, M.N.Q. The effect of molar ratio on structural and magnetic properties of BaFe12O19 nanoparticles prepared by sol-gel auto-combustion method. Int. J. Nano Dimens. 2013, 3, 191–197. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Boil. Chem. 1951, 193, 265–275. [Google Scholar]
- Pencreac’h, G.; Baratti, J.C. Hydrolysis of p-nitrophenyl palmitate in n-heptane by the Pseudomonas cepacia lipase: A simple test for the determination of lipase activity in organic media. Enzym. Microb. Technol. 1996, 18, 417–422. [Google Scholar] [CrossRef]
- Liu, X.; Guan, Y.; Shen, R.; Liu, H. Immobilization of lipase onto micron-size magnetic beads. J. Chromatogr. B 2005, 822, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-Q.; Li, Q.; Yin, J.-Z.; Guo, D.; Qiao, B.-Q. Continuous production of biodiesel from soybean flakes by extraction coupling with transesterification under supercritical conditions. Fuel Process. Technol. 2016, 144, 37–41. [Google Scholar] [CrossRef]
- Milina, R.; Mustafa, Z. Gas chromatographic investigations of compositional profiles of biodiesel from different origin. Pet. Coal 2013, 55, 12–19. [Google Scholar]
- Snedecor, G.; Cochran, W. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Kaur, T.; Srivastava, A. Effect of pH on Magnetic Properties of Doped Barium Hexaferrite. Int. J. Res. Mech. Eng. Technol. 2013, 3, 171–173. [Google Scholar]
- Chauhan, C.; Jotania, R.; Jotania, K. Structural properties of cobalt substituted barium hexaferrite nanoparticles prepared by a thermal treatment method. J. Nanosyst. Phys. Chem. Math. 2013, 4, 363–369. [Google Scholar]
- Wang, J.; Meng, G.; Tao, K.; Feng, M.; Zhao, X.; Li, Z.; Xi, H.; Xia, D.; Lu, J.R. Immobilization of lipases on alkyl silane modified magnetic nanoparticles: Effect of alkyl chain length on enzyme activity. PLoS ONE 2012, 7, e43478. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.; Goyal, S.; Chauhan, C. Novel approach of alkaline protease mediated biodegradation analysis of mustard oil driven emulsified bovine serum albumin nanospheres for controlled release of entrapped Pennisetum glaucum (Pearl Millet) amylase. Am. J. Adv. Drug Deliv. 2015, 3, 135–148. [Google Scholar]
- Lee, D.-G.; Ponvel, K.M.; Kim, M.; Hwang, S.; Ahn, I.S.; Lee, C.H. Immobilization of lipase on hydrophobic nano-sized magnetite particles. J. Mol. Catal. B Enzym. 2009, 57, 62–66. [Google Scholar] [CrossRef]
- Ting, W.-J.; Tung, K.-Y.; Giridhar, R.; Wu, W.T. Application of binary immobilized Candida rugosa lipase for hydrolysis of soybean oil. J. Mol. Catal. B Enzym. 2006, 42, 32–38. [Google Scholar] [CrossRef]
- Ferrario, V.; Veny, H.; De Angelis, E.; Navarini, L.; Ebert, C.; Gardossi, L. Lipases immobilization for effective synthesis of biodiesel starting from coffee waste oils. Biomolecules 2013, 3, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Koria, L.; Nithya, G. Analysis of Datura stramonium Linn. biodiesel by gas chromatography-mass spectrometry (gc-ms) and influence of fatty acid composition on the fuel related characteristics. J. Phytol. 2012, 4, 6–9. [Google Scholar]
