Haptic Feedback Systems for Lower-Limb Prosthetic Applications: A Review of System Design, User Experience, and Clinical Insights
Abstract
1. Introduction
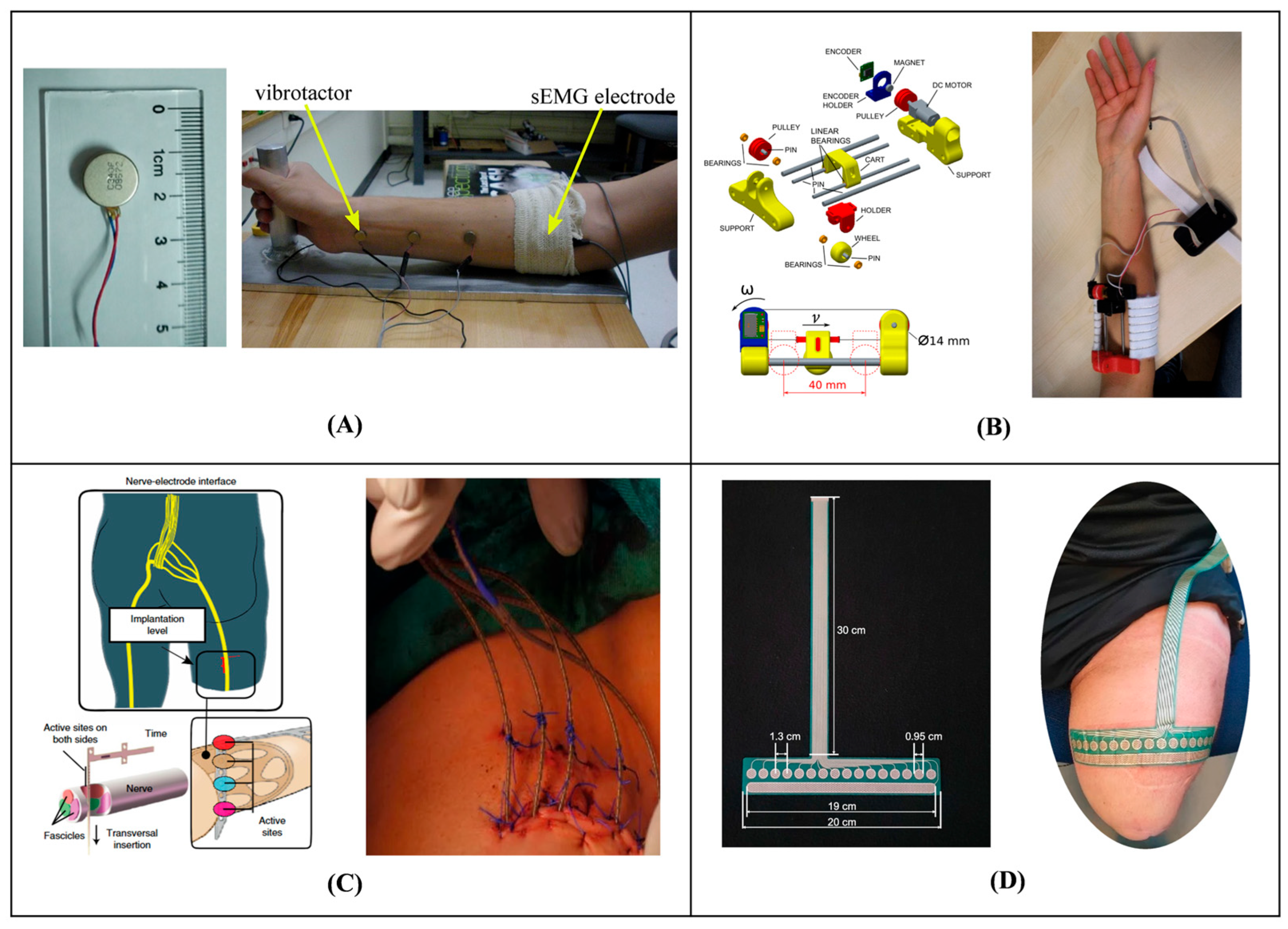
2. Materials and Methods
3. Clinical and Functional Outcomes
3.1. Gait Symmetry
3.2. Balance and Postural Stability
3.3. Spatial Awareness and Proprioception
3.4. Phantom Limb Pain
4. Issues Regarding the Design of Haptic Feedback Systems
4.1. Actuator Placement and Feedback Modality
4.2. Perceptual Illusions
4.3. Technical Parameters
4.4. Practical Considerations
5. Devices and User Experience
5.1. Training and User Adaptation
5.2. Usability
5.3. Cognitive Load
6. Discussion and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| sEMG | Surface Electromyography |
| AB | Able-Bodied |
| TFA | Transfemoral Amputee |
| TTA | Transtibial Amputee |
| TRA | Transradial Amputee |
| SSD | Sensory Substitution Device |
| IOI | Intensity Order Illusion |
| SUS | System Usability Scale |
| NASA-TLX | NASA Task Load Index |
| VAS | Visual Analog Scale |
| EEG | Electroencephalography |
| fNIRS | functional Near-Infrared Spectroscopy |
| IMU | Inertial Measurement Unit |
References
- Eakin, C.L.; Quesada, P.M.; Skinner, H. Lower-Limb Proprioception in above-Knee Amputees. Clin. Orthop. Relat. Res. 1992, 284, 239–246. [Google Scholar] [CrossRef]
- Buckley, J.G.; O’Driscoll, D.; Bennett, S.J. Postural sway and active balance performance in highly active lower-limb amputees. Am. J. Phys. Med. Rehabil. 2002, 81, 13–20. [Google Scholar] [CrossRef]
- Maqbool, H.F.; Mahmood, I.; Ali, A.; Iqbal, N.; Seong, J.T.; Dehghani-Sanij, A.A.; Alaziz, S.N.; Awad, M.I. Gait asymmetrical evaluation of lower limb amputees using wearable inertial sensors. Heliyon 2024, 10, e32207. [Google Scholar] [CrossRef]
- Nolan, L.; Wit, A.; Dudziñski, K.; Lees, A.; Lake, M.; Wychowañski, M. Adjustments in gait symmetry with walking speed in trans-femoral and trans-tibial amputees. Gait Posture 2003, 17, 142–151. [Google Scholar] [CrossRef]
- Carse, B.; Hebenton, J.; Brady, L.; Davie-Smith, F. Absent loading response knee flexion: The impact on gait kinetics and centre of mass motion in individuals with unilateral transfemoral amputation, and the effect of microprocessor controlled knee provision. Clin. Biomech. 2023, 108, 106061. [Google Scholar] [CrossRef] [PubMed]
- Sepp, L.A.; Baum, B.S.; Nelson-Wong, E.; Silverman, A.K. Hip Joint Contact Loading and Muscle Forces During Running With a Transtibial Amputation. ASME J. Biomech. Eng. 2021, 143, 031012. [Google Scholar] [CrossRef] [PubMed]
- Struyf, P.A.; van Heugten, C.M.; Hitters, M.W.; Smeets, R.J. The Prevalence of Osteoarthritis of the Intact Hip and Knee Among Traumatic Leg Amputees. Arch. Phys. Med. Rehabil. 2009, 90, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Norvell, D.C.; Czerniecki, J.M.; Reiber, G.E.; Maynard, C.; Pecoraro, J.A.; Weiss, N.S. The prevalence of knee pain and symptomatic knee osteoarthritis among veteran traumatic amputees and nonamputees. Arch. Phys. Med. Rehabil. 2005, 86, 487–493. [Google Scholar] [CrossRef]
- Gailey, R.; Allen, K.; Castles, J.; Kucharik, J.; Roeder, M. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. J. Rehabil. Res. Dev. 2008, 45, 15–29. [Google Scholar] [CrossRef]
- Bates, T.J.; Fergason, J.R.; Pierrie, S.N. Technological Advances in Prosthesis Design and Rehabilitation Following Upper Extremity Limb Loss. Curr. Rev. Musculoskelet. Med. 2020, 13, 485–493. [Google Scholar] [CrossRef]
- Asif, M.; Tiwana, M.I.; Khan, U.S.; Qureshi, W.S.; Iqbal, J.; Rashid, N.; Naseer, N. Advancements, Trends and Future Prospects of Lower Limb Prosthesis. IEEE Access 2021, 9, 85956–85977. [Google Scholar] [CrossRef]
- Moisan, G.; Ma, C.Z. Advances in prosthetics and orthotics. BMC Musculoskelet. Disord. 2024, 25, 135. [Google Scholar] [CrossRef]
- Varaganti, P.; Seo, S. Recent Advances in Biomimetics for the Development of Bio-Inspired Prosthetic Limbs. Biomimetics 2024, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Vujaklija, I.; Brånemark, R.; Bull, A.M.J.; Dietl, H.; Graimann, B.; Hargrove, L.J.; Hoffmann, K.-P.; Huang, H.; Ingvarsson, T.; et al. Toward higher-performance bionic limbs for wider clinical use. Nat. Biomed. Eng. 2023, 7, 473–485. [Google Scholar] [CrossRef]
- Basla, C.; Chee, L.; Valle, G.; Raspopovic, S. A non-invasive wearable sensory leg neuroprosthesis: Mechanical, electrical and functional validation. J. Neural. Eng. 2022, 19, 016008. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.M.; O’Brien, R.; Laciar, E.; Maglione, L.S.; Molisani, L. Vibrotactile Stimulation in the Upper-Arm for Restoring Individual Finger Sensations in Hand Prosthesis. J. Med. Biol. Eng. 2018, 38, 782–789. [Google Scholar] [CrossRef]
- Kristjánsson, Á.; Moldoveanu, A.; Jóhannesson, Ó.I.; Balan, O.; Spagnol, S.; Valgeirsdóttir, V.V.; Unnthorsson, R. Designing sensory-substitution devices: Principles, pitfalls and potential 1. Restor. Neurol. Neurosci. 2016, 34, 769–787. [Google Scholar] [CrossRef]
