Nanoparticle-Based Delivery Systems for Synergistic Therapy in Lung Cancers
Abstract
1. Introduction
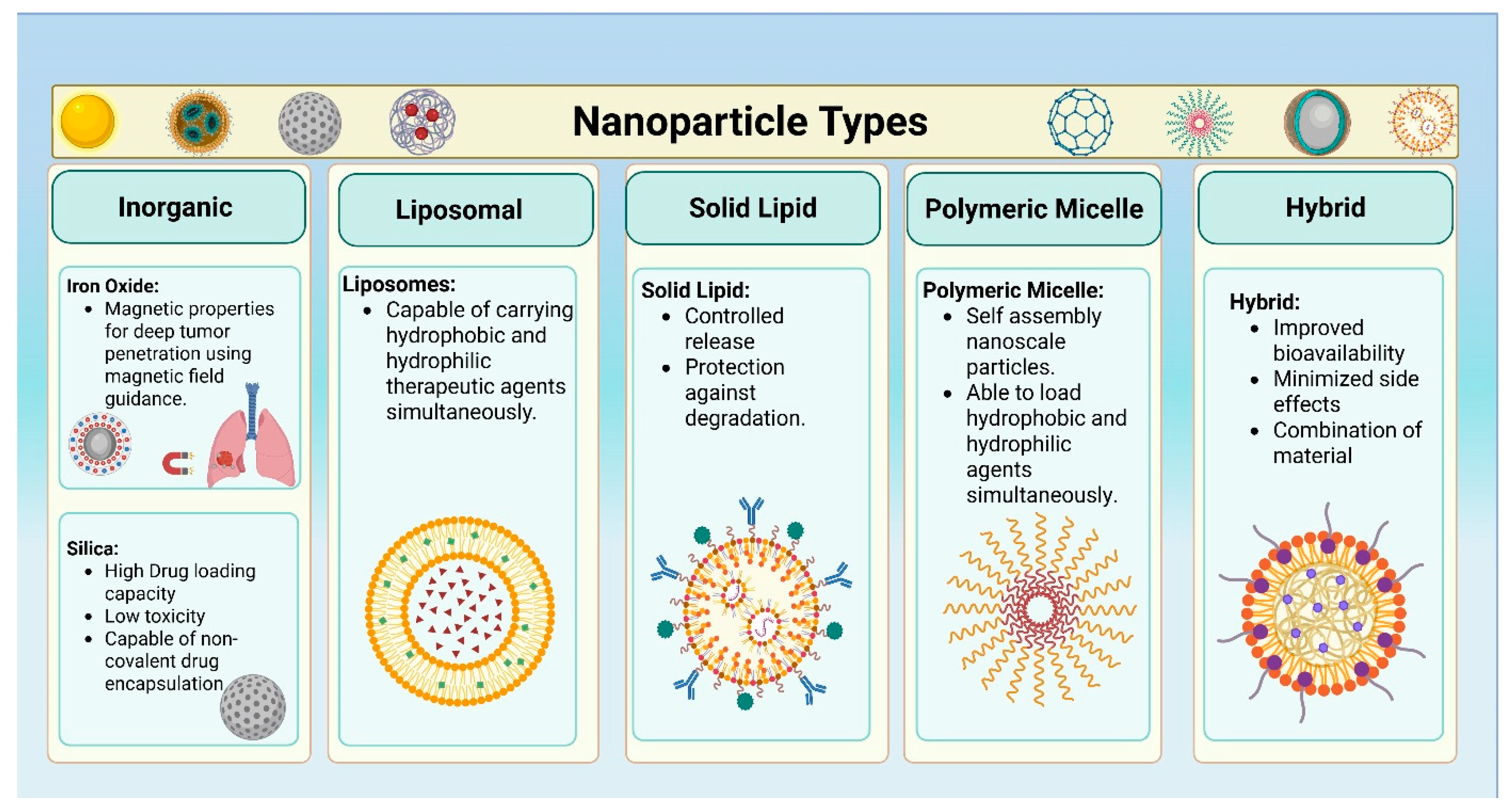
2. Different Types of Nanoparticles for Simultaneous Delivery of Therapeutic Agents in Lung Cancers
2.1. Liposomes
2.2. Solid Lipid Nanoparticles
2.3. Polymeric Micelles
2.4. Inorganic Nanoparticles
2.5. Hybrid Nanoparticles
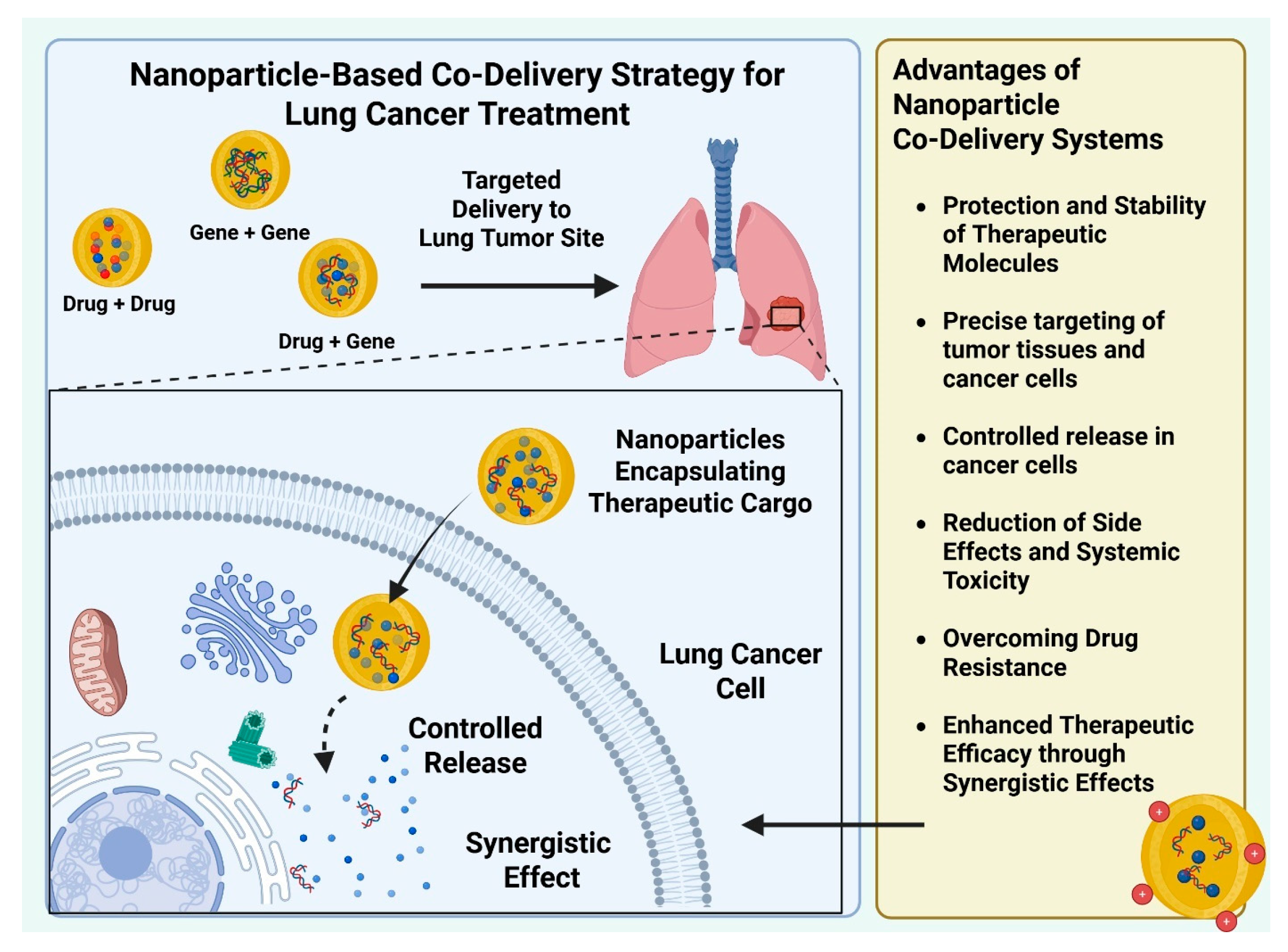
3. Lung Cancer Synergistic Therapy Using Nanoparticle-Based Co-Delivery Systems
3.1. Nanoparticle-Based “Drug–Drug” Co-Delivery Systems
3.2. Nanoparticle-Based “Gene-Gene” Co-Delivery Systems
3.3. Nanoparticle-Based “Drug-Gene” Co-Delivery Systems
4. Current Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Adenosine triphosphate | ATP |
| Enhanced permeability and retention | EPR |
| Epidermal growth factor receptor | EGFR |
| Nanoparticles | NPs |
| Non-small cell lung cancer | NSCLC |
| Solid lipid nanoparticles | SLN |
| Berberine | BBR |
| Magnolol | MAG |
| Small interfering RNA | siRNA |
| Paclitaxel | PTX |
| Docetaxel | DTX |
| Polyethylene glycol | PEG |
| Poly(lactic-co-glycolic acid) | PLGA |
| Mesoporous silica nanoparticles | MSNs |
| Amphiphilic poly(lactic-co-glycolic acid)–polyethylene glycol | PLGA-PEG |
| Superparamagnetic iron oxide nanoparticles | SPIONs |
| Adipic acid dihydrazide | ADH |
| Hyaluronic acid | HA |
| Erlotinib | ERL |
| Bevacizumab | BEV |
| Transferrin | Tf |
| Methoxy poly (ethylene glycol)-poly(ethylenimine)-poly(l-glutamate) | mPEG-OEI-PLG |
| Doxorubicin | DXR |
| Cisplatin | CP |
| Tocopherol polyethylene glycol 1000 succinate | TPGS |
| Polyvinyl imine | PEI |
| Polycaprolactone | PCL |
| Polyvinyl alcohol | PVA |
| Poly (β-Amino ester) | PBAE |
| Near-infrared | NIR |
| Conjugated linoleic acid | CLA |
| Lipid-coated iron oxide NPS | IONP-C/O@LP |
| Cytosine-phosphate-guanine | CPG |
| Genistein | GNS |
| All-trans retinoic acid | ATRA |
| PGAM1 siRNA | siPGAM1 |
| Multidrug resistance | MDR |
| P-glycoprotein | P-gp |
| General control non-repressed 5 protein | GCN5 |
| Inhibitors of apoptosis proteins | IAPs |
| Small hairpin RNAs | shRNA |
| Hypoxia-inducible factors | HIFs |
| ATP-binding cassette | ABC |
| Caspase 8 | CASP8 |
| Liposome-incorporated poly | ε-caprolactone |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Ganesh, K.; Massague, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- Merjaneh, N.; Hajjar, M.; Lan, Y.W.; Kalinichenko, V.V.; Kalin, T.V. The promise of combination therapies with foxm1 inhibitors for cancer treatment. Cancers 2024, 16, 756. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Sharma, A.; Shambhwani, D.; Pandey, S.; Singh, J.; Lalhlenmawia, H.; Kumarasamy, M.; Singh, S.K.; Chellappan, D.K.; Gupta, G.; Prasher, P.; et al. Advances in lung cancer treatment using nanomedicines. ACS Omega 2023, 8, 10–41. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Milewski, D.; Balli, D.; Ustiyan, V.; Le, T.; Dienemann, H.; Warth, A.; Breuhahn, K.; Whitsett, J.A.; Kalinichenko, V.V.; Kalin, T.V. Foxm1 activates agr2 and causes progression of lung adenomas into invasive mucinous adenocarcinomas. PLoS Genet. 2017, 13, e1007097. [Google Scholar] [CrossRef]
- Milewski, D.; Pradhan, A.; Wang, X.; Cai, Y.; Le, T.; Turpin, B.; Kalinichenko, V.V.; Kalin, T.V. Foxf1 and foxf2 transcription factors synergistically promote rhabdomyosarcoma carcinogenesis by repressing transcription of p21(cip1) cdk inhibitor. Oncogene 2017, 36, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Milewski, D.; Pradhan, A.; Rama, N.; Rice, K.; Le, T.; Flick, M.J.; Vaz, S.; Zhao, X.; Setchell, K.D.; et al. The foxm1 inhibitor rcm-1 decreases carcinogenesis and nuclear beta-catenin. Mol. Cancer Ther. 2019, 18, 1217–1229. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.X.; Lyu, M.; Deng, Z.C.; Shi, D.L. Transparent porphyrin-based hybrid films for spectral selective solar harvesting and energy generation. Sol. Energy Mater. Sol. Cells 2022, 243, 111788. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, B. Amoxicillin degradation and antimutagenic potential of phytofabricated silver nanoparticles-doped polyurethane membrane for wastewater treatment. Nano LIFE 2023, 13, 2350009. [Google Scholar] [CrossRef]
- Pradhan, A.; Dunn, A.; Ustiyan, V.; Bolte, C.; Wang, G.; Whitsett, J.A.; Zhang, Y.; Porollo, A.; Hu, Y.C.; Xiao, R.; et al. The s52f foxf1 mutation inhibits stat3 signaling and causes alveolar capillary dysplasia. Am. J. Respir. Crit. Care Med. 2019, 200, 1045–1056. [Google Scholar] [CrossRef]