- Felizardo, P.; Correia, M.J.N.; Raposo, I.; Mendes, J.F.; Rui, B.; Bordado, J.M. Production of biodiesel from waste frying oils. Waste Manag. 2006, 26, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, A.B.; Watts, K.C.; Islam, M.R. Waste cooking oil as an alternate feedstock for biodiesel production. Energies 2008, 1, 3–18. [Google Scholar] [CrossRef]
- Hilber, T.; Ahn, E.; Mittelbach, M.; Schmitd, E. Animal fats perform well in biodiesel. Render Magazine, February 2006; 16–18. [Google Scholar]
- Shrestha, D.; Van Gerpen, J.; Thompson, J. Effectiveness of cold flow additives on various biodiesels, diesel, and their blends. Trans. ASABE 2008, 51, 1365–1370. [Google Scholar] [CrossRef]
- Dunn, R.O. Cold flow properties of biodiesel: A guide to getting an accurate analysis. Biofuels 2015, 6, 115–128. [Google Scholar] [CrossRef]
- Ibiari, N.N.; El-Enin, S.A.A.; Attia, N.K.; El-Diwani, G. Ultrasonic comparative assessment for biodiesel production from rapeseed. J. Am. Sci. 2010, 6, 937–943. [Google Scholar]
- Brown, E.K.; Palmquist, M.; Luning Prak, D.J.; Mueller, S.S.; Bowen, L.L.; Sweely, K.; Ruiz, O.N.; Trulove, P.C. Interaction of Selected Fuels with Water: Impact on Physical Properties and Microbial Growth. J. Pet. Environ. Biotechnol. 2015, 6. [Google Scholar] [CrossRef]
- Cancela, Á.; Maceiras, R.; Alfonsín, V.; Sánchez, Á. Transesterification of waste frying oil under ultrasonic irradiation. Eur. J. Sustain. Dev. 2015, 4, 401–406. [Google Scholar] [CrossRef]
- Akanawa, T.T.; Moges, H.G.; Babu, R.; Bisrat, D.; Akanawa, T.T.; Babu, R.; Bisrat, D. Castor Seed from Melkasa Agricultural Research Centre, East Showa, Ethiopia and it’s biodiesel performance in Four Stroke Diesel Engine. Int. J. Renew. Energy Dev. 2014, 3, 99–105. [Google Scholar]
| Initial Lipase Concentration (mg/mL) | Activity of Immobilized Lipase (U/mL) | Protein Immobilized (mg/g Support) | Specific Activity (U/mg Protein) |
|---|---|---|---|
| 00.2 | 4.8a ± 0.56 | 9.0a ± 0.70 | 6.15a ± 0.10 |
| 00.4 | 12.5b ± 0.35 | 15.7b ± 0.49 | 7.30b ± 0.21 |
| 00.6 | 23.9c ± 0.63 | 24.8c ± 0.42 | 8.82c ± 0.57 |
| 00.8 | 27.0d ± 0.56 | 36.5d ± 1.06 | 9.44de ± 0.39 |
| 01.0 | 30.5f ± 0.77 | 49.0e ± 0.91 | 10.10e ± 0.61 |
| 01.4 | 28.6e ± 0.77 | 57.8g ± 0.56 | 8.92cd ± 0.26 |
| 01.8 | 28.4e ± 0.98 | 52.6f ± 0.91 | 9.75de ± 0.53 |
| 02.0 | 28.5e ± 0.35 | 52.5f ± 0.56 | 9.60de ± 0.35 |
| LSD | 01.70 | 03.25 | 1.01 |
| Fatty Acids | Formula | Common Acronym | Methyl Esters | % Composition by Mass |
|---|---|---|---|---|
| Oleic acid | C17H33COOH | C18: 0 | Methyl oleate | 46.5d ± 0.91 |
| Palmitic acid | C15H31COOH | C16: 0 | Methyl palmitate | 30.9c ± 0.77 |
| Stearic acid | C17H35COOH | C18: 0 | Methyl strearate | 09.0b ± 0.56 |