- Lauretti, C.; Pinzari, G.; Ciancio, A.L.; Davalli, A.; Sacchetti, R.; Sterzi, S.; Guglielmelli, E.; Zollo, L. A vibrotactile stimulation system for improving postural control and knee joint proprioception in lower-limb amputees. In Proceedings of the 26th IEEE International Symposium on Robot and Human Interactive Communication (RO-MAN), Lisbon, Portugal, 28 August–1 September 2017; pp. 88–93. [Google Scholar]
- Marinelli, A.; Boccardo, N.; Canepa, M.; Di Domenico, D.; Gruppioni, E.; Laffranchi, M.; De Michieli, L.; Chiappalone, M.; Semprini, M.; Dosen, S. A compact solution for vibrotactile proprioceptive feedback of wrist rotation and hand aperture. J. NeuroEng. Rehabil. 2024, 21, 142. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Bianchi, M.; Battaglia, E.; Catalano, M.G.; Bicchi, A. HapPro: A Wearable Haptic Device for Proprioceptive Feedback. IEEE Trans. Biomed. Eng. 2019, 66, 138–149. [Google Scholar] [CrossRef]
- Chai, G.H.; Wang, H.; Li, G.Y.; Sheng, X.J.; Zhu, X.Y. Electrotactile Feedback Improves Grip Force Control and Enables Object Stiffness Recognition While Using a Myoelectric Hand. IEEE Trans. Neural. Syst. Rehabil. Eng. 2022, 30, 1310–1320. [Google Scholar] [CrossRef]
- Valgeirsdóttir, V.V.; Sigurdardóttir, J.S.; Jóhannesson, O.I.; Alexandersson, A.; Kristjánsson, A. Multistakeholder Perceptions on Lower-Limb Prosthetic User Requirements and the Development of Neuroprostheses: A Contextual Inquiry. J. Prosthet. Orthot. 2023, 35, 92–105. [Google Scholar] [CrossRef]
- Valgeirsdóttir, V.V.; Sigurðardóttir, J.S.; Lechler, K.; Tronicke, L.; Jóhannesson, Ó.I.; Alexandersson, Á.; Kristjánsson, Á. How Do We Measure Success? A Review of Performance Evaluations for Lower-Limb Neuroprosthetics. J. Prosthet. Orthot. 2022, 34, e20–e36. [Google Scholar] [CrossRef]
- Maldonado-Contreras, J.; Marayong, P.; Khoo, I.-H.; Rivera, R.; Ruhe, B.; Wu, W. Proprioceptive improvements of lower-limb amputees under training with a vibrotactile device—A pilot study. In Proceedings of the 2017 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT), Bethesda, MD, USA, 6–8 November 2017; pp. 229–232. [Google Scholar]
- Martini, E.; Cesini, I.; D’Abbraccio, J.; Arnetoli, G.; Doronzio, S.; Giffone, A.; Meoni, B.; Oddo, C.M.; Vitiello, N.; Crea, S. Increased Symmetry of Lower-Limb Amputees Walking With Concurrent Bilateral Vibrotactile Feedback. IEEE Trans. Neural. Syst. Rehabil. Eng. 2021, 29, 74–84. [Google Scholar] [CrossRef]
- Chen, B.; Feng, Y.; Wang, Q. Combining Vibrotactile Feedback with Volitional Myoelectric Control for Robotic Transtibial Prostheses. Front. Neurorobot. 2016, 10, 8. [Google Scholar] [CrossRef]
- Erwin, A.; Sup, F.C. A Haptic Feedback Scheme to Accurately Position a Virtual Wrist Prosthesis Using a Three-Node Tactor Array. PLoS ONE 2015, 10, e0134095. [Google Scholar] [CrossRef]
- Petrini, F.M.; Bumbasirevic, M.; Valle, G.; Ilic, V.; Mijovic, P.; Cvancara, P.; Barberi, F.; Katic, N.; Bortolotti, D.; Andreu, D.; et al. Sensory feedback restoration in leg amputees improves walking speed, metabolic cost and phantom pain. Nat. Med. 2019, 25, 1356–1363. [Google Scholar] [CrossRef]
- Valette, R.; Gonzalez-Vargas, J.; Dosen, S. The impact of walking on the perception of multichannel electrotactile stimulation in individuals with lower-limb amputation and able-bodied participants. J. NeuroEng. Rehabil. 2023, 20, 108. [Google Scholar] [CrossRef]
- Preatoni, G.; Valle, G.; Petrini, F.M.; Raspopovic, S. Lightening the Perceived Prosthesis Weight with Neural Embodiment Promoted by Sensory Feedback. Curr. Biol. 2021, 31, 1065–1071.e1064. [Google Scholar] [CrossRef]
- Hartcher-O’Brien, J.; Auvray, M. The Process of Distal Attribution Illuminated Through Studies of Sensory Substitution. Multisens. Res. 2014, 27, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Ung, G.; Ayaz, H.; Brown, J.D. Neurophysiological Evaluation of Haptic Feedback for Myoelectric Prostheses. IEEE Trans. Hum. Mach. Syst. 2021, 51, 253–264. [Google Scholar] [CrossRef]
- Shehata, A.W.; Rehani, M.; Jassat, Z.E.; Hebert, J.S. Mechanotactile Sensory Feedback Improves Embodiment of a Prosthetic Hand During Active Use. Front. Neurosci. 2020, 14, 263. [Google Scholar] [CrossRef]
- Escamilla-Nunez, R.; Michelini, A.; Andrysek, J. Biofeedback Systems for Gait Rehabilitation of Individuals with Lower-Limb Amputation: A Systematic Review. Sensors 2020, 20, 1628. [Google Scholar] [CrossRef]
- Raspopovic, S.; Valle, G.; Petrini, F.M. Sensory feedback for limb prostheses in amputees. Nat. Mater. 2021, 20, 925–939. [Google Scholar] [CrossRef]
- Masteller, A.; Sankar, S.; Kim, H.B.; Ding, K.Q.; Liu, X.G.; All, A.H. Recent Developments in Prosthesis Sensors, Texture Recognition, and Sensory Stimulation for Upper Limb Prostheses. Ann. Biomed. Eng. 2021, 49, 57–74. [Google Scholar] [CrossRef]
- Rathore, R.; Singh, A.K.; Chaudhary, H.; Kandan, K. Gait Abnormality Detection in Unilateral Trans-Tibial Amputee in Real-Time Gait Using Wearable Setup. IEEE Sens. J. 2023, 23, 12567–12573. [Google Scholar] [CrossRef]
