Label Free Detection of L-Glutamate Using Microfluidic Based Thermal Biosensor
Abstract
:1. Introduction
1.1. L-Glutamate Sensors: State of the Art and Limitations
1.2. Calorimetric Biosensing
2. Experimental
2.1. Materials and Chemicals
2.2. Device Fabrication
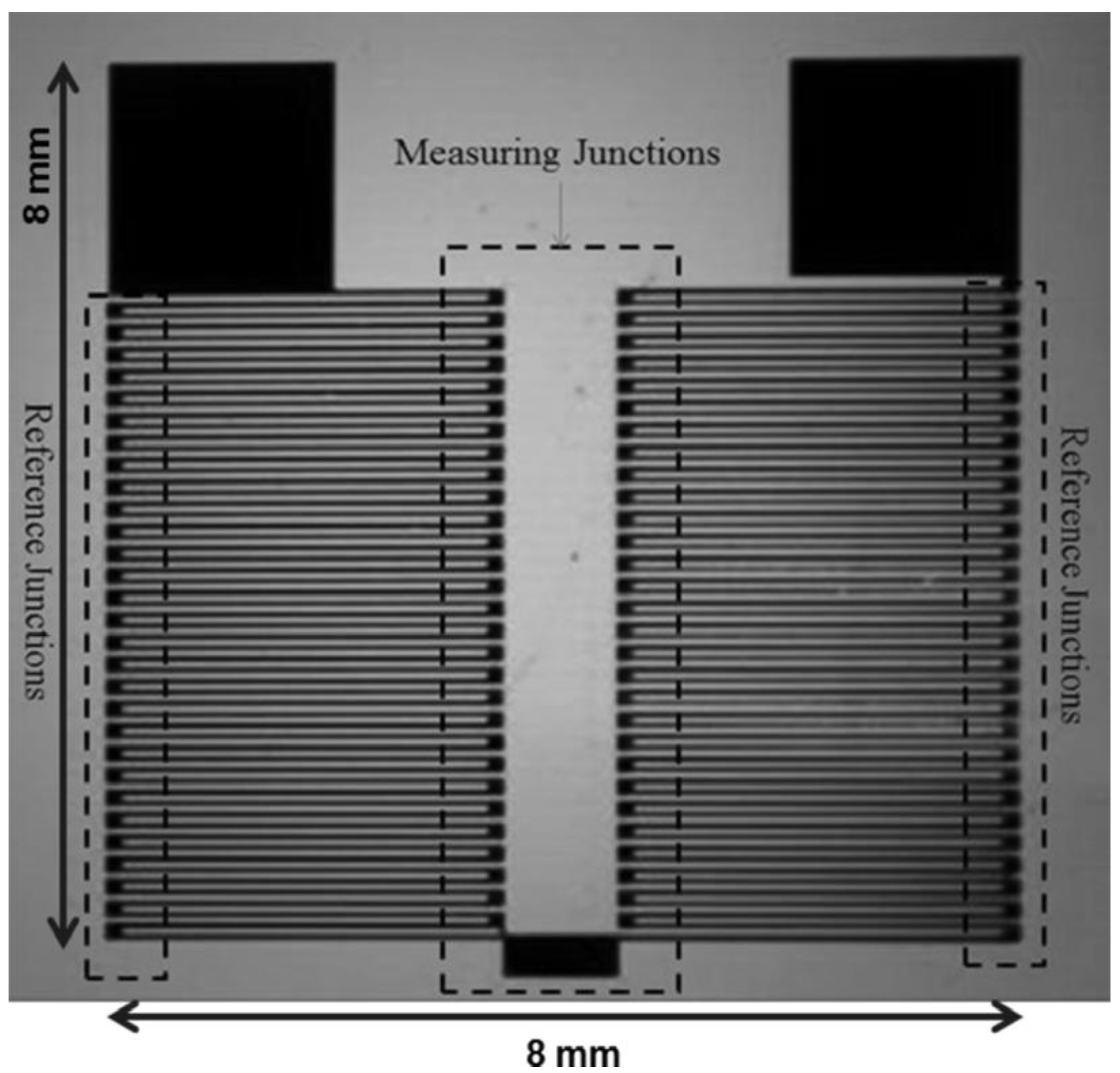
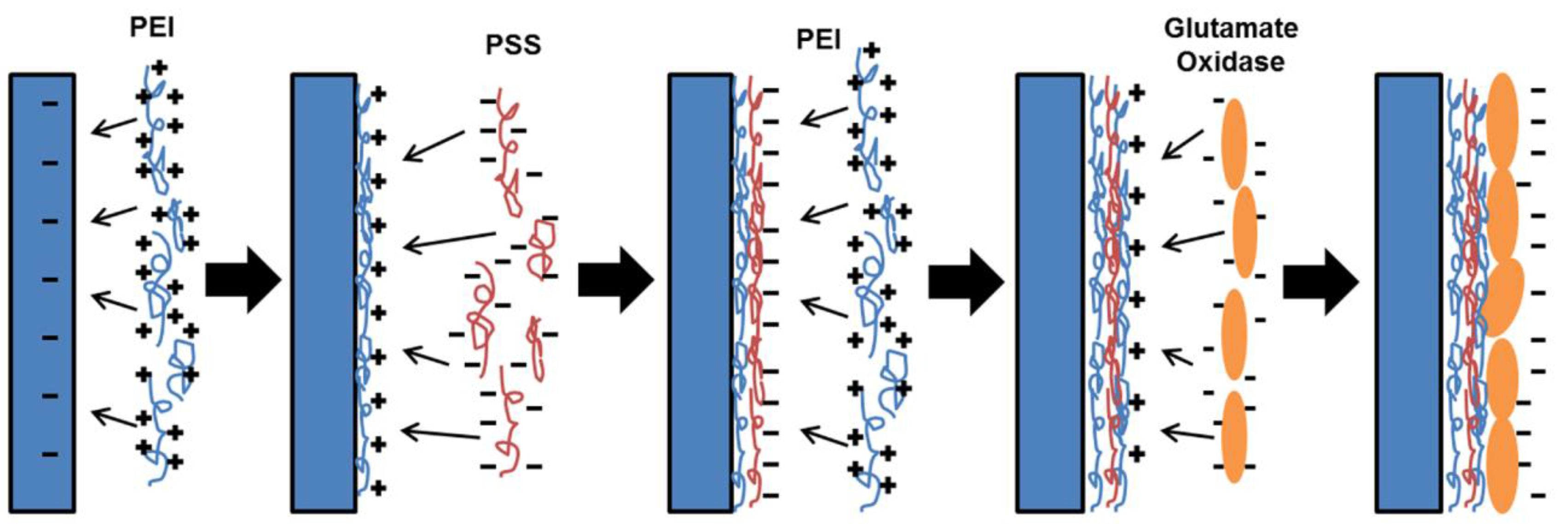
2.3. Enzyme Immobilization
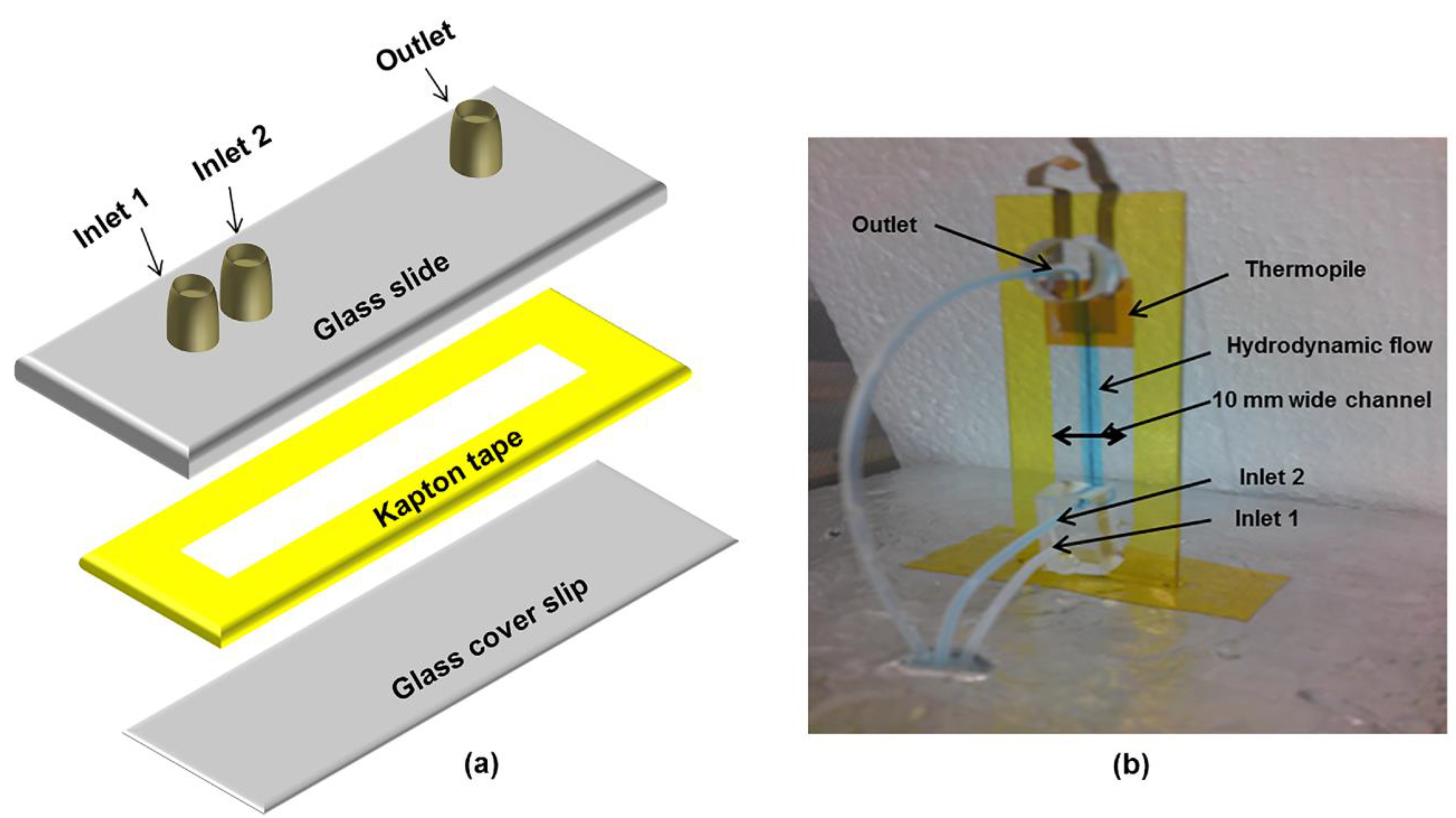
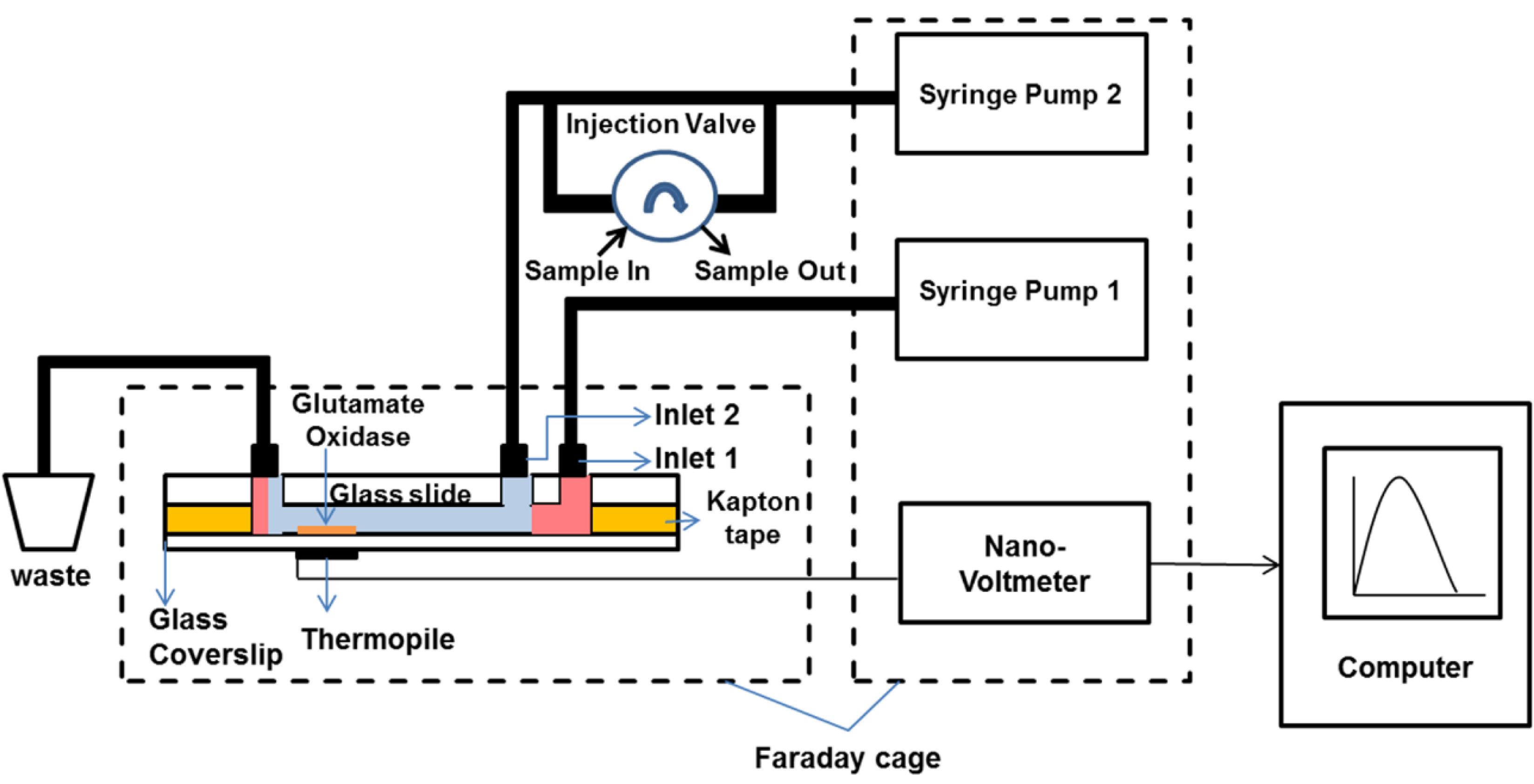
2.4. Measurement System
3. Results and Discussion
3.1. Activity and Storage Stability of Immobilized GLOD
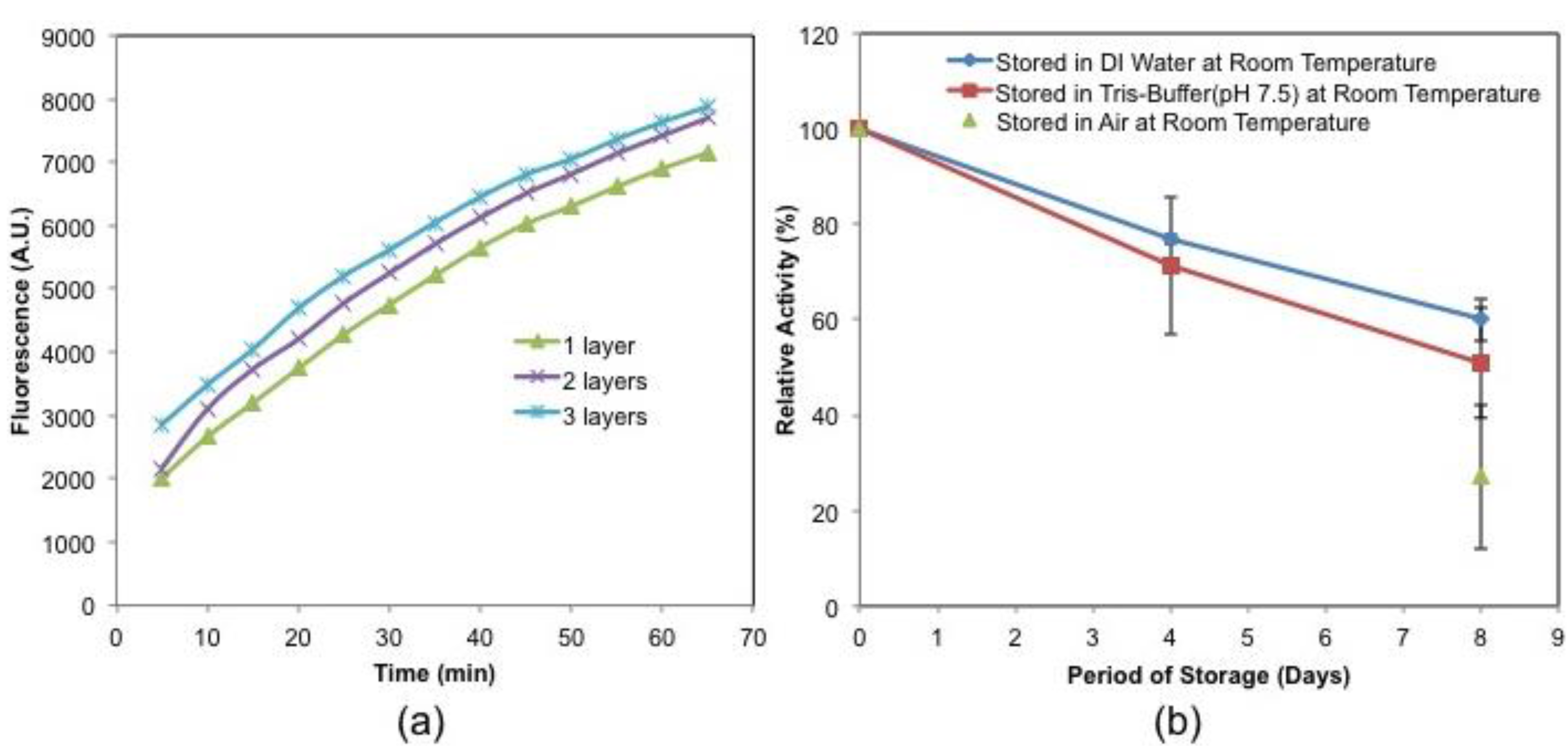
3.2. L-Glutamate Detection
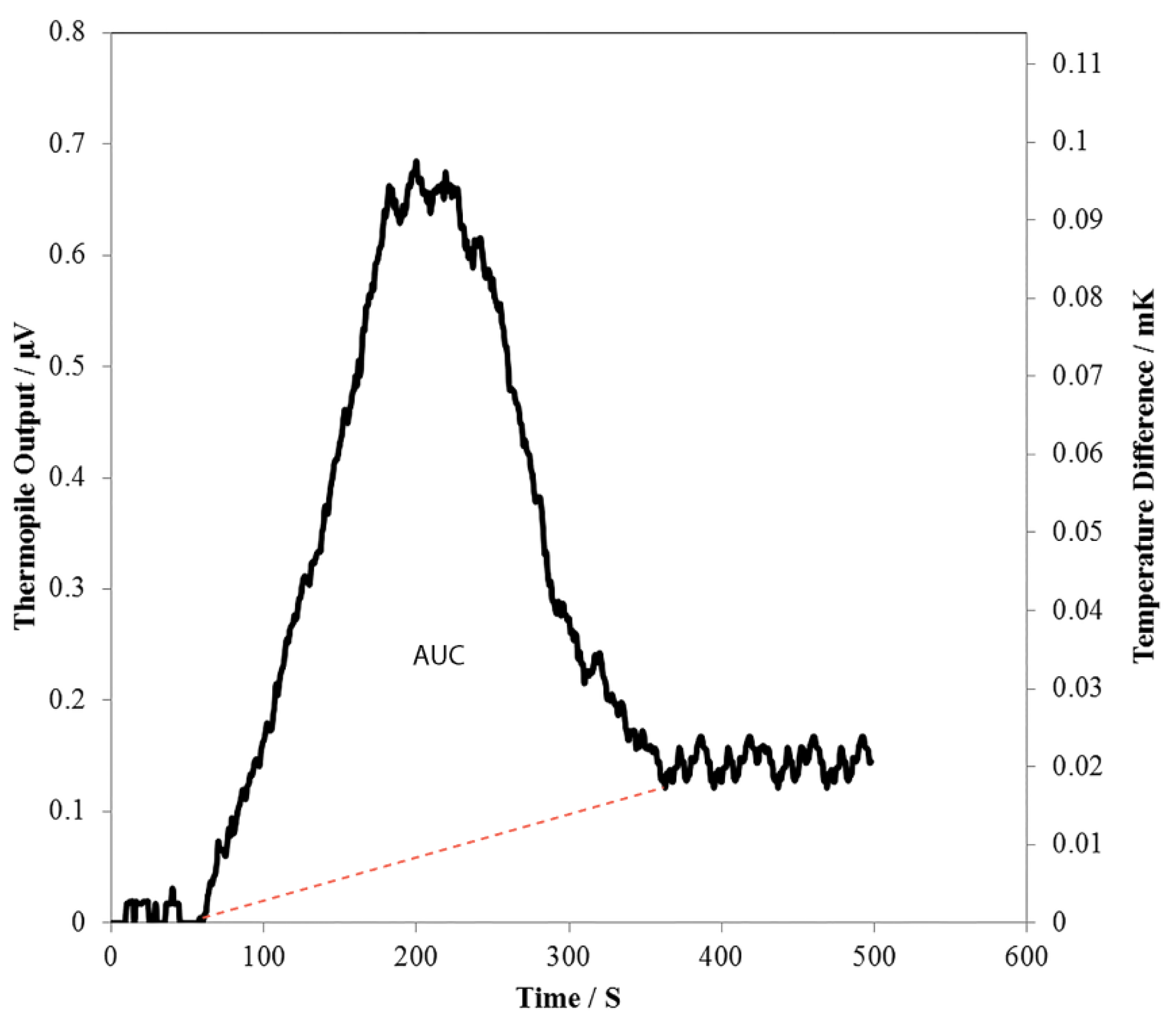
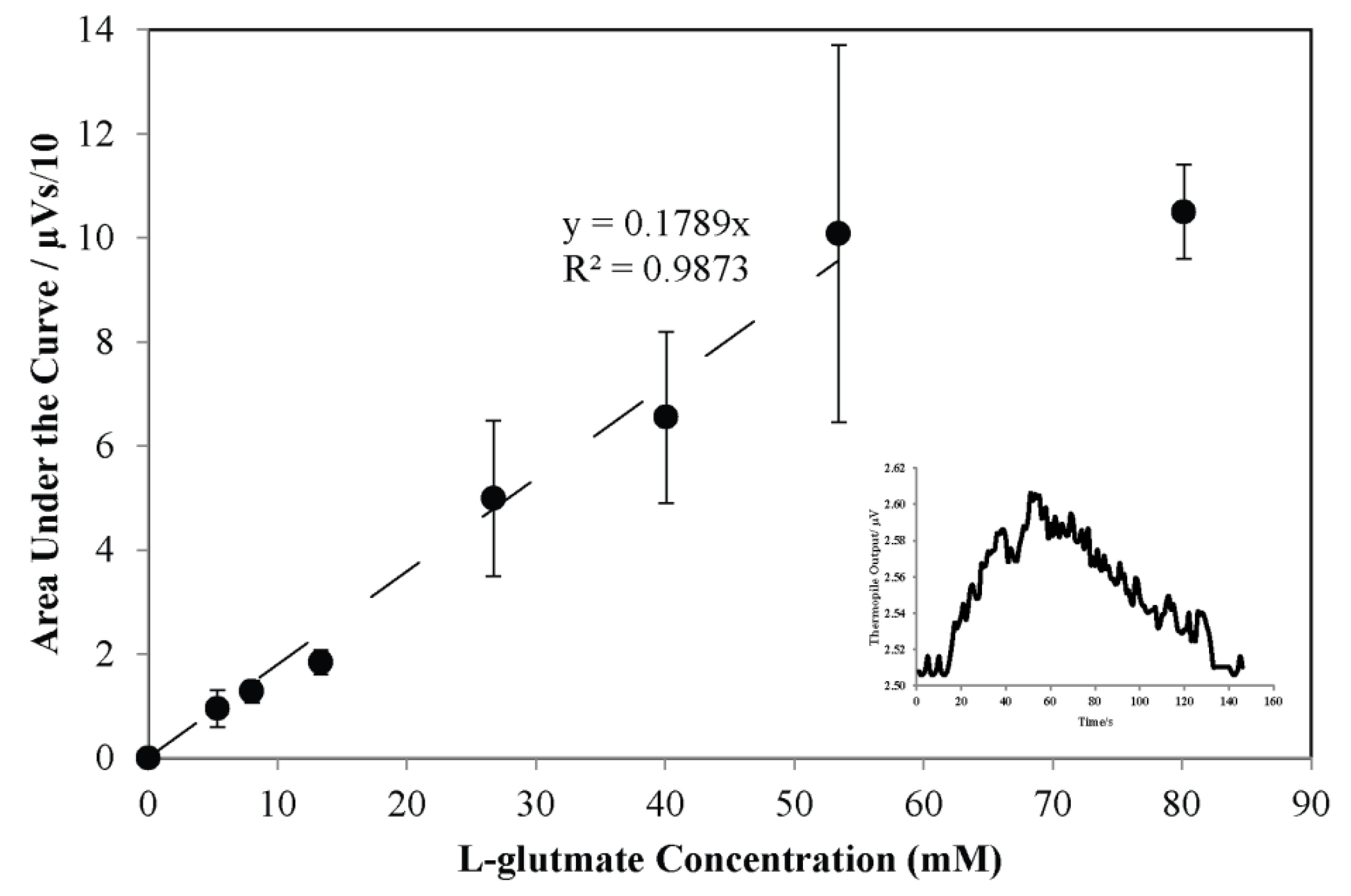
3.3. Potential Uses of the Thermoelectric L-Glutamate Sensor

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R. The molecular biology of memory storage: A dialogue between genes and synapses. Science 2001, 294, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Hamberger, A.; Berthold, C.-H.; Karlsson, B.; Lehmann, A.; Nystrom, B. Extracellular GABA, glutamate and glutamine in vivo perfusion-dialysis of rabbit hippocampus. Neurol. Neurobiol. 1983, 7, 473–492. [Google Scholar]