- Wang, G.; Wen, B.; Deng, Z.; Zhang, Y.; Kolesnichenko, O.A.; Ustiyan, V.; Pradhan, A.; Kalin, T.V.; Kalinichenko, V.V. Endothelial progenitor cells stimulate neonatal lung angiogenesis through foxf1-mediated activation of bmp9/acvrl1 signaling. Nat. Commun. 2022, 13, 2080. [Google Scholar] [CrossRef]
- Deng, Z.; Kalin, G.T.; Shi, D.; Kalinichenko, V.V. Nanoparticle delivery systems with cell-specific targeting for pulmonary diseases. Am. J. Respir. Cell Mol. Biol. 2021, 64, 292–307. [Google Scholar] [CrossRef]
- Bian, F.; Lan, Y.W.; Zhao, S.; Deng, Z.; Shukla, S.; Acharya, A.; Donovan, J.; Le, T.; Milewski, D.; Bacchetta, M.; et al. Lung endothelial cells regulate pulmonary fibrosis through foxf1/r-ras signaling. Nat. Commun. 2023, 14, 2560. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shan, X.; Chen, Z.; Gao, N.; Zeng, W.; Zeng, X.; Mei, L. Applications of surface modification technologies in nanomedicine for deep tumor penetration. Adv. Sci. 2020, 8, 2002589. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, J.; Li, Q. Preparation of bifunctional nanoparticles and evaluation of therapeutic function in glioma cells. Nano LIFE 2023, 14, 2350017. [Google Scholar] [CrossRef]
- Bian, F.; Goda, C.; Wang, G.; Lan, Y.W.; Deng, Z.; Gao, W.; Acharya, A.; Reza, A.A.; Gomez-Arroyo, J.; Merjaneh, N.; et al. Foxf1 promotes tumor vessel normalization and prevents lung cancer progression through fzd4. EMBO Mol. Med. 2024, 16, 1063–1090. [Google Scholar] [CrossRef]
- Radeva, L.; Yoncheva, K. Nanogels-innovative drug carriers for overcoming biological membranes. Gels 2025, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Vandesompele, J.; Braeckmans, K.; De Smedt, S.C.; Remaut, K. Nucleic acid degradation as barrier to gene delivery: A guide to understand and overcome nuclease activity. Chem. Soc. Rev. 2024, 53, 317–360. [Google Scholar] [CrossRef]
- Micura, R.; Hobartner, C. Fundamental studies of functional nucleic acids: Aptamers, riboswitches, ribozymes and dnazymes. Chem. Soc. Rev. 2020, 49, 7331–7353. [Google Scholar] [CrossRef] [PubMed]
- Dolai, J.; Mandal, K.; Jana, N.R. Nanoparticle size effects in biomedical applications. ACS Appl. Nano Mater. 2021, 4, 6471–6496. [Google Scholar] [CrossRef]
- Wu, J. The enhanced permeability and retention (epr) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpinski, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, S.; Wang, Y.; Shi, D. Circulating tumor cell isolation for cancer diagnosis and prognosis. eBioMedicine 2022, 83, 104237. [Google Scholar] [CrossRef]
- Han, X.; Deng, Z.; Yang, Z.; Wang, Y.; Zhu, H.; Chen, B.; Cui, Z.; Ewing, R.C.; Shi, D. Biomarkerless targeting and photothermal cancer cell killing by surface-electrically-charged superparamagnetic Fe3O4 composite nanoparticles. Nanoscale 2017, 9, 1457–1465. [Google Scholar] [CrossRef]
- Deng, Z.; Lin, J.; Bud′ko, S.L.; Webster, B.; Kalin, T.V.; Kalinichenko, V.V.; Shi, D. Dual targeting with cell surface electrical charge and folic acid via superparamagnetic Fe3O4@Cu2−xS for photothermal cancer cell killing. Cancers 2021, 13, 5275. [Google Scholar] [CrossRef]
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Ren, L.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; et al. Targeting negative surface charges of cancer cells by multifunctional nanoprobes. Theranostics 2016, 6, 1887–1898. [Google Scholar] [CrossRef]
- Essa, M.L.; Elashkar, A.A.; Hanafy, N.A.N.; Saied, E.M.; El-Kemary, M. Dual targeting nanoparticles based on hyaluronic and folic acids as a promising delivery system of the encapsulated 4-methylumbelliferone (4-mu) against invasiveness of lung cancer in vivo and in vitro. Int. J. Biol. Macromol. 2022, 206, 467–480. [Google Scholar] [CrossRef]
- Moradi, R.; Mohammadzadeh, R.; Akbari, A. Kappa-carrageenan crosslinked magnetic folic acid-conjugated chitosan nanocomposites for arginase encapsulation, delivery and cancer therapy. Nano LIFE 2021, 11, 2140005. [Google Scholar] [CrossRef]
- Kohram, F.; Deng, Z.; Zhang, Y.; Al Reza, A.A.; Li, E.; Kolesnichenko, O.A.; Shukla, S.; Ustiyan, V.; Gomez-Arroyo, J.; Acharya, A.; et al. Demonstration of safety in wild type mice of npfoxf1, a novel nanoparticle-based gene therapy for alveolar capillary dysplasia with misaligned pulmonary veins. Biologics 2023, 17, 43–55. [Google Scholar] [CrossRef]
- Sun, F.; Wang, G.; Pradhan, A.; Xu, K.; Gomez-Arroyo, J.; Zhang, Y.; Kalin, G.T.; Deng, Z.; Vagnozzi, R.J.; He, H.; et al. Nanoparticle delivery of stat3 alleviates pulmonary hypertension in a mouse model of alveolar capillary dysplasia. Circulation 2021, 144, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Bolte, C.; Ustiyan, V.; Ren, X.; Dunn, A.W.; Pradhan, A.; Wang, G.; Kolesnichenko, O.A.; Deng, Z.; Zhang, Y.; Shi, D.; et al. Nanoparticle delivery of proangiogenic transcription factors into the neonatal circulation inhibits alveolar simplification caused by hyperoxia. Am. J. Respir. Crit. Care Med. 2020, 202, 100–111. [Google Scholar] [CrossRef]