- Persine, S.; Simoneau-Buessinger, E.; Charlaté, F.; Bassement, J.; Gillet, C.; Découfour, N.; Leteneur, S. Transfemoral amputees adapt their gait during cross-slope walking with specific upper-lower limb coordination. Gait Posture 2023, 105, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, A.H.; van Keeken, H.G.; Schoppen, T.; Otten, E.; Hof, A.L.; Halbertsma, J.P.K.; Postema, K. Balance control on a moving platform in unilateral lower limb amputees. Gait Posture 2008, 28, 222–228. [Google Scholar] [CrossRef]
- Dietrich, C.; Nehrdich, S.; Seifert, S.; Blume, K.R.; Miltner, W.H.R.; Hofmann, G.O.; Weiss, T. Leg Prosthesis With Somatosensory Feedback Reduces Phantom Limb Pain and Increases Functionality. Front. Neurol. 2018, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Crea, S.; Edin, B.B.; Knaepen, K.; Meeusen, R.; Vitiello, N. Time-Discrete Vibrotactile Feedback Contributes to Improved Gait Symmetry in Patients With Lower Limb Amputations: Case Series. Phys. Ther. 2017, 97, 198–207. [Google Scholar] [CrossRef]
- Fan, R.E.; Culjat, M.O.; King, C.H.; Franco, M.L.; Boryk, R.; Bisley, J.W.; Dutson, E.; Grundfest, W.S. A haptic feedback system for lower-limb prostheses. IEEE Trans. Neural. Syst. Rehabil. Eng. 2008, 16, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Husman, M.A.B.; Maqbool, H.F.; Awad, M.I.; Abouhossein, A.; Dehghani-Sanij, A.A. A wearable skin stretch haptic feedback device: Towards improving balance control in lower limb amputees. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2120–2123. [Google Scholar]
- Rusaw, D.; Hagberg, K.; Nolan, L.; Ramstrand, N. Can vibratory feedback be used to improve postural stability in persons with transtibial limb loss? J. Rehabil. Res. Dev. 2012, 49, 1239–1253. [Google Scholar] [CrossRef]
- Chen, L.J.; Feng, Y.G.; Chen, B.J.; Wang, Q.N.; Wei, K.L. Improving postural stability among people with lower-limb amputations by tactile sensory substitution. J. NeuroEng. Rehabil. 2021, 18, 159. [Google Scholar] [CrossRef]
- Peng, Y.H.; Sakai, Y.; Nakagawa, K.; Funabora, Y.; Aoyama, T.; Yokoe, K.; Doki, S. Funabot-Suit: A bio-inspired and McKibben muscle-actuated suit for natural kinesthetic perception. Biomim. Intell. Robot. 2023, 3, 100127. [Google Scholar] [CrossRef]
- Rokhmanova, N.; Rombokas, E. Vibrotactile feedback improves foot placement perception on stairs for lower-limb prosthesis users. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 1215–1220. [Google Scholar]
- Sie, A.; Boe, D.; Rombokas, E. Design and evaluation of a wearable haptic feedback system for lower limb prostheses during stair descent. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Enschede, The Netherlands, 26–29 August 2018; pp. 219–224. [Google Scholar]
- Petrini, F.M.; Valle, G.; Bumbasirevic, M.; Barberi, F.; Bortolotti, D.; Cvancara, P.; Hiairrassary, A.; Mijovic, P.; Sverrisson, A.O.; Pedrocchi, A.; et al. Enhancing functional abilities and cognitive integration of the lower limb prosthesis. Sci. Transl. Med. 2019, 11, eaav8939. [Google Scholar] [CrossRef] [PubMed]
- Valle, G.; Mazzoni, A.; Iberite, F.; D’Anna, E.; Strauss, I.; Granata, G.; Controzzi, M.; Clemente, F.; Rognini, G.; Cipriani, C.; et al. Biomimetic Intraneural Sensory Feedback Enhances Sensation Naturalness, Tactile Sensitivity, and Manual Dexterity in a Bidirectional Prosthesis. Neuron 2018, 100, 37–45. [Google Scholar] [CrossRef]
- George, J.A.; Kluger, D.T.; Davis, T.S.; Wendelken, S.M.; Okorokova, E.V.; He, Q.; Duncan, C.C.; Hutchinson, D.T.; Thumser, Z.C.; Beckler, D.T.; et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot. 2019, 4, eaax2352. [Google Scholar] [CrossRef]
- D’Anna, E.; Valle, G.; Mazzoni, A.; Strauss, I.; Iberite, F.; Patton, J.; Petrini, F.M.; Raspopovic, S.; Granata, G.; Di Iorio, R.; et al. A closed-loop hand prosthesis with simultaneous intraneural tactile and position feedback. Sci. Robot. 2019, 4, eaau8892. [Google Scholar] [CrossRef]
- Skervin, A.; Levy, B. Management of common surgical complications. Surgery 2023, 41, 76–80. [Google Scholar] [CrossRef]
- Paisa, R.; Nilsson, N.C.; Serafin, S. Tactile displays for auditory augmentation-A scoping review and reflections on music applications for hearing impaired users. Front. Comput. Sci. 2023, 5, 1085539. [Google Scholar] [CrossRef]
- Johannesson, O.I.; Hoffmann, R.; Valgeirsdottir, V.V.; Unnthornorsson, R.; Moldoveanu, A.; Kristjansson, A. Relative vibrotactile spatial acuity of the torso. Exp. Brain Res. 2017, 235, 3505–3515. [Google Scholar] [CrossRef]
- Plaisier, M.A.; Vleeshouwers, C.S.J.M.; Boonstra, N.; Shi, Y.Y.; van der Velden, S.J.I.; Vos, W.K.; Kappers, A.M.L. Vibrotactile spatial acuity on the back. Perception 2024, 53, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.H.; Wong, D.W.; Ma, C.Z.; Zhang, M.; Lee, W.C. Wearable Vibrotactile Biofeedback Device Allowing Identification of Different Floor Conditions for Lower-Limb Amputees. Arch. Phys. Med. Rehabil. 2016, 97, 1210–1213. [Google Scholar] [CrossRef]
- Yeganeh, N.; Makarov, I.; Stefánsson Thors, S.S.; Kristjánsson, Á.; Unnthorsson, R. Evaluating the Optimum Distance between Voice Coil Actuators Using the Relative Point Localization Method on the Forearm. Actuators 2023, 12, 6. [Google Scholar] [CrossRef]
