The Influence of Reaming Velocity During Preparation of the Femoral Canal—An In Vitro Analysis of Two Straight Femoral Revision Stems with a Fluted Tapered Design
Abstract
1. Introduction
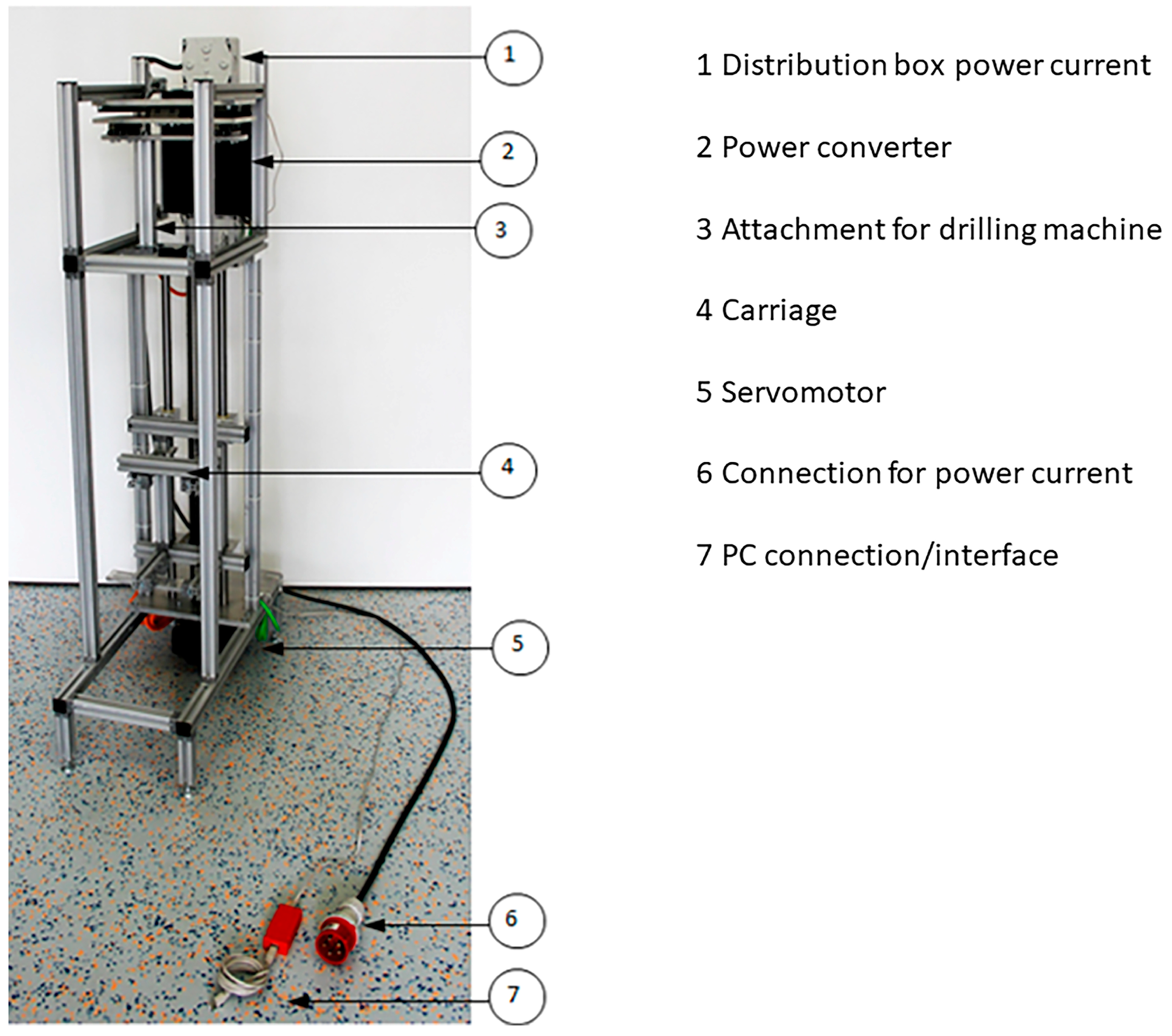
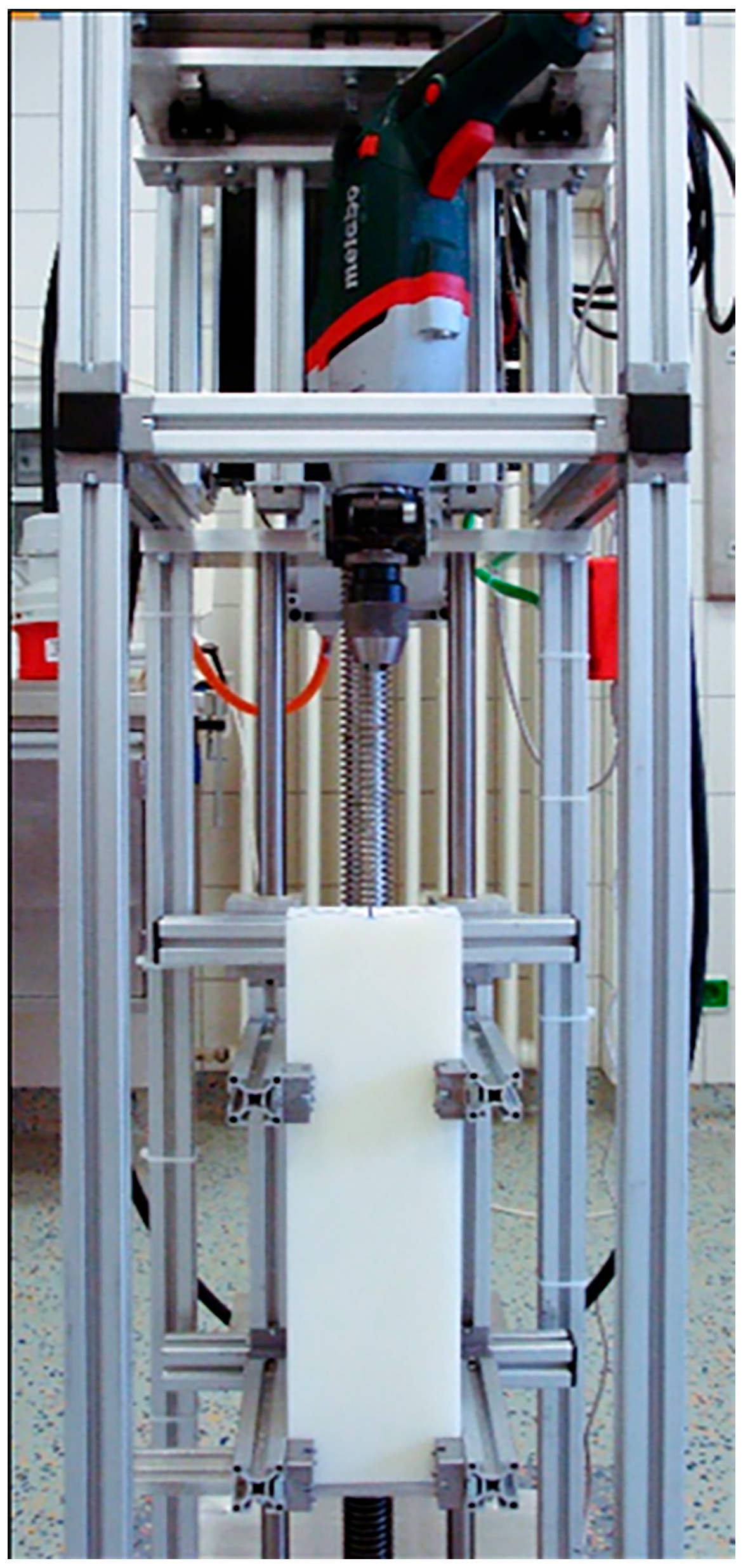
2. Materials and Methods
3. Results
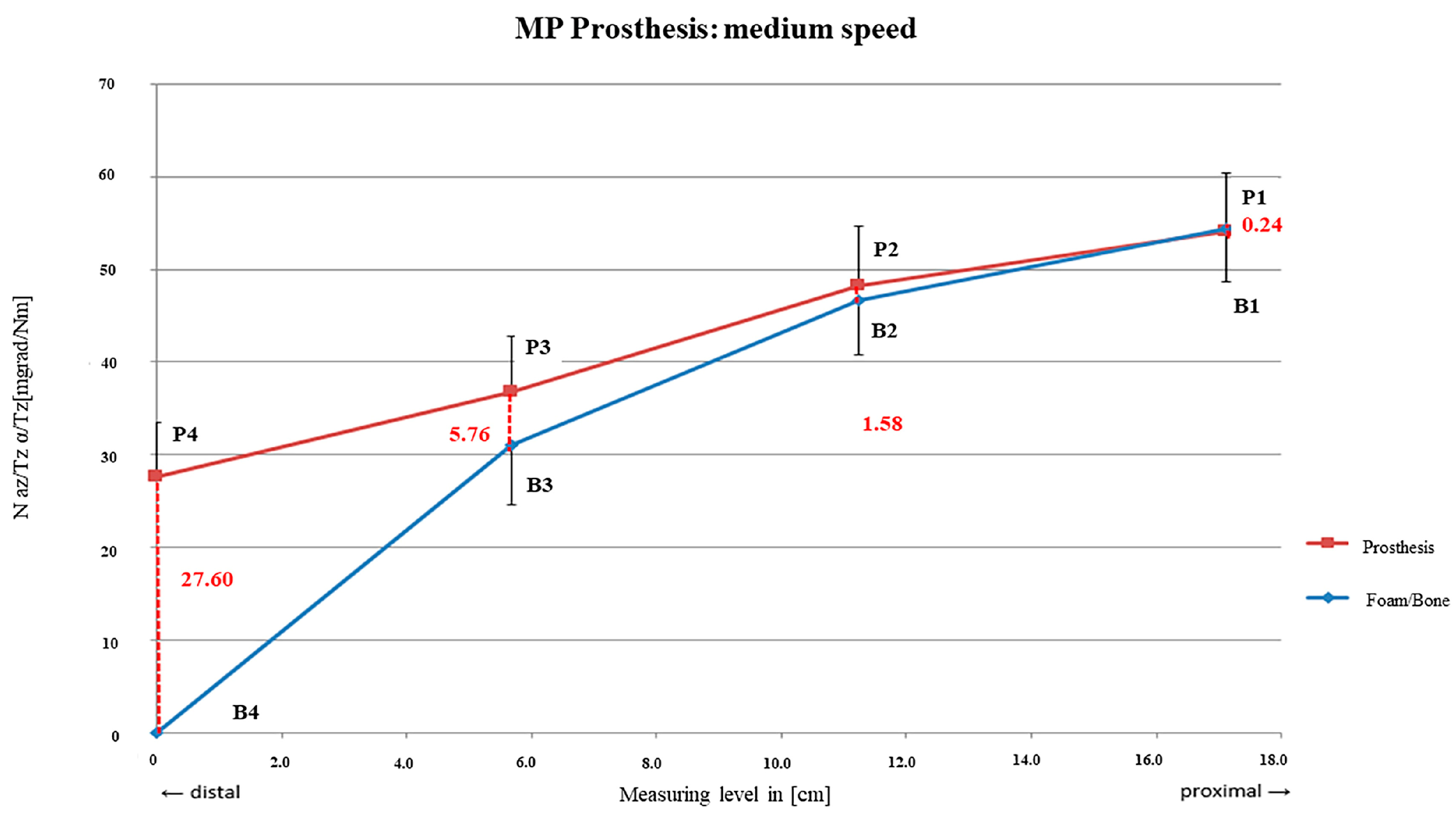
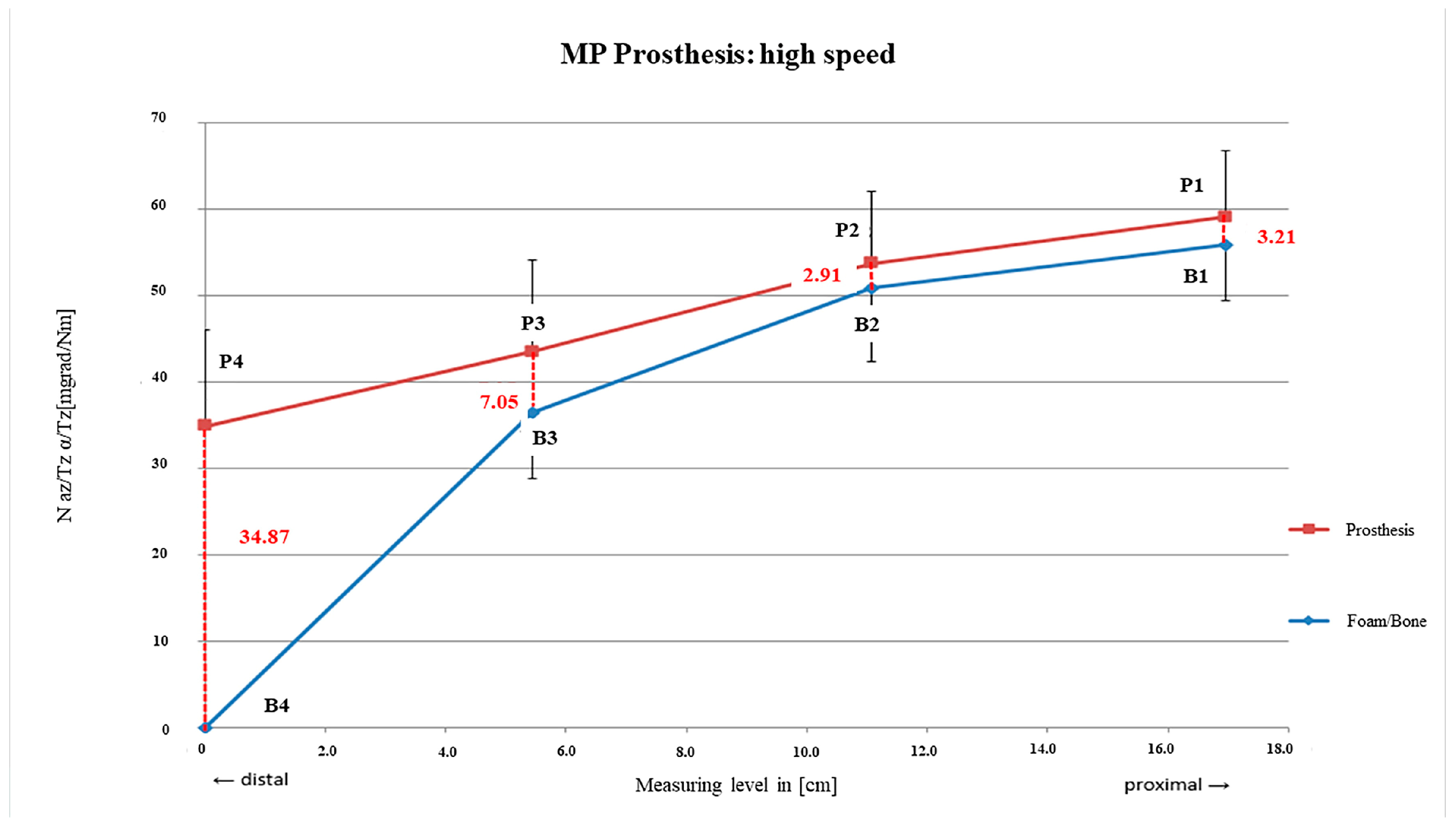
3.1. Link MP® Reconstruction Stem
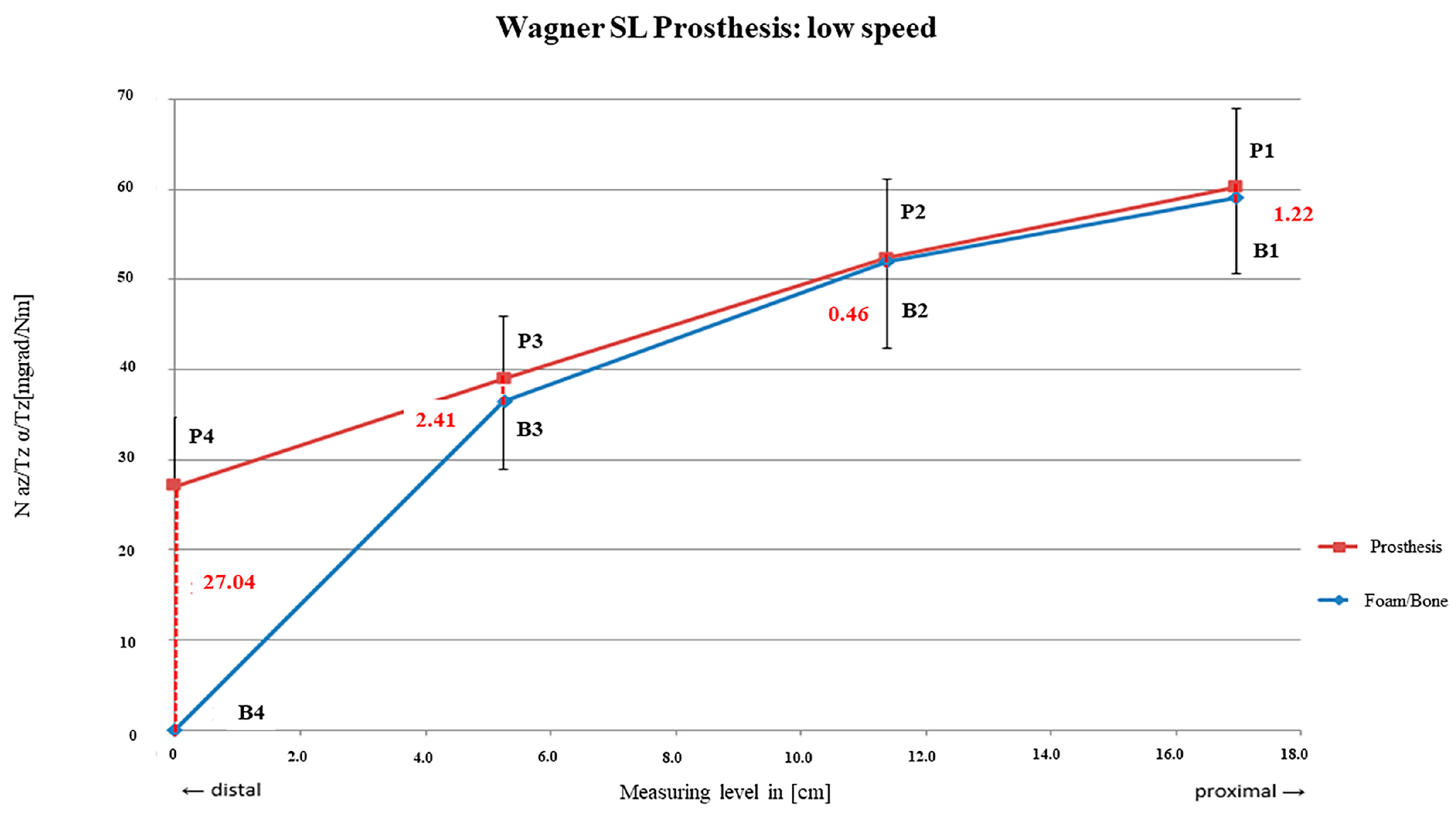
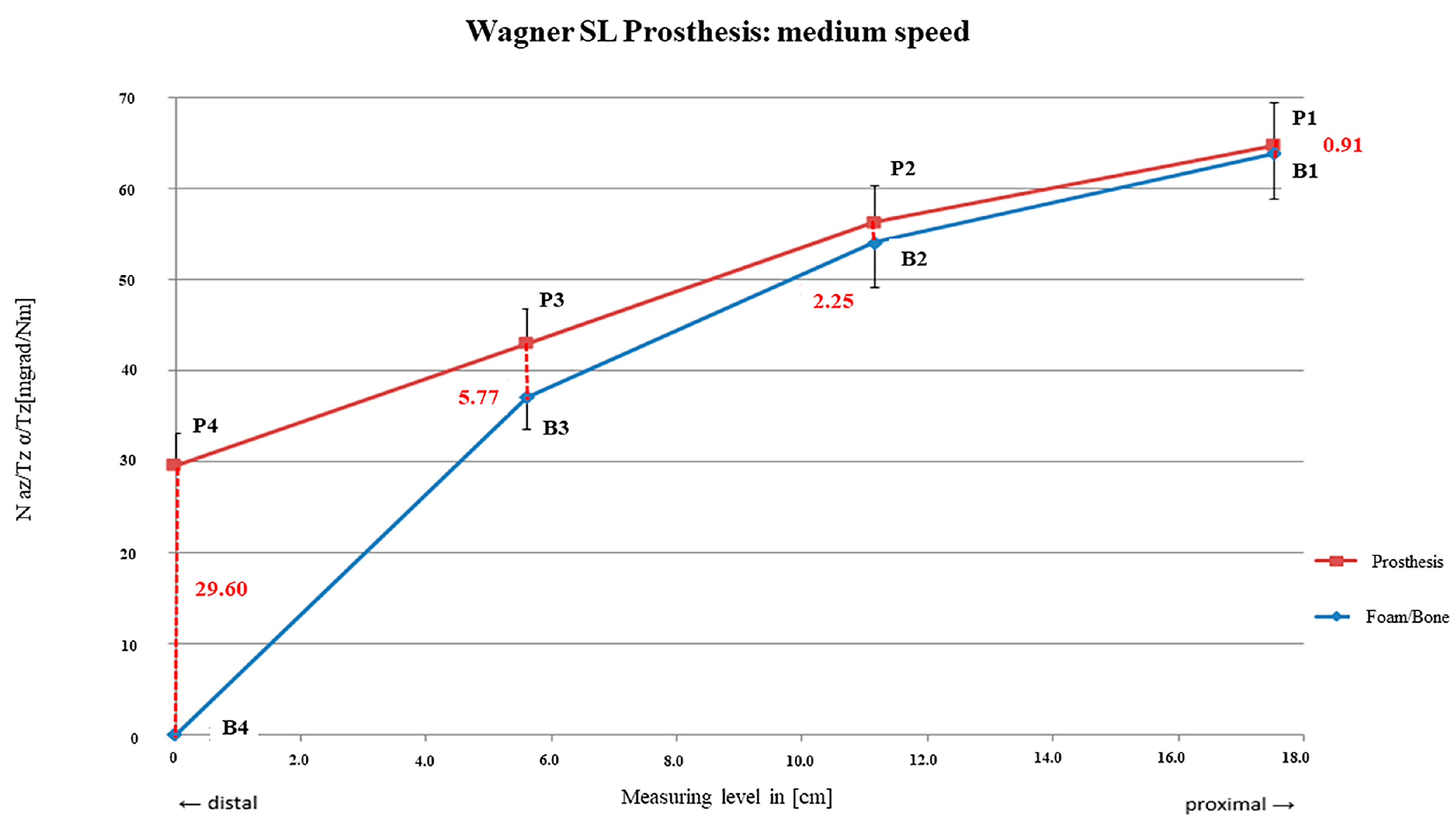
3.2. Wagner SL® Revision Stem
3.3. Comparison of the Two Systems
3.4. Post Hoc Power Analysis
4. Discussion
4.1. Link MP® Reconstruction Stem
4.2. Wagner SL® Revision Stem
4.3. Comparison of Both Systems
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lakstein, D.; Backstein, D.; Safir, O.; Kosashvili, Y.; Gross, A.E. Revision total hip arthroplasty with a porous-coated modular stem: 5 to 10 years follow-up. Clin. Orthop. Relat. Res. 2010, 468, 1310–1315. [Google Scholar]