- Hamberger, A.; NystrÃm, B. Extra-and intracellular amino acids in the hippocampus during development of hepatic encephalopathy. Neurochem. Res. 1984, 9, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Isacsson, H.; Hamberger, A. Effects of in vivo administration of kainic acid on the extracellular amino acid pool in the rabbit hippocampus. J. Neurochem. 1983, 40, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Takano, T.; Hansen, A.J. Opinion: Beyond the role of glutamate as a neurotransmitter. Nat. Rev. Neurosci. 2002, 3, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Niwa, O.; Horiuchi, T.; Kurita, R.; Tabei, H.; Torimitsu, K. Microfabricated on-line sensor for continuous monitoring of L-glutamate. Anal. Sci. 1998, 14, 947–953. [Google Scholar] [CrossRef]
- Niwa, O.; Kurita, R.; Horiuchi, T.; Torimitsu, K. Continuous monitoring of L-glutamate released from cultured rat nerve cells with a microfabricated on-line sensor at a slow flow rate. Electroanalysis 1999, 11, 356–361. [Google Scholar] [CrossRef]
- Niwa, O.; Horiuchi, T.; Torimitsu, K. Continuous monitoring of L-glutamate released from cultured nerve cells by an online sensor coupled with micro-capillary sampling. Biosens. Bioelectron. 1997, 12, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Niwa, O.; Torimitsu, K.; Morita, M.; Osborne, P.; Yamamoto, K. Concentration of extracellular L-glutamate released from cultured nerve cells measured with a small-volume online sensor. Anal. Chem. 1996, 68, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Blöchl, A.; Dennison, S.; Schuhmann, W.; Csöregi, E. Glutamate detection from nerve cells using a planar electrodes array integrated in a microtiter plate. Biosens. Bioelectron. 2005, 20, 2116–2119. [Google Scholar] [CrossRef] [PubMed]
- Pasco, N.; Jeffries, C.; Davies, Q.; Downard, A.J.; Roddick-Lanzilotta, A.D.; Gorton, L. Characterisation of a thermophilic L-glutamate dehydrogenase biosensor for amperometric determination of L-glutamate by flow injection analysis. Biosens. Bioelectron. 1999, 14, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.K.; Chattopadhyay, P.; Roychudhuri, U.; Chakraborty, R. A biosensor based on co-immobilized L-glutamate oxidase and L-glutamate dehydrogenase for analysis of monosodium glutamate in food. Biosens. Bioelectron. 2006, 21, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.T.C.; Yao, J.; Chan, W.