- Elsayed, H.; Weigel, M.; Müller, F.; Schmitz, M.; Marky, K.; Günther, S.; Riemann, J.; Mühlhäuser, M. VibroMap: Understanding the Spacing of Vibrotactile Actuators across the Body. Proc. ACM Interact. Mob. Wearable. Ubiquitous. Technol. 2020, 4, 1–16. [Google Scholar] [CrossRef]
- Friedman, R.M.; Chen, L.M.; Roe, A.W. Modality maps within primate somatosensory cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 12724–12729. [Google Scholar] [CrossRef]
- Huang, H.; Li, T.; Bruschini, C.; Enz, C.; Justiz, J.; Antfolk, C.; Koch, V.M. Multi-modal sensory feedback system for upper limb amputees. In Proceedings of the 2017 New Generation of CAS (NGCAS), Genova, Italy, 6–9 September 2017; pp. 193–196. [Google Scholar]
- Hoffmann, R.; Valgeirsdottir, V.V.; Johannesson, O.I.; Unnthorsson, R.; Kristjansson, A. Measuring relative vibrotactile spatial acuity: Effects of tactor type, anchor points and tactile anisotropy. Exp. Brain Res. 2018, 236, 3405–3416. [Google Scholar] [CrossRef]
- Yeganeh, N.; Makarov, I.; Unnthorsson, R.; Kristjánsson, Á. Effects of Stimulus Frequency and Location on Vibrotactile Discrimination Performance Using Voice Coil Actuators on the Forearm. Actuators 2023, 12, 224. [Google Scholar] [CrossRef]
- Cody, F.W.J.; Garside, R.A.D.; Lloyd, D.; Poliakoff, E. Tactile spatial acuity varies with site and axis in the human upper limb. Neurosci. Lett. 2008, 433, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Leineweber, M.J.; Andrysek, J. Exploring the Tactor Configurations of Vibrotactile Feedback Systems for Use in Lower-Limb Prostheses. ASME J. Vib. Acoust. 2019, 141, 051009. [Google Scholar] [CrossRef]
- Geldard, F.A.; Sherrick, C.E. The cutaneous “rabbit”: A perceptual illusion. Science 1972, 178, 178–179. [Google Scholar] [CrossRef]
- Flach, R.; Haggard, P. The cutaneous rabbit revisited. J. Exp. Psychol. Hum. Percept. Perform. 2006, 32, 717–732. [Google Scholar] [CrossRef]
- Miyazaki, M.; Hirashima, M.; Nozaki, D. The “Cutaneous Rabbit” Hopping out of the Body. J. Neurosci. 2010, 30, 1856–1860. [Google Scholar] [CrossRef]
- Gardner, E.P.; Spencer, W.A. Sensory funneling. I. Psychophysical observations of human subjects and responses of cutaneous mechanoreceptive afferents in the cat to patterned skin stimuli. J. Neurophysiol. 1972, 35, 925–953. [Google Scholar] [CrossRef]
- Rahal, L.; Cha, J.; Saddik, A.E.; Kammerl, J.; Steinbach, E. Investigating the influence of temporal intensity changes on apparent movement phenomenon. In Proceedings of the 2009 IEEE International Conference on Virtual Environments, Human-Computer Interfaces and Measurements Systems VECIMS, Hong Kong, China, 11–13 May 2009; pp. 310–313. [Google Scholar]
- Jeannin, M.; Dhiab, A.B.; Pantera, L.; Hudin, C.; Panëels, S. The Funneling Illusion Using the Confinement of Vibrotactile Stimuli in Narrow Plates. In Proceedings of the 2021 IEEE World Haptics Conference (WHC), Montreal, QC, Canada, 6–9 July 2021; p. 1147. [Google Scholar]
- Helson, H. The Tau Effect--an Example of Psychological Relativity. Science 1930, 71, 536–537. [Google Scholar] [CrossRef] [PubMed]
- Lechelt, E.C.; Borchert, R. The interdependence of time and space in somesthesis: The Tau effect reexamined. Bull. Psychon. Soc. 1977, 10, 191–193. [Google Scholar] [CrossRef][Green Version]
- Yoji, S. The effect of space on time estimation (S-effect) in tactual space (I). Jpn. J. Psychol. 1952, 22, 189–201. [Google Scholar] [CrossRef]
- Jones, B.; Huang, Y.L. Space-Time Dependencies in Psychophysical Judgment of Extent and Duration—Algebraic Models of the Tau and Kappa Effects. Psychol. Bull. 1982, 91, 128–142. [Google Scholar] [CrossRef]
- Sherrick, C.E.; Rogers, R. Apparent haptic movement. Percept. Psychophys. 1966, 1, 175–180. [Google Scholar] [CrossRef]
- Carter, O.; Konkle, T.; Wang, Q.; Hayward, V.; Moore, C. Tactile rivalry demonstrated with an ambiguous apparent-motion quartet. Curr. Biol. 2008, 18, 1050–1054. [Google Scholar] [CrossRef]
- Hoffmann, R.; Brinkhuis, M.A.B.; Unnthorsson, R.; Kristjánsson, A. The intensity order illusion: Temporal order of different vibrotactile intensity causes systematic localization errors. J. Neurophysiol. 2019, 122, 1810–1820. [Google Scholar] [CrossRef]
- Makarov, I.; Thors, S.S.S.; Ævarsson, E.A.; Jörgensson, F.K.P.; Yeganeh, N.; Kristjánsson, Á.; Unnthorsson, R. The Haptic Intensity Order Illusion Is Caused by Amplitude Changes. ACM Trans. Appl. Percept. 2023, 21, 1–18. [Google Scholar] [CrossRef]
- Yeganeh, N.; Makarov, I.; Kristjánsson, Á.; Unnthorsson, R. Discrimination Accuracy of Sequential Versus Simultaneous Vibrotactile Stimulation on the Forearm. Appl. Sci. 2024, 14, 43. [Google Scholar] [CrossRef]
- Ævarsson, E.A.; Ásgeirsdóttir, T.; Pind, F.; Kristjánsson, Á.; Unnthorsson, R. Vibrotactile Threshold Measurements at the Wrist Using Parallel Vibration Actuators. ACM Trans. Appl. Percept. 2022, 19, 1–11. [Google Scholar] [CrossRef]
- Haq, A.U.; Reddy, N.S.K. A brief review on various high energy absorbing materials. Mater. Today Proc. 2021, 38, 3198–3204. [Google Scholar] [CrossRef]
- Rungruangkitkrai, N.; Phromphen, P.; Chartvivatpornchai, N.; Srisa, A.; Laorenza, Y.; Wongphan, P.; Harnkarnsujarit, N. Water Repellent Coating in Textile, Paper and Bioplastic Polymers: A Comprehensive Review. Polymers 2024, 16, 2790. [Google Scholar] [CrossRef]