- Dunn, A.W.; Kalinichenko, V.V.; Shi, D. Highly efficient in vivo targeting of the pulmonary endothelium using novel modifications of polyethylenimine: An importance of charge. Adv. Healthc. Mater. 2018, 7, e1800876. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Rivas-Garcia, L.; Baptista, P.V.; Fernandes, A.R. Gene therapy in cancer treatment: Why go nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef]
- Miron-Barroso, S.; Domenech, E.B.; Trigueros, S. Nanotechnology-based strategies to overcome current barriers in gene delivery. Int. J. Mol. Sci. 2021, 22, 8537. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y.; Wu, Z.; Zhang, L.; He, D.; Li, X.; Wang, Z. Synergistic combination therapy of lung cancer: Cetuximab functionalized nanostructured lipid carriers for the co-delivery of paclitaxel and 5-demethylnobiletin. Biomed. Pharmacother. 2019, 118, 109225. [Google Scholar] [CrossRef]
- Kapoor, D.U.; Gandhi, S.M.; Swarn, S.; Lal, B.; Prajapati, B.G.; Khondee, S.; Mangmool, S.; Singh, S.; Chittasupho, C. Polymeric nanoparticles for targeted lung cancer treatment: Review and perspectives. Pharmaceutics 2025, 17, 1091. [Google Scholar] [CrossRef]
- Omidian, H.; Gill, E.J.; Cubeddu, L.X. Lipid nanoparticles in lung cancer therapy. Pharmaceutics 2024, 16, 644. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.; Zhou, H.; Liu, C. Advancements in nanocarrier delivery systems for photodynamic therapy in lung cancer. Int. J. Nanomed. 2025, 20, 6853–6874. [Google Scholar] [CrossRef]
- Nair, N.U.; Greninger, P.; Zhang, X.; Friedman, A.A.; Amzallag, A.; Cortez, E.; Sahu, A.D.; Lee, J.S.; Dastur, A.; Egan, R.K.; et al. A landscape of response to drug combinations in non-small cell lung cancer. Nat. Commun. 2023, 14, 3830. [Google Scholar] [CrossRef]
- Rahman, M.A. Exploration of nanomedicine-based dry powder inhalation formulation co-loaded with erlotinib and curcumin for treatment of non-small cell lung cancer. Nano LIFE 2024, 15, 24500144. [Google Scholar] [CrossRef]
- Afsharzadeh, M.; Hashemi, M.; Mokhtarzadeh, A.; Abnous, K.; Ramezani, M. Recent advances in co-delivery systems based on polymeric nanoparticle for cancer treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1095–1110. [Google Scholar] [CrossRef]
- Fumoto, S.; Nishida, K. Co-delivery systems of multiple drugs using nanotechnology for future cancer therapy. Chem. Pharm. Bull. 2020, 68, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Zhang, Z.; Wang, C.H.; Fan, Y.S.; Meng, Q.Y.; You, Y.Z. Interactions in DNA condensation: An important factor for improving the efficacy of gene transfection. Bioconjug Chem. 2019, 30, 284–292. [Google Scholar] [CrossRef]
- Majid, A.; Memon, N.H.; Arain, S.Q.; Channa, U.; Ali, M.; Phull, A.R. Bioinspired cellular membrane vesicles derived from bacterial and erythrocyte ghosts: Development and applications as a smart drug delivery system. Nano LIFE 2024, 2441001. [Google Scholar] [CrossRef]
- Nel, J.; Elkhoury, K.; Velot, E.; Bianchi, A.; Acherar, S.; Francius, G.; Tamayol, A.; Grandemange, S.; Arab-Tehrany, E. Functionalized liposomes for targeted breast cancer drug delivery. Bioact. Mater. 2023, 24, 401–437. [Google Scholar] [CrossRef]
- Carvalho, B.G.; Vit, F.F.; Carvalho, H.F.; Han, S.W.; de la Torre, L.G. Recent advances in co-delivery nanosystems for synergistic action in cancer treatment. J. Mater. Chem. B 2021, 9, 1208–1237. [Google Scholar] [CrossRef]
- Li, Y.; Cong, H.; Wang, S.; Yu, B.; Shen, Y. Liposomes modified with bio-substances for cancer treatment. Biomater. Sci. 2020, 8, 6442–6468. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Jazani, R.S.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by design-driven zeta potential optimisation study of liposomes with charge imparting membrane additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, R.; Rezayat, S.M.; Faridi-Majidi, R.; Sadri, K.; Jaafari, M.R. Optimization of docetaxel loading conditions in liposomes: Proposing potential products for metastatic breast carcinoma chemotherapy. Sci. Rep. 2020, 10, 5569. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.E.; Benkstein, K.D.; Cleveland, T.E.T.; Anderson, K.W.; Carrier, M.J.; Vreeland, W.N. Particle metrology approach to understanding how storage conditions affect long-term liposome stability. Langmuir 2023, 39, 12313–12323. [Google Scholar] [CrossRef]