-C. Selective enzyme immobilization on arrayed microelectrodes for the application of sensing neurotransmitters. Biochem. Eng. J. 2013, 78, 146–153. [Google Scholar] [CrossRef]
- Tseng, T.T.C.; Monbouquette, H.G. Implantable microprobe with arrayed microsensors for combined amperometric monitoring of the neurotransmitters, glutamate and dopamine. J. Electroanal. Chem. 2012, 682, 141–146. [Google Scholar] [CrossRef]
- Hu, Y.; Mitchell, K.M.; Albahadily, F.N.; Michaelis, E.K.; Wilson, G.S. Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 1994, 659, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Day, B.K.; Pomerleau, F.; Burmeister, J.J.; Huettl, P.; Gerhardt, G.A. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J. Neurochem. 2006, 96, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Kulagina, N.V.; Shankar, L.; Michael, A.C. Monitoring glutamate and ascorbate in the extracellular space of brain tissue with electrochemical microsensors. Anal. Chem. 1999, 71, 5093–5100. [Google Scholar] [CrossRef] [PubMed]
- Wassum, K.M.; Tolosa, V.M.; Tseng, T.C.; Balleine, B.W.; Monbouquette, H.G.; Maidment, N.T. Transient extracellular glutamate events in the basolateral amygdala track reward-seeking actions. J. Neurosci. 2012, 32, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Abe, C.; Awazu, C.; Tanaka, K. Long-term hypergravity induces plastic alterations in vestibulo-cardiovascular reflex in conscious rats. Neurosci. Lett. 2007, 412, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Cairns, B.E.; Dong, X.; Mann, M.K.; Svensson, P.; Sessle, B.J.; Arendt-Nielsen, L.; McErlane, K.M. Systemic administration of monosodium glutamate elevates intramuscular glutamate levels and sensitizes rat masseter muscle afferent fibers. Pain 2007, 132, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Blaha, C.D. Apparatus and Method for Modulating Neurochemical Levels in the Brain. U.S. Patent WO/2006/041871; IPC: A61N 1/36 (2006.01), 2006. [Google Scholar]
- Tseng, T.T.C.; Chang, C.-F.; Chan, W.-C. Fabrication of implantable, enzyme-immobilized glutamate sensors for the monitoring of glutamate concentration changes in vitro and in vivo. Molecules 2014, 19, 7341–7355. [Google Scholar] [CrossRef] [PubMed]