- Jibodh, S.R.; Schwarzkopf, R.; Anthony, S.G.; Malchau, H.; Dempsey, K.E.; Estok, D.M., 2nd. Revision hip arthroplasty with a modular cementless stem: Mid-term follow up. J. Arthroplasty. 2013, 28, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.S.; Shepherd, D.E.; Hukins, D.W. Compressive properties of commercially available polyurethane foams as mechanical models for osteoporotic human cancellous bone. BMC Musculoskelet. Disord. 2008, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Zerdzicki, K.; Znaczko, A.; Kondrusik, A.; Korbut, W. Compressive and tensile properties of polyurethane foam mimicking trabecular tissue in artificial femoral head bones. Front. Bioeng. Biotechnol. 2025, 12, 1482165. [Google Scholar] [CrossRef] [PubMed]
- Horak, Z.; Dvorak, K.; Zarybnicka, L.; Vojackova, H.; Dvorakova, J.; Vilimek, M. Experimental Measurements of Mechanical Properties of PUR Foam Used for Testing Medical Devices and Instruments Depending on Temperature, Density and Strain Rate. Materials 2020, 13, 4560. [Google Scholar] [CrossRef]
- Liska, F.; Eichhorn, S.; Schreiber, U.; Burgkart, R.R.G. (Eds.) Die Mechanischen Eigenschaften Verschiedener Künstlicher Spongiöser Knochenersatzmaterialien im Vergleich zu Humanen Materiall; DKOU: Berlin, Germany, 2008. [Google Scholar]
- Kuhn, J.L.; Goldstein, S.A.; Ciarelli, M.J.; Matthews, L.S. The limitations of canine trabecular bone as a model for human. A biomechanical study. J. Biomech. 1989, 22, 95–107. [Google Scholar] [CrossRef]
- Martens, M.V.A.R.; Van Audekercke, R.; Delport, P.; De Meester, P.; Mulier, J.C. The mechanical characteristics of cancellous bone at the upper femoral region. J. Biomech. 1983, 16, 971–983. [Google Scholar] [CrossRef]
- Schmidbauer, U.; Brendel, T.; Kunze, K.G.; Nietert, M.; Ecke, H. Dynamic force measurement in implantation of total endoprostheses of the hip joint. Unfallchirurgie 1993, 19, 11–15. [Google Scholar] [CrossRef]
- Bergmann, G.; Graichen, F.; Rohlmann, A. Hip joint loading during walking and running, measured in two patients. J. Biomech. 1993, 26, 969–990. [Google Scholar] [CrossRef]
