Selenium-Containing Nano-Micelles Delay the Cellular Senescence of BMSCs Under Oxidative Environment and Maintain Their Regenerative Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of mPEG-b-P(TMC-co-MSeSe) Polymer
2.2. Preparation and Characterization of SeSe Nano-Micelles and FITC Loaded SeSe Nano-Micelles
2.3. Cell Culture in an Oxidative Microenvironment
2.4. Detection of Cell Proliferation
2.5. Detection of Cellular Uptake of Nano-Micelles
2.6. Detection of ROS Levels in BMSCs
2.7. SA-β-gal Staining of BMSCs
2.8. Osteogenic Induction of BMSCs
2.9. Detection of ALP Activity in BMSCs
2.10. RNA Analysis Using RT-qPCR
2.11. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of mPEG-b-P(TMC-co-MSeSe) Polymer
3.2. Characterization of NMSe Nano-Micelles
3.3. NMSe Regulates Intracellular ROS Levels in BMSCs in Oxidative Microenvironment