- Doong, R.-A.; Shih, H.-M. Glutamate optical biosensor based on the immobilization of glutamate dehydrogenase in titanium dioxide sol-gel matrix. Biosens. Bioelectron. 2006, 22, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Laiwattanapaisal, W.; Yakovleva, J.; Bengtsson, M.; Laurell, T.; Wiyakrutta, S.; Meevootisom, V.; Chailapakul, O.; Emneus, J. On-chip microfluidic systems for determination of L-glutamate based on enzymatic recycling of substrate. Biomicrofluidics 2009, 3, 014104–014112. [Google Scholar] [CrossRef]
- Ligler, F.S.; Rowe Taitt, C.A. Optical Biosensors—Present & Future; Elsevier: Amsterdam, The Netherlands, 2002; p. 646. [Google Scholar]
- Qin, S.; van der Zeyden, M.; Oldenziel, W.; Cremers, T.; Westerink, B. Microsensors for in vivo measurement of glutamate in brain tissue. Sensors 2008, 8, 6860–6884. [Google Scholar] [CrossRef]
- Maalouf, R.; Chebib, H.; Saïkali, Y.; Vittori, O.; Sigaud, M.; Jaffrezic-Renault, N. Amperometric and impedimetric characterization of a glutamate biosensor based on Nafion® and a methyl viologen modified glassy carbon electrode. Biosens. Bioelectron. 2007, 22, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Muehlbauer, M.J.; Guilbeau, E.J.; Towe, B.C.; Brandon, T.A. Thermoelectric enzyme sensor for measuring blood glucose. Biosens. Bioelectron. 