- Jakubowitz, E.; Bitsch, R.G.; Heisel, C.; Lee, C.; Kretzer, J.P.; Thomsen, M.N. Primary rotational stability of cylindrical and conical revision hip stems as a function of femoral bone defects: An in vitro comparison. J. Biomech. 2008, 41, 3078–3084. [Google Scholar] [CrossRef]
- Wagner, H.; Wagner, M. [Femur revision prosthesis]. Z Orthop. Ihre Grenzgeb. 1993, 131, 574–577. [Google Scholar] [CrossRef]
- Park, Y.; Choi, D.; Hwang, D.S.; Yoon, Y.S. Statistical analysis of interfacial gap in a cementless stem FE model. J. Biomech. Eng. 2009, 131, 021016. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, B.; Cristofolini, L.; Taddei, F.; Viceconti, M. Sensitivity of the primary stability of a cementless hip stem to its position and orientation. Artif. Organs. 2008, 32, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Reimeringer, M.; Nuno, N. The influence of contact ratio and its location on the primary stability of cementless total hip arthroplasty: A finite element analysis. J. Biomech. 2016, 49, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Al-Azzawi, A.; Riyadh, H.; Mukka, S.; Sayed-Noor, A. Cementless, modular, distally fixed stem in hip revision arthroplasty: A single-center study of 132 consecutive hips. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 45–50. [Google Scholar] [CrossRef]
- Kwong, L.M.; Miller, A.J.; Lubinus, P. A modular distal fixation option for proximal bone loss in revision total hip arthroplasty: A 2- to 6-year follow-up study. J. Arthroplasty. 2003, 18 (Suppl. S1), 94–97. [Google Scholar] [CrossRef]
- Reimeringer, M.; Nuno, N.; Desmarais-Trepanier, C.; Lavigne, M.; Vendittoli, P.A. The influence of uncemented femoral stem length and design on its primary stability: A finite element analysis. Comput. Methods Biomech. Biomed. Engin. 2013, 16, 1221–1231. [Google Scholar] [CrossRef]
- Weiss, R.J.; Beckman, M.O.; Enocson, A.; Schmalholz, A.; Stark, A. Minimum 5-year follow-up of a cementless, modular, tapered stem in hip revision arthroplasty. J. Arthroplasty. 2011, 26, 16–23. [Google Scholar] [CrossRef]
- Wirtz, D.C.; Heller, K.D.; Holzwarth, U.; Siebert, C.; Pitto, R.P.; Zeiler, G.; Blencke, B.A.; Forst, R. A modular femoral implant for uncemented stem revision in THR. Int. Orthop. 2000, 24, 134–138. [Google Scholar] [CrossRef]