- Ma, X.X.; Sui, X.Y.; Liu, C.; Li, H.; Han, C.Y.; Xu, T.; Zhang, H.Y.; Zhang, Y.J.; Cai, D.F.; Li, Y.J.; et al. Co-delivery of berberine and magnolol targeted liposomes for synergistic anti-lung cancer. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131773. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and strategies of cationic liposomes for cancer gene therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Do, J.; Verma, G.; Maurice, Y.; Abdumanobova, M.; Deng, Z.; Kalin, T.V.; Kalinichenko, V.V. Protocol for minicircle production for gene therapy without subsequent cleanup steps. STAR Protoc. 2025, 6, 103982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.M.; Zhang, S.B.; Zhi, D.F.; Zhao, Y.N.; Cui, S.H.; Cui, J.N. Co-delivery of paclitaxel and survivin sirna with cationic liposome for lung cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124054. [Google Scholar] [CrossRef]
- Gu, Z.; Da Silva, C.G.; Van der Maaden, K.; Ossendorp, F.; Cruz, L.J. Liposome-based drug delivery systems in cancer immunotherapy. Pharmaceutics 2020, 12, 1054. [Google Scholar] [CrossRef] [PubMed]
- Magar, K.T.; Boafo, G.F.; Li, X.T.; Chen, Z.J.; He, W. Liposome-based delivery of biological drugs. Chin. Chem. Lett. 2022, 33, 587–596. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Catucci, L. Liposomes containing nanoparticles: Preparation and applications. Colloids Surf. B Biointerfaces 2022, 218, 112737. [Google Scholar] [CrossRef]
- Qu, M.H.; Zeng, R.F.; Fang, S.; Dai, Q.S.; Li, H.P.; Long, J.T. Liposome-based co-delivery of sirna and docetaxel for the synergistic treatment of lung cancer. Int. J. Pharm. 2014, 474, 112–122. [Google Scholar] [CrossRef]
- Wang, S.; Gou, J.; Wang, Y.; Tan, X.; Zhao, L.; Jin, X.; Tang, X. Synergistic antitumor efficacy mediated by liposomal co-delivery of polymeric micelles of vinorelbine and cisplatin in non-small cell lung cancer. Int. J. Nanomed. 2021, 16, 2357–2372. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Sivadasan, D.; Ramakrishnan, K.; Mahendran, J.; Ranganathan, H.; Karuppaiah, A.; Rahman, H. Solid lipid nanoparticles: Applications and prospects in cancer treatment. Int. J. Mol. Sci. 2023, 24, 6199. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, J.E.N.; Omidi, Y. Solid lipid-based nanocarriers as efficient targeted drug and gene delivery systems. Trac-Trends Anal. Chem. 2016, 77, 100–108. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Badawi, N.; El-Say, K.; Attia, D.; El-Nabarawi, M.; Elmazar, M.; Teaima, M. Development of pomegranate extract-loaded solid lipid nanoparticles: Quality by design approach to screen the variables affecting the quality attributes and characterization. ACS Omega 2020, 5, 21712–21721. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid lipid nanoparticles (slns): An advanced drug delivery system targeting brain through bbb. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid nanoparticles for drug delivery. Adv. NanoBiomed Res. 2021, 2, 202100109. [Google Scholar] [CrossRef]
- Li, M.; Fang, G.; Zahid, F.; Saleem, R.; Ishrat, G.; Ali, Z.; Naeem, M.; Din, F.U. Co-delivery of paclitaxel and curcumin loaded solid lipid nanoparticles for improved targeting of lung cancer: In vitro and in vivo investigation. Heliyon 2024, 10, e30290. [Google Scholar] [CrossRef]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. Ph-responsive polymer nanomaterials for tumor therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Wang, L.; Brey, E.M.; Uribe, G.R.; Tang, L. Smart nanoparticles for chemo-based combinational therapy. Pharmaceutics 2021, 13, 853. [Google Scholar] [CrossRef]
- Gupta, B.; Poudel, B.K.; Regmi, S.; Pathak, S.; Ruttala, H.B.; Gautam, M.; An, G.J.; Jeong, J.H.; Choi, H.G.; Yong, C.S.; et al. Paclitaxel and erlotinib-co-loaded solid lipid core nanocapsules: Assessment of physicochemical characteristics and cytotoxicity in non-small cell lung cancer. Pharm. Res. 2018, 35, 96. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, P.; Chen, Y.; Sun, J.; Kong, F. Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy. Int. J. Mol. Med. 2014, 34, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Li, N.; Liu, Y.; Su, H. Combination lung cancer chemotherapy: Design of a ph-sensitive transferrin-peg-hz-lipid conjugate for the co-delivery of docetaxel and baicalin. Biomed. Pharmacother. 2017, 95, 548–555. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Yt, K. Progress in polymeric micelles for drug delivery applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, Y.; Guo, Z.; Chen, J.; Lin, L.; Wu, J.; Tian, H.; Chen, X. Pulmonary delivery by exploiting doxorubicin and cisplatin co-loaded nanoparticles for metastatic lung cancer therapy. J. Control. Release 2019, 295, 153–163. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Yao, Y.; Zhang, S.; Gu, Z. Reverse micelle-based water-soluble nanoparticles for simultaneous bioimaging and drug delivery. Org. Biomol. Chem. 2017, 15, 3232–3238. [Google Scholar] [CrossRef]