1990, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Muehlbauer, M.J.; Guilbeau, E.J.; Towe, B.C. Applications and stability of a thermoelectric enzyme sensor. Sens. Actuator B. Chem. 1990, 2, 223–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Tadigadapa, S. Calorimetric biosensors with integrated microfluidic channels. Biosens. Bioelectron. 2004, 19, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Fon, W.; Axelrod, B.W.; Roukes, M.L. High-sensitivity microfluidic calorimeters for biological and chemical applications. Proc. Nat. Acad. Sci. USA 2009, 106, 15225–15230. [Google Scholar] [CrossRef] [PubMed]
- Kopparthy, V.L.; Tangutooru, S.M.; Nestorova, G.G.; Guilbeau, E.J. Thermoelectric microfluidic sensor for bio-chemical applications. Sens. Actuator B. Chem. 2012, 166–167, 608–615. [Google Scholar]
- Lvov, Y.; Ariga, K.; Ichinose, I.; Kunitake, T. Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J. Am. Chem. Soc. 2002, 117, 6117–6123. [Google Scholar] [CrossRef]
- Miller, S.L.; Smith-Magowan, D. The thermodynamics of the Krebs cycle and related compounds. J. Phys. Chem. Ref. Data 1990, 19, 1049–1073. [Google Scholar] [CrossRef]