- Plauché, A.; Villarreal, D.; Gregg, R.D. A Haptic Feedback System for Phase-Based Sensory Restoration in Above-Knee Prosthetic Leg Users. IEEE Trans. Haptics 2016, 9, 421–426. [Google Scholar] [CrossRef]
- Marayong, P.; Khoo, I.-H.; Nguyen, K.; Bharti, N.; Ruhe, B.; Craig, D.; Wu, W. Vibrotactile device for rehabilitative training of persons with lower-limb amputation. In Proceedings of the 2014 IEEE Healthcare Innovation Conference (HIC), Seattle, WA, USA, 8–10 October 2014; pp. 157–160. [Google Scholar]
- Canino, J.M.; Fite, K.B. Haptic feedback in lower-limb prosthesis: Combined haptic feedback and EMG control of a powered prosthesis. In Proceedings of the 2016 IEEE EMBS International Student Conference (ISC), Ottawa, ON, Canada, 29–31 May 2016; pp. 1–4. [Google Scholar]
- Leal, J.M.C.; Gyllinsky, J.V.; Zamudio, A.A.A.; Mankodiya, K. Hapticlink: A force-based haptic feedback system for single and double lower-limb amputees. In Proceedings of the 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society, EMBC, Glasgow, UK, 11–15 July 2022; pp. 4226–4229. [Google Scholar]
- Hoffmann, R.; Spagnol, S.; Kristjánsson, A.; Unnthorsson, R. Evaluation of an Audio-haptic Sensory Substitution Device for Enhancing Spatial Awareness for the Visually Impaired. Optom. Vis. Sci. 2018, 95, 757–765. [Google Scholar] [CrossRef]
- Jóhannesson, O.I.; Balan, O.; Unnthorsson, R.; Moldoveanu, A.; Kristjánsson, A. The Sound of Vision Project: On the Feasibility of an Audio-Haptic Representation of the Environment, for the Visually Impaired. Brain Sci. 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R. The System Usability Scale: Past, Present, and Future. Int. J. Hum.-Comput. Int. 2018, 34, 577–590. [Google Scholar] [CrossRef]
- Valgeirsdóttir, V.V.; Alexandersson, A.; Lechler, K.; Jóhannesson, O.I.; Kristjánsson, A. What a Knee Should Be: A Pilot Study on the Perspectives of Highly Active Prosthetic Users. J. Prosthet. Orthot. 2024, 36, 33–41. [Google Scholar] [CrossRef]
- Vimal, A.K.; Verma, V.; Khanna, N.; Joshi, D. Investigating the Effect of Vibrotactile Feedback in Transfemoral Amputee With and Without Movable Ankle Joint. IEEE Trans. Neural. Syst. Rehabil. Eng. 2020, 28, 2890–2900. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Bensmaïa, S.J.; Hsiao, S.S.; Johnson, K.O. Time-course of vibratory adaptation and recovery in cutaneous mechanoreceptive afferents. J. Neurophysiol. 2005, 94, 3037–3045. [Google Scholar] [CrossRef]
- Domenici, N.; Tonelli, A.; Gori, M. The Development of Adaptation Aftereffects in the Vibrotactile Domain. J. Exp. Psychol. Gen. 2022, 151, 3134–3143. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.G. Nasa-Task Load Index (NASA-TLX); 20 Years Later. Proc. Hum. Factors. Ergon. Soc. Annu. Meet. 2006, 50, 904–908. [Google Scholar] [CrossRef]
- Zanetti, R.; Arza, A.; Aminifar, A.; Atienza, D. Real-Time EEG-Based Cognitive Workload Monitoring on Wearable Devices. IEEE Trans. Biomed. Eng. 2022, 69, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Fishburn, F.A.; Norr, M.E.; Medvedev, A.V.; Vaidya, C.J. Sensitivity of fNIRS to cognitive state and load. Front. Hum. Neurosci. 2014, 8, 76. [Google Scholar] [CrossRef]
- Amann, L.K.; Casasnovas, V.; Hainke, J.; Gail, A. Optimality of multisensory integration while compensating for uncertain visual target information with artificial vibrotactile cues during reach planning. J. NeuroEng. Rehabil. 2024, 21, 155. [Google Scholar] [CrossRef]
- Fratini, A.; Cesarelli, M.; Bifulco, P.; Romano, M. Relevance of motion artifact in electromyography recordings during vibration treatment. J. Electromyogr. Kinesiol. 2009, 19, 710–718. [Google Scholar] [CrossRef]
- Abercromby, A.F.J.; Amonette, W.E.; Layne, C.S.; Mcfarlin, B.K.; Hinman, M.R.; Paloski, W.H. Variation in neuromuscular responses during acute whole-body vibration exercise. Med. Sci. Sports Exerc. 2007, 39, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Dosen, S.; Amsuess, S.; Farina, D. Closed-Loop Control of Myoelectric Prostheses With Electrotactile Feedback: Influence of Stimulation Artifact and Blanking. IEEE Trans. Neural. Syst. Rehabil. Eng. 2015, 23, 807–816. [Google Scholar] [CrossRef]
- Zbinden, J.; Lendaro, E.; Ortiz-Catalan, M. A multi-dimensional framework for prosthetic embodiment: A perspective for translational research. J. NeuroEng. Rehabil. 2022, 19, 122. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, J.; Lendaro, E.; Ortiz-Catalan, M. Prosthetic embodiment: Systematic review on definitions, measures, and experimental paradigms. J. NeuroEng. Rehabil. 2022, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Bekrater-Bodmann, R. Factors Associated With Prosthesis Embodiment and Its Importance for Prosthetic Satisfaction in Lower Limb Amputees. Front. Neurorobot. 2021, 14, 604376. [Google Scholar] [CrossRef]
- Bernstein, R.M.; Watts, H.G.; Setoguchi, Y. The lengthening of short upper extremity amputation stumps. J. Pediatr. Orthop. 2008, 28, 86–90. [Google Scholar] [CrossRef]