- Walters, S.H.; Birchfield, A.S.; Fuglestad, B. Advances in utilizing reverse micelles to investigate membrane proteins. Biochem. Soc. Trans. 2024, 52, 2499–2511. [Google Scholar] [CrossRef]
- Sankaran, R.; Bong, J.H.; Chow, Y.H.; Wong, F.W.F.; Ling, T.C.; Show, P.L. Reverse micellar system in protein recovery—A review of the latest developments. Curr. Protein Pept. Sci. 2019, 20, 1012–1026. [Google Scholar] [CrossRef]
- Gill, K.K.; Kaddoumi, A.; Nazzal, S. Mixed micelles of peg(2000)-dspe and vitamin-e tpgs for concurrent delivery of paclitaxel and parthenolide: Enhanced chemosenstization and antitumor efficacy against non-small cell lung cancer (nsclc) cell lines. Eur. J. Pharm. Sci. 2012, 46, 64–71. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A review of polymeric micelles and their applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantu, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Gao, W.; Kohram, F.; Li, E.; Kalin, T.V.; Shi, D.; Kalinichenko, V.V. Fluorinated amphiphilic poly(beta-amino ester) nanoparticle for highly efficient and specific delivery of nucleic acids to the lung capillary endothelium. Bioact. Mater. 2024, 31, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.; Deng, Z.; Bian, F.; Shukla, S.; Gomez-Arroyo, J.; Shi, D.; Kalinichenko, V.V.; Kalin, T.V. Improving anti-tumor efficacy of low-dose vincristine in rhabdomyosarcoma via the combination therapy with foxm1 inhibitor rcm1. Front. Oncol. 2023, 13, 1112859. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Polymeric micellar systems—A special emphasis on “smart” drug delivery. Pharmaceutics 2023, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yu, J.; Hao, T.; Wang, W.; Wei, M.; Li, G. Advances in polymeric micelles: Responsive and targeting approaches for cancer immunotherapy in the tumor microenvironment. Pharmaceutics 2023, 15, 2622. [Google Scholar] [CrossRef] [PubMed]
- Tea, L.; Willner, L.; Waldorf, C.; Matsarskaia, O.; Schweins, R.; Förster, S.; Willner, L.; Stellbrink, J. Surface charged polymeric micelles─a tunable model system studied by sans. Macromolecules 2024, 57, 5818–5830. [Google Scholar] [CrossRef]
- Sun, C.; Lu, J.; Wang, J.; Hao, P.; Li, C.; Qi, L.; Yang, L.; He, B.; Zhong, Z.; Hao, N. Redox-sensitive polymeric micelles with aggregation-induced emission for bioimaging and delivery of anticancer drugs. J. Nanobiotechnol. 2021, 19, 14. [Google Scholar] [CrossRef]
- Jin, M.; Jin, G.; Kang, L.; Chen, L.; Gao, Z.; Huang, W. Smart polymeric nanoparticles with ph-responsive and peg-detachable properties for co-delivering paclitaxel and survivin sirna to enhance antitumor outcomes. Int. J. Nanomed. 2018, 13, 2405–2426. [Google Scholar] [CrossRef]
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.; Lee, Y.W.; Nagaraj, H.; Rotello, V.M. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020, 156, 188–213. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Zhou, R.; Deng, Z.; Han, Y.; Han, X.; Tao, W.; Yang, Z.; Shi, C.; Hong, D.; et al. Targeting and regulating of an oncogene via nanovector delivery of microrna using patient-derived xenografts. Theranostics 2017, 7, 677–693. [Google Scholar] [CrossRef]
- Sharma, P.; Holliger, N.; Pfromm, P.H.; Liu, B.; Chikan, V. Size-controlled synthesis of iron and iron oxide nanoparticles by the rapid inductive heating method. ACS Omega 2020, 5, 19853–19860. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Haq, I.U.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, T.; Gao, J. Biocompatible iron oxide nanoparticles for targeted cancer gene therapy: A review. Nanomaterials 2022, 12, 3323. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine 2018, 13, 929–952. [Google Scholar] [CrossRef]
- Ngema, L.M.; Adeyemi, S.A.; Marimuthu, T.; Ubanako, P.; Wamwangi, D.; Choonara, Y.E. Synthesis of novel conjugated linoleic acid (cla)-coated superparamagnetic iron oxide nanoparticles (spions) for the delivery of paclitaxel with enhanced in vitro anti-proliferative activity on a549 lung cancer cells. Pharmaceutics 2022, 14, 829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Wang, Q.W.; Xu, Y.J.; Sun, H.M.; Wang, L.; Zhang, L.X. Co-delivery of cisplatin and oleanolic acid by silica nanoparticles-enhanced apoptosis and reverse multidrug resistance in lung cancer. Kaohsiung J. Med. Sci. 2021, 37, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric lipid hybrid nanoparticles (plns) as emerging drug delivery platform—A comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, P.; Chen, Q.; Wang, Z.; Gu, Y.; Ma, J.; Li, W.; Yang, C.; Qiao, Y.; Hou, Y.; et al. Two-pronged intracellular co-delivery of antigen and adjuvant for synergistic cancer immunotherapy. Adv. Mater. 