- Tamvakopoulos, G.S.; Servant, C.T.; Clark, G.; Ivory, J.P. Medium-term follow-up series using a modular distal fixation prosthesis to address proximal femoral bone deficiency in revision total hip arthroplasty. A 5- to 9-year follow-up study. Hip Int. 2007, 17, 143–149. [Google Scholar] [CrossRef]
- Ducheyne, P.; De Meester, P.; Aernoudt, E. Influence of a functional dynamic loading on bone ingrowth into surface pores of orthopedic implants. J. Biomed. Mater. Res. 1977, 11, 811–838. [Google Scholar] [CrossRef]
- Pilliar, R.M.; Lee, J.M.; Maniatopoulos, C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin. Orthop. Relat. Res. 1986, 208, 108–113. [Google Scholar] [CrossRef]
- Baca, V.; Horak, Z.; Mikulenka, P.; Dzupa, V. Comparison of an inhomogeneous orthotropic and isotropic material models used for FE analyses. Med. Eng. Phys. 2008, 30, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Brusi, G.; Pancanti, A.; Cristofolini, L. Primary stability of an anatomical cementless hip stem: A statistical analysis. J. Biomech. 2006, 39, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Tarala, M.; Janssen, D.; Verdonschot, N. Toward a method to simulate the process of bone ingrowth in cementless THA using finite element method. Med. Eng. Phys. 2013, 35, 543–548. [Google Scholar] [CrossRef]
- Böhm, P.; Bischel, O. The use of tapered stems for femoral revision surgery. Clin. Orthop. Relat. Res. 2004, 420, 148–159. [Google Scholar] [CrossRef]
- Paulsen, K. [Histological examinations about possible heat lesions of bone and cartilage during boring and grinding (author’s transl)]. Arch. Otorhinolaryngol. 1976, 212, 35–41. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bischel, O.E.; Seeger, J.B.; Jung, M.K.; Dörfler, S.; Höppchen, A.J.; Jahnke, A.; Jakubowitz, E. The Influence of Reaming Velocity During Preparation of the Femoral Canal—An In Vitro Analysis of Two Straight Femoral Revision Stems with a Fluted Tapered Design. Bioengineering 2025, 12, 984. https://doi.org/10.3390/bioengineering12090984