- Templeton, C.A.; Strzalkowski, N.D.J.; Galvin, P.; Bent, L.R. Cutaneous sensitivity in unilateral trans-tibial amputees. PLoS ONE 2018, 13, e0197557. [Google Scholar] [CrossRef]
- Ellenberg, M.A.X. Diabetic neuropathy presenting as the initial clinical manifestation of diabetes. Ann. Intern. Med. 1958, 49, 620–631. [Google Scholar] [CrossRef] [PubMed]

| Haptic Feedback Method | Principle of Operation | Advantages | Challenges |
|---|---|---|---|
| Vibrotactile | Uses actuators to generate vibrations on the skin, simulating touch sensations | Compact, energy-efficient, widely studied | Limited spatial resolution, possible desensitization over time |
| Mechanotactile | Applies direct mechanical pressure or skin stretch to convey sensory information | Can mimic natural touch more closely, useful for proprioception | Can be bulky, actuator complexity, potential user discomfort |
| Electrotactile | Delivers electrical pulses to stimulate sensory nerves, creating artificial touch sensations | Can provide precise and varied sensory signals | May cause discomfort or irritation, requires careful calibration |
| Invasive Approaches (Intraneural, Implanted Electrodes) | Non-Invasive Approaches (Vibrotactile, Mechanotactile, Electrotactile) | |
|---|---|---|
| Precision of feedback | High (direct neural stimulation, naturalistic sensation) | Moderate to low (surface-level cues, limited spatial resolution) |
| Embodiment and externalization | Strong, prosthesis often perceived as part of body | Moderate, may feel “gadget-like” without extensive training |
| Surgical risks | Present (implantation required, risk of infection/complications) | None |
| Long-term stability | Potential issues (signal degradation, electrode encapsulation) | High (no implants, easier maintenance) |
| Cost | High (surgery, device development, maintenance) | Lower (wearable actuators, simpler electronics) |
| Accessibility | Limited to specialized clinics and research settings | Broad (portable, wearable, can be mass-produced) |
| Clinical adoption | Early-stage, limited trials | Already implemented in several experimental and prototype devices |
| Study | Participants | Key Findings | Limitations | Body Locations | System Source |
|---|---|---|---|---|---|
| Electrotactile | |||||
| Valette et al. (2023) [29] | 11 AB, 3 TTA, 3 TFA | Reduced electrotactile perception, higher sensation and discomfort thresholds, lower spatial discrimination accuracy during walking | Small amputee sample size, perception variability across individuals | Thigh, residual limb | Force sensors |
| Basla et al. (2022) [15] | 3 AB, 3 TFA | Enhanced walking symmetry | Small sample size, requires long-term and home-based assessment | Thigh, stump, hip | Knee angles, Force sensors |
| Dietrich et al. (2018) [40] | 14 TTA | Reduced phantom limb pain, increased prosthesis functionality, and improved walking stability | Small sample size, short training duration, feedback system design limitations | Thigh | Force sensors |
| Intraneural electrodes | |||||
| Preatoni et al. (2021) [30] | 1 TFA | Sensory feedback reduced perceived prosthesis weight, increased embodiment and confidence, and maintained walking speed under cognitive load | Single-subject study | Residual limb | Force sensors |
| Petrini et al. (2019) [28] | 2 TFA | Improved walking speed, reduced metabolic cost, increased confidence, and decreased phantom limb pain | Small sample size, limited duration of the study (3 months), need for larger trials to assess long-term clinical benefits | Residual limb | Knee angles, force sensors |
| Petrini et al. (2019) [49] | 3 TFA | Improved mobility, fall prevention, agility, embodiment, and reduced cognitive load | Small sample size, short study duration (3 months per subject), non-implantable system tested in a lab setting | Residual limb | Knee angles, force sensors |
| Mechanotactile | |||||
| Husman et al. (2016) [43] | 3 AB | High perceptibility for balance control | Tested only in static conditions, no amputee participants | Thigh | Inertial measurement unit (IMU) |
| Canino et al. (2016) [86] | 2 AB | Sustained pressure feedback significantly improved EMG control in absence of visual feedback, vibration feedback less effective due to desensitization | Vibrotactile feedback prone to desensitization, more complex trajectories require improved magnitude encoding | Thigh | EMG |
| Fan et al. (2008) [42] | 6 AB | Demonstrating its potential to improve balance and gait in prosthetic users | Not tested on amputees, requires further optimization for portability and clinical use | Thigh | Force sensors |
| Vibrotactile | |||||
| Leal et al. (2022) [87] | 2 TFA | Participants achieved high accuracy in interpreting directional and intensity-based haptic feedback | Only 2 participants, limited generalizability, COVID-19 disrupted further testing | Arm, thigh | Force sensors |
| Martini et al. (2021) [25] | 3 TFA | Improved temporal gait symmetry with bilateral feedback, one subject retained improvement post-training | Small sample size, variability in individual responses | Waist | Force sensors |