2022, 34, e2202168. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhang, Z.; Wang, B.; Chen, G.; Zhang, Y.; Deng, H.; Tang, Z.; Mao, J.; Wang, L. Combination chemotherapy of lung cancer—Co-delivery of docetaxel prodrug and cisplatin using aptamer-decorated lipid-polymer hybrid nanoparticles. Drug Des. Dev. Ther. 2020, 14, 2249–2261. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Xing, H.; Sun, Y.; Feng, S.; Wang, S. Non-small cell lung cancer combination therapy: Hyaluronic acid modified, epidermal growth factor receptor targeted, ph sensitive lipid-polymer hybrid nanoparticles for the delivery of erlotinib plus bevacizumab. Biomed. Pharmacother. 2020, 125, 109861. [Google Scholar] [CrossRef]
- Kamel, N.M.; Helmy, M.W.; Abdelfattah, E.Z.; Khattab, S.N.; Ragab, D.; Samaha, M.W.; Fang, J.Y.; Elzoghby, A.O. Inhalable dual-targeted hybrid lipid nanocore-protein shell composites for combined delivery of genistein and all-trans retinoic acid to lung cancer cells. ACS Biomater. Sci. Eng. 2020, 6, 71–87. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, C.; Chen, Z.; Li, M.; Li, Y.; Gao, J. Tumor microenvironment-activated cancer cell membrane-liposome hybrid nanoparticle-mediated synergistic metabolic therapy and chemotherapy for non-small cell lung cancer. J. Nanobiotechnol. 2021, 19, 339. [Google Scholar] [CrossRef]
- Al Bostami, R.D.; Abuwatfa, W.H.; Husseini, G.A. Recent advances in nanoparticle-based co-delivery systems for cancer therapy. Nanomaterials 2022, 12, 2672. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of atp-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, Y.; Kim, J.; Cho, H.; Kim, K. Overcoming cancer drug resistance with nanoparticle strategies for key protein inhibition. Molecules 2024, 29, 3994. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, J.; Yu, X.; Liu, X.; Cheng, Y.; Zhou, C.; Li, M.; Shi, L.; Deng, Y.; Liu, H.; et al. Tumor-targeting ph/redox dual-responsive nanosystem epigenetically reverses cancer drug resistance by co-delivering doxorubicin and gcn5 sirna. Acta Biomater. 2021, 135, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yin, Q.; Chen, L.; Zhang, Z.; Li, Y. Co-delivery of paclitaxel and survivin shrna by pluronic p85-pei/tpgs complex nanoparticles to overcome drug resistance in lung cancer. Biomaterials 2012, 33, 8613–8624. [Google Scholar] [CrossRef]
- Wicks, E.E.; Semenza, G.L. Hypoxia-inducible factors: Cancer progression and clinical translation. J. Clin. Investig. 2022, 132, e159839. [Google Scholar] [CrossRef]
- Lee, P.W.T.; Koseki, L.R.; Haitani, T.; Harada, H.; Kobayashi, M. Hypoxia-inducible factor-dependent and independent mechanisms underlying chemoresistance of hypoxic cancer cells. Cancers 2024, 16, 1729. [Google Scholar] [CrossRef]
- Bui, B.P.; Nguyen, P.L.; Lee, K.; Cho, J. Hypoxia-inducible factor-1: A novel therapeutic target for the management of cancer, drug resistance, and cancer-related pain. Cancers 2022, 14, 6054. [Google Scholar] [CrossRef]
- Jalouli, M. Emerging role of hypoxia-inducible factors (hifs) in modulating autophagy: Perspectives on cancer therapy. Int. J. Mol. Sci. 2025, 26, 1752. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mei, H.; Gao, Y.; Xie, X.; Nie, H.; Li, T.; Zhang, H.; Jia, L. Co-delivery of oxygen and erlotinib by aptamer-modified liposomal complexes to reverse hypoxia-induced drug resistance in lung cancer. Biomaterials 2017, 145, 56–71. [Google Scholar] [CrossRef]
- Li, Y.; Thambi, T.; Lee, D.S. Co-delivery of drugs and genes using polymeric nanoparticles for synergistic cancer therapeutic effects. Adv. Healthc. Mater. 2018, 7, 1700886. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; TanTai, J. Co-delivery anticancer drug nanoparticles for synergistic therapy against lung cancer cells. Drug Des. Dev. Ther. 2020, 14, 4503–4510. [Google Scholar] [CrossRef]
- Zhong, T.; Liu, X.; Li, H.; Zhang, J. Co-delivery of sorafenib and crizotinib encapsulated with polymeric nanoparticles for the treatment of in vivo lung cancer animal model. Drug Deliv. 2021, 28, 2108–2118. [Google Scholar] [CrossRef]
- Fan, X.; Wang, T.; Ji, Z.; Li, Q.; Shen, H.; Wang, J. Synergistic combination therapy of lung cancer using lipid-layered cisplatin and oridonin co-encapsulated nanoparticles. Biomed. Pharmacother. 2021, 141, 111830. [Google Scholar] [CrossRef]