Bischel OE, Seeger JB, Jung MK, Dörfler S, Höppchen AJ, Jahnke A, Jakubowitz E. The Influence of Reaming Velocity During Preparation of the Femoral Canal—An In Vitro Analysis of Two Straight Femoral Revision Stems with a Fluted Tapered Design. Bioengineering. 2025; 12(9):984. https://doi.org/10.3390/bioengineering12090984
Chicago/Turabian StyleBischel, Oliver E., Jörn B. Seeger, Matthias K. Jung, Stefan Dörfler, Arnold J. Höppchen, Alexander Jahnke, and Eike Jakubowitz. 2025. "The Influence of Reaming Velocity During Preparation of the Femoral Canal—An In Vitro Analysis of Two Straight Femoral Revision Stems with a Fluted Tapered Design" Bioengineering 12, no. 9: 984. https://doi.org/10.3390/bioengineering12090984
APA StyleBischel, O. E., Seeger, J. B., Jung, M. K., Dörfler, S., Höppchen, A. J., Jahnke, A., & Jakubowitz, E. (2025). The Influence of Reaming Velocity During Preparation of the Femoral Canal—An In Vitro Analysis of Two Straight Femoral Revision Stems with a Fluted Tapered Design. Bioengineering, 12(9), 984. https://doi.org/10.3390/bioengineering12090984