| L. J. Chen et al. (2021) [45] | 8 AB, 7 TTA | Improved postural stability, reducing body sway | Tested only in a controlled lab, limited to TTA, used only center of pressure as stability measure | Forearm | Force sensors |
| Vimal et al. (2020) [92] | 5 TFA | Improved limit of stability, particularly with movable ankle joints | Only anterior–posterior center of pressure analyzed, ankle joint condition not randomized | Residual limb | Force sensors |
| Rokhmanova et al. (2019) [47] | 10 AB, 2 TTA | Improved foot placement awareness | Only tested vibrotactile not modality-matched (pressure) feedback | Thigh | Force sensors |
| Shi et al. (2019) [65] | 10 AB, 3 TFA | Inner socket tactors improved perception, higher intensity and spacing enhanced accuracy | Tested under static conditions (sitting), needs real-world mobility assessment | Thigh, residual limb | Simulator |
| Sie et al. (2018) [48] | 28 AB | Improved step-edge detection, reduced localization error in visually obstructed stair descent tasks | Tested only on able-bodied subjects, possible sensor bias from boot placement and sole curvature, actuator calibration inconsistencies | Thigh | Force sensors |
| Lauretti et al. (2017) [18] | 16 AB, 1 (TFA, TTA) | Capable of improving postural control and knee-joint proprioception | Needs further validation on a larger amputee population | Forearm, low back | Knee angles, force sensors |
| Maldonado et al. (2017) [24] | 2 TTA | Improved proprioception and postural control; 17% faster response time in trained amputee | Small sample size, device design limitations | Thigh | Knee angles |
| Crea et al. (2017) [41] | 3 TFA | Improved temporal gait symmetry after training, even under dual-task conditions | Small sample size, short follow-up, limited generalizability | Lower abdomen | Force sensors |
| B. Chen et al. (2016) [26] | 8 AB, 2 TTA | Improved the perception of ankle joint position and enhances prosthetic control | Tested only in seated conditions | Thigh | Ankle angles |
| Wan et al. (2016) [57] | 8 AB, 2 TFA, 3 TTA | Improved amputees’ ability to identify different floor conditions | Limited to standing conditions only | Hand | Force sensors |
| Plauché et al. (2016) [84] | 9 AB | Reduced stride length, step width, and trunk sway variability, indicating improved gait stability | Tested only on able-bodied users with simulated prosthesis | Thigh | Force sensors |
| Canino et al. (2016) [86] | 2 AB | Sustained pressure feedback significantly improved EMG control in absence of visual feedback, vibration feedback less effective due to desensitization | Vibrotactile feedback prone to desensitization, more complex trajectories require improved magnitude encoding | Thigh | EMG |
| Marayong et al. (2014) [85] | 1 TTA | Participant could perceive and distinguish feedback types | Delay between activation and actuator output, inaccurate knee angle readings affected timing | Residual limb | Knee angles |
| Rusaw et al. (2012) [44] | 24 TTA | Improved postural stability | Feedback only responded to normal (vertical) forces, not shear forces; feedback only provided on the prosthetic side | Thigh | Force sensors |
| Study | Participants | Key Findings | Limitations | Body Locations | System Source |
|---|---|---|---|---|---|
| Electrotactile | |||||
| Chai et al. (2022) [21] | 10 AB, 2 TRA | Improved grip force control and stiffness recognition | No intraneural feedback due to lack of amputees, stiffness discrimination should include object deformation data | Forearm | Force sensors |
| Intraneural electrodes | |||||
| George et al. (2019) [51] | 1 TRA | Improved object manipulation, grasp control, and prosthesis usability | Limited patient time, daily living activities not tested with biomimetic feedback | Residual limb | Force sensors |
| D’Anna et al. (2019) [52] | 2 TRA | Improved task performance in prosthetic hand use, participants were able to identify object size | Limited to two channels of proprioceptive feedback, does not cover all five fingers, future research needed for wrist and elbow feedback | Residual limb | Fingers angles, Force sensors |
| Valle et al. (2018) [50] | 2 TRA | Improved sensation naturalness, tactile sensitivity, manual dexterity, and prosthesis embodiment | Case study with a small sample size, findings need validation in a larger population | Residual limb | Force sensors |
| Mechanotactile | |||||
| Shehata et al. (2020) [33] | 21 AB | Improved embodiment (ownership and location) with synchronous feedback, asynchronous feedback reduced agency | Tested only on able-bodied participants, subjective embodiment measures | Hand | Force sensors |
| Rossi et al. (2019) [20] | 43 AB, 1 TRA | Provided accurate proprioceptive feedback on hand aperture | Large device size, not integrated into prosthetic socket | Forearm | EMG |
| Huang et al. (2017) [61] | 3 TRA | Improved localization and intensity recognition | System requires miniaturization, high power consumption | Residual limb | Force sensors |
| Vibrotactile | |||||
| Marinelli et al. (2024) [19] | 10 AB (control group), 10 AB, 4 TRA | Compact feedback with fewer motors, amputees performed similarly to able-bodied participants | Test and control conditions in separate groups of participants, long single session affects mental fatigue | Forearm | Wrist angles, EMG |