- Tian, J.; Min, Y.; Rodgers, Z.; Au, K.M.; Hagan, C.T.T.; Zhang, M.; Roche, K.; Yang, F.; Wagner, K.; Wang, A.Z. Co-delivery of paclitaxel and cisplatin with biocompatible plga-peg nanoparticles enhances chemoradiotherapy in non-small cell lung cancer models. J. Mater. Chem. B 2017, 5, 6049–6057. [Google Scholar] [CrossRef]
- Zhang, M.; Hagan, C.T.T.; Foley, H.; Tian, X.; Yang, F.; Au, K.M.; Mi, Y.; Medik, Y.; Roche, K.; Wagner, K.; et al. Co-delivery of etoposide and cisplatin in dual-drug loaded nanoparticles synergistically improves chemoradiotherapy in non-small cell lung cancer models. Acta Biomater. 2021, 124, 327–335. [Google Scholar] [CrossRef]
- Wan, X.; Min, Y.; Bludau, H.; Keith, A.; Sheiko, S.S.; Jordan, R.; Wang, A.Z.; Sokolsky-Papkov, M.; Kabanov, A.V. Drug combination synergy in worm-like polymeric micelles improves treatment outcome for small cell and non-small cell lung cancer. ACS Nano 2018, 12, 2426–2439. [Google Scholar] [CrossRef]
- Yang, T.; Yu, S.; Liu, L.; Sun, Y.; Lan, Y.; Ma, X.; Zhu, R.; Li, L.; Hou, Y.; Liu, Y. Dual polymeric prodrug co-assembled nanoparticles with precise ratiometric co-delivery of cisplatin and metformin for lung cancer chemoimmunotherapy. Biomater. Sci. 2020, 8, 5698–5714. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sarkar, S.S.; Saproo, S.; Yadav, S.; Antil, D.; Das, B.; Naidu, S. Apoptosis-targeted gene therapy for non-small cell lung cancer using chitosan-poly-lactic-co-glycolic acid -based nano-delivery system and casp8 and mirs 29a-b1 and 34a. Front. Bioeng. Biotechnol. 2023, 11, 1188652. [Google Scholar] [CrossRef] [PubMed]
- Mujokoro, B.; Adabi, M.; Sadroddiny, E.; Adabi, M.; Khosravani, M. Nano-structures mediated co-delivery of therapeutic agents for glioblastoma treatment: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Kordbacheh, H.; Bahmani, E.; Bybordi, S.; Rezaee, A.; Dehghanian, Z.; Ehsanfar, N.; Goleij, P.; Sharifianjazi, F.; Irani, M. Co-delivery of bcl-2 sirna and doxorubicin using liposome-incorporated poly(ε-caprolactone)/chitosan nanofibers for the treatment of lung cancer. J. Drug Deliv. Sci. Technol. 2024, 99, 105994. [Google Scholar] [CrossRef]
- Xu, C.; Tian, H.; Wang, P.; Wang, Y.; Chen, X. The suppression of metastatic lung cancer by pulmonary administration of polymer nanoparticles for co-delivery of doxorubicin and survivin sirna. Biomater. Sci. 2016, 4, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Monteiro, P.F.; Gurnani, P.; Stolnik, S.; Ungaro, F.; Quaglia, F.; Clarke, P.; Grabowska, A.; Kavallaris, M.; Alexander, C. Multi-component bioresponsive nanoparticles for synchronous delivery of docetaxel and tubb3 sirna to lung cancer cells. Nanoscale 2021, 13, 11414–11426. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Y.; Zhang, E.; Jiang, M.; Zhi, D.; Chen, H.; Cui, S.; Zhen, Y.; Cui, J.; Zhang, S. Co-delivery of paclitaxel and anti-vegf sirna by tripeptide lipid nanoparticle to enhance the anti-tumor activity for lung cancer therapy. Drug Deliv. 2020, 27, 1397–1411. [Google Scholar] [CrossRef] [PubMed]

| NP | Cargo | Efficacy | Toxicity | Reference |
|---|---|---|---|---|
| Liposome | Berberine (BBR) and Magnolol (MAG) | An in vitro study showed improved cellular uptake and enhanced cytotoxicity on lung cancer A549 cells. | Reduced systemic toxicity (No significant body weight and pathological changes observed in treated mice) | [57] |
| Liposome | Survivin-specific siRNA and paclitaxel (PTX) | Enhanced siRNA uptake by approximately 1.4-fold. Increased Cytotoxicity on NCI-H460 lung cancer cells. | A decreased PTX dosage was required to limit toxic effects. | [60] |
| Liposome | Docetaxel (DTX) and BCL-2 siRNA | Inhibited cell proliferation in A549 and H226 lung cancer cells in a time-dependent and dose-dependent manner, with significant apoptosis observed. | 100% survival rate for mice treated with lipo-DTX/siRNA. | [65] |
| Solid lipid | Paclitaxel (PTX) and Erlotinib (ERL) | Enhanced cellular uptake and improved inhibition of NCI-H23 cells. | Not mentioned. | [78] |
| Solid lipid | Docetaxel (DTX) and baicalin (BA) | The tumor volumes in mice treated with Tf-D/B-SLNs were significantly smaller compared to free drugs. | Reduced systemic toxicity (No significant body weight change observed in treated mice) | [80] |
| Polymeric micelles | Doxorubicin (DOX) and cis-platinum (CDDP) | Co-NPs exhibited higher anti-tumor efficiency for metastatic lung cancer than single treatments of DOX or CDDP. | Reduced systemic toxicity (No obvious body weight loss and organ damage observed in treated mice) | [82] |
| Polymeric micelles | Paclitaxel (PTX) and survivin siRNA | Accumulation of nanoparticles at the tumor site led to pronounced inhibition of tumor growth in treated mice. | Reduced systemic toxicity (Minor effect on normal cell progression) | [95] |