| Linoleic acid | C17H31COOH | C18: 2 | Methyl linoleate | 08.5b ± 0.35 |
| Linolenic acid | C17H29COOH | C18: 3 | Methyl linolenate | 05.1a ± 0.28 |
| LSD | 02.95 |
| Factors Affecting Biodiesel Production | Biodiesel Yield (%) | |
|---|---|---|
| Methanol/oil ratio (mole/mole) | 1:1 | 42a ± 1.41 |
| 2:1 | 65b ± 2.12 | |
| 3:1 | 87d ± 0.707 | |
| 4:1 | 90e ± 1.76 | |
| 5:1 | 76c ± 0.707 | |
| LSD | 03.75 | |
| Enzyme concentration (%) | 3 | 69a ± 0.707 |
| 5 | 89b ± 1.06 | |
| 10 | 91bc ± 1.41 | |
| 15 | 94c ± 0.707 | |
| 20 | 97d ± 1.909 | |
| LSD | 02.25 | |
| Reaction temperature (°C) | 15 | 55a ± 0.494 |
| 25 | 73b ± 1.41 | |
| 35 | 81c ± 0.636 | |
| 45 | 91d ± 1.41 | |
| 55 | 75b ± 0.707 | |
| LSD | 05.95 | |
| Reaction time (h) | 2 | 50a ± 0.707 |
| 4 | 88d ± 1.06 | |
| 6 | 88d ± 1.41 | |
| 8 | 61c ± 0.848 | |
| 10 | 58b ± 0.707 | |
| LSD | 03.10 | |
| Shaking speed (rpm) | 100 | 43a ± 1.41 |
| 200 | 52b ± 1.48 | |
| 300 | 71c ± 1.41 | |
| 400 | 88d ± 0.636 | |
| 500 | 69c ± 1.13 | |
| LSD | 09.05 | |
| No. | Characteristics | Result | Unit | Test Method |
|---|---|---|---|---|
| 1 | Kinematic viscosity at 40 °C | 5.83 | mm2·s−1 | ASTM D445 |
| 2 | Density at 15.5 °C | 0.850 | g·cm−3 | ASTM D1298 |
| 3 | Calorific value | 43.1 | MJ/Kg | ASTM D-224 |
| 4 | Total sulfur content | 0.050 | mass% | ASTM D4294 |
| 5 | Flash point | 188 | °C | ASTM D92 |
| 6 | Pour point | −9 | °C | ASTM D97 |
| 7 | Cloud point | −3 | °C | ASTM D2500 |
| 8 | Cetane number | 59.5 | — | ASTM D613 |
| 9 | Water content | 0.091 | vol% | ASTM D6304 |
| 10 | Acid number | 0.182 | mg KOH g−1 | ASTM D664 |
| 11 | Distillation temperature (DT) | 95% Recovery at 340 | °C | ASTM D86 |
| 12 | Iodine number | 102 | mg I2/100 g oil | ASTM D4737 |
| 13 | Saponification value | 206 | mg KOH/g oil | ASTM D 5558 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Batal, A.I.; Farrag, A.A.; Elsayed, M.A.; El-Khawaga, A.M. Biodiesel Production by Aspergillus niger Lipase Immobilized on Barium Ferrite Magnetic Nanoparticles. Bioengineering 2016, 3, 14. https://doi.org/10.3390/bioengineering3020014
El-Batal AI, Farrag AA, Elsayed MA, El-Khawaga AM. Biodiesel Production by Aspergillus niger Lipase Immobilized on Barium Ferrite Magnetic Nanoparticles. Bioengineering. 2016; 3(2):14. https://doi.org/10.3390/bioengineering3020014
Chicago/Turabian StyleEl-Batal, Ahmed I., Ayman A. Farrag, Mohamed A. Elsayed, and Ahmed M. El-Khawaga. 2016. "Biodiesel Production by Aspergillus niger Lipase Immobilized on Barium Ferrite Magnetic Nanoparticles" Bioengineering 3, no. 2: 14. https://doi.org/10.3390/bioengineering3020014
APA StyleEl-Batal, A. I., Farrag, A. A., Elsayed, M. A., & El-Khawaga, A. M. (2016). Biodiesel Production by Aspergillus niger Lipase Immobilized on Barium Ferrite Magnetic Nanoparticles. Bioengineering, 3(2), 14. https://doi.org/10.3390/bioengineering3020014