- Okumura, W.; Moridera, N.; Kanazawa, E.; Shoji, A.; Hirano-Iwata, A.; Sugawara, M. Visualizing L-glutamate fluxes in acute hippocampal slices with glutamate oxidase-immobilized coverslips. Anal. Biochem. 2009, 385, 326–333. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopparthy, V.L.; Tangutooru, S.M.; Guilbeau, E.J. Label Free Detection of L-Glutamate Using Microfluidic Based Thermal Biosensor. Bioengineering 2015, 2, 2-14. https://doi.org/10.3390/bioengineering2010002
Kopparthy VL, Tangutooru SM, Guilbeau EJ. Label Free Detection of L-Glutamate Using Microfluidic Based Thermal Biosensor. Bioengineering. 2015; 2(1):2-14. https://doi.org/10.3390/bioengineering2010002
Chicago/Turabian StyleKopparthy, Varun Lingaiah, Siva Mahesh Tangutooru, and Eric J. Guilbeau. 2015. "Label Free Detection of L-Glutamate Using Microfluidic Based Thermal Biosensor" Bioengineering 2, no. 1: 2-14. https://doi.org/10.3390/bioengineering2010002
APA StyleKopparthy, V. L., Tangutooru, S. M., & Guilbeau, E. J. (2015). Label Free Detection of L-Glutamate Using Microfluidic Based Thermal Biosensor. Bioengineering, 2(1), 2-14. https://doi.org/10.3390/bioengineering2010002