| Inorganic nanoparticles | Conjugated linoleic acid (CLA) and Paclitaxel (PTX) | Highly suppressed A549 cell proliferation. | Not mentioned. | [103] |
| Inorganic nanoparticles | Cisplatin and oleanolic acid | Increased Apoptosis of cancer cells in treated mice | Reduced systemic toxicity (No obvious body weight loss observed in treated mice) | [104] |
| Hybrid nanoparticles | Peptide antigen and adjuvant (CpG DNA) | IONP-C/O@LP treatment synergistically improved the efficacy of DC activation and enhanced antigen-specific T cell responses. Significantly inhibited tumor growth and remarkably prolonged animal survival rates. | Reduced systemic toxicity (No obvious body weight loss and organ damage observed in treated mice) | [106] |
| Hybrid nanoparticles | Docetaxel prodrug (DTXp2) and cisplatin (DDP) | LPHNs showed significantly higher distribution in the tumor than free drugs and profound tumor inhibition ability. | No systemic toxicity. Hematological parameters, including blood urea nitrogen (BUN), serum aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels, were all within the normal range for LPHNs groups. | [107] |
| Hybrid nanoparticles | Erlotinib (ERL) and bevacizumab (BEV) | Strong tumor suppression with markedly smaller tumor volumes. | Low cardio-renal toxicity. | [108] |
| Hybrid nanoparticles | Genistein (GNS) and all-trans retinoic acid (ATRA). | The treated group exhibited reduced lung lesion numbers, a lower average count of microscopic metastatic lung adenomatous foci, and decreased tumor biomarker levels in vivo. | Reduced systemic toxicity (No significant body weight change observed in treated mice) | [109] |
| Hybrid nanoparticles | Docetaxel (DTX) and Phosphoglycerate mutase 1 (PGAM1) siRNA (siPGAM1) | NP highly accumulated in A549 tumor xenografts in vivo. | Reduced systemic toxicity (No obvious body weight loss and organ damage observed in treated mice) | [110] |
| Liposome | Erlotinib (ERL) and perfluorooctyl bromide | Reversal of hypoxia-driven drug resistance in cancer | Not mentioned | [120] |
| Lipid | Cisplatin (CP) and Oridonin | Increased cell accumulation due to the cell membrane and lipid-based NP. With an increase in stability. | Cell toxicity was studied using A549/DDP cells, and found that blank NP did not showcase any cell toxicity. Loaded NP increases in toxicity as the concentration of the drugs increases. | [124] |
| Polymeric Micelle | Hyaluronic acid-cisplatin/polystyrene and Polymetformin | Dual-Prodrug exhibited self-assembly with antitumor effects in lung cancer models. | Not mentioned. | [128] |
| Chitosan and poly(lactic-co-glycolic acid) (PLGA) | miR-29A-B1, miR-34A, and CASP8. | Delivering three agents effective at restoring apoptotic signaling in tumor cells. | The highest amount of dose given at 80 ug/mL induced an 88% survival rate of cells | [129] |
| cationic liposome-incorporated poly(ε-caprolactone)/chitosan | Bcl-2 siRNA and Doxorubicin (DXR) | A2780/AD was utilized and demonstrated the co-loaded NP enhanced cancer cell death. | Not mentioned. | [131] |
| PEG-disulfide-PLGA and oligo(β-aminoesters). | TUBB3 siRNA and Docetaxel (DTX) | Enhanced the cytotoxic effect on NSCLC by bypassing the drug resistance mechanism. | Gene knockdown was successful and showcased no observable cytotoxicity to nearby cells. | [133] |
| Lipid | Paclitaxel (PTX) and VEGF small interfering RNA (siRNA) | Controlled tumor growth using NCI-H460 cells. | A low dose of PTX was required to reduce toxicity levels. | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Siraj, A.A.; Lowry, I.; Ruan, E.; Patel, R.; Gao, W.; Kalin, T.V.; Kalinichenko, V.V. Nanoparticle-Based Delivery Systems for Synergistic Therapy in Lung Cancers. Bioengineering 2025, 12, 968. https://doi.org/10.3390/bioengineering12090968
Deng Z, Siraj AA, Lowry I, Ruan E, Patel R, Gao W, Kalin TV, Kalinichenko VV. Nanoparticle-Based Delivery Systems for Synergistic Therapy in Lung Cancers. Bioengineering. 2025; 12(9):968. https://doi.org/10.3390/bioengineering12090968
Chicago/Turabian StyleDeng, Zicheng, Ali Al Siraj, Isabella Lowry, Ellen Ruan, Rohan Patel, Wen Gao, Tanya V. Kalin, and Vladimir V. Kalinichenko. 2025. "Nanoparticle-Based Delivery Systems for Synergistic Therapy in Lung Cancers" Bioengineering 12, no. 9: 968. https://doi.org/10.3390/bioengineering12090968
APA StyleDeng, Z., Siraj, A. A., Lowry, I., Ruan, E., Patel, R., Gao, W., Kalin, T. V., & Kalinichenko, V. V. (2025). Nanoparticle-Based Delivery Systems for Synergistic Therapy in Lung Cancers. Bioengineering, 12(9), 968. https://doi.org/10.3390/bioengineering12090968