| Thomas et al. (2021) [32] | 10 AB | Haptic feedback improved stiffness discrimination accuracy and reduced cognitive load (measured via fNIRS) | Tested only on able-bodied participant, task was simple and may not generalize to real-world use | Upper arm | Force sensors |
| Fontana et al. (2018) [16] | 30 AB | 94% accuracy in finger sensation discrimination, 85% in grasping pattern recognition | Tested only on able-bodied subjects, needs validation with amputees | Arm | Simulator |
| Huang et al. (2017) [61] | 3 TRA | Improved localization and intensity recognition | System requires miniaturization, high power consumption | Residual limb | Force sensors |
| Erwin et al. (2015) [27] | 8 AB | Improved virtual wrist positioning accuracy compared to no feedback | Not tested on amputees, feedback limited to one degree of freedom | Forearm | EMG |
| Study | Participants | Key Findings | Limitations | Body Locations | System Source |
|---|---|---|---|---|---|
| Vibrotactile | |||||
| Plaisier et al. (2024) [56] | 13 AB | Spatial acuity on the back is significantly higher in the horizontal direction than in the vertical direction | N/A | Back | Simulator |
| Yeganeh et al. (2024) [80] | 8 AB | Sequential stimulation had higher accuracy than simultaneous, with better performance for shorter patterns and learning effects over time. | Differences in timing between conditions need further study | Forearm | Simulator |
| Amann et al. (2024) [98] | 31 AB | Participants learned to interpret vibrotactile cues and integrated them with visual info to improve accuracy | Performance varied across participants, limited training, potential skin vibration overlap | Arm | Simulator |
| Yeganeh et al. (2023) [58] | 8 AB | 20 mm was identified as the optimal interspacing for voice coil actuators on the forearm | N/A | Forearm | Simulator |
| Yeganeh et al. (2023) [63] | 8 AB | Placing actuators near the wrist and elbow improves accuracy, frequency variations have minimal effects | Need to determine the impact of anisotropies in vibrotactile localization and the effect of denser actuator placements near anatomical landmarks | Forearm | Simulator |
| Makarov et al. (2023) [79] | 17 AB | The intensity order illusion is caused by amplitude changes rather than frequency differences; the illusion occurs in the vertical direction but not in the horizontal direction | N/A | Waist | Simulator |
| Ævarsson et al. (2022) [81] | 30 AB | Sensitivity was in higher frequency on the inner wrist, suggest need for personalized calibration | Further testing is needed due to demographic imbalances (e.g., age/ gender distribution) | Wrist | Simulator |
| Hoffmann et al. (2019) [78] | 16 AB | Varying the temporal and intensity order of vibrotactile stimuli causes systematic localization errors; strong-to-weak stimuli increase downward perception and vice versa | Frequency and amplitude are linked, further research is needed to test other body parts and determine optimal parameters | Low back | Simulator |
| Hoffmann et al. (2018) [62] | 17 AB | Spatial acuity depends on tactor type, better discrimination accuracy for horizontal presentation and normal eccentric rotating mass tactors | Limited to lower thoracic region, different tactor types under load may yield varying results | Low back | Simulator |
| Johannesson et al. (2017) [55] | 30 AB | Spatial acuity for vibrotactile stimuli on the torso is below 13 mm; accuracy decreased as inter-tactor spacing decreased | Need to compare different tactor types | Back | Simulator |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi, M.; Yeganeh, N.; Makarov, I.; Sverrisson, A.Ö.; Gunnarsson, K.F.; Briem, K.; Brynjólfsson, S.; Kristjánsson, Á.; Unnthorsson, R. Haptic Feedback Systems for Lower-Limb Prosthetic Applications: A Review of System Design, User Experience, and Clinical Insights. Bioengineering 2025, 12, 989. https://doi.org/10.3390/bioengineering12090989
Karimi M, Yeganeh N, Makarov I, Sverrisson AÖ, Gunnarsson KF, Briem K, Brynjólfsson S, Kristjánsson Á, Unnthorsson R. Haptic Feedback Systems for Lower-Limb Prosthetic Applications: A Review of System Design, User Experience, and Clinical Insights. Bioengineering. 2025; 12(9):989. https://doi.org/10.3390/bioengineering12090989
Chicago/Turabian StyleKarimi, Mohammadmahdi, Nashmin Yeganeh, Ivan Makarov, Atli Örn Sverrisson, Karl Fannar Gunnarsson, Kristín Briem, Sigurður Brynjólfsson, Árni Kristjánsson, and Runar Unnthorsson. 2025. "Haptic Feedback Systems for Lower-Limb Prosthetic Applications: A Review of System Design, User Experience, and Clinical Insights" Bioengineering 12, no. 9: 989. https://doi.org/10.3390/bioengineering12090989
APA StyleKarimi, M., Yeganeh, N., Makarov, I., Sverrisson, A. Ö., Gunnarsson, K. F., Briem, K., Brynjólfsson, S., Kristjánsson, Á., & Unnthorsson, R. (2025). Haptic Feedback Systems for Lower-Limb Prosthetic Applications: A Review of System Design, User Experience, and Clinical Insights. Bioengineering, 12(9), 989. https://doi.org/10.3390/bioengineering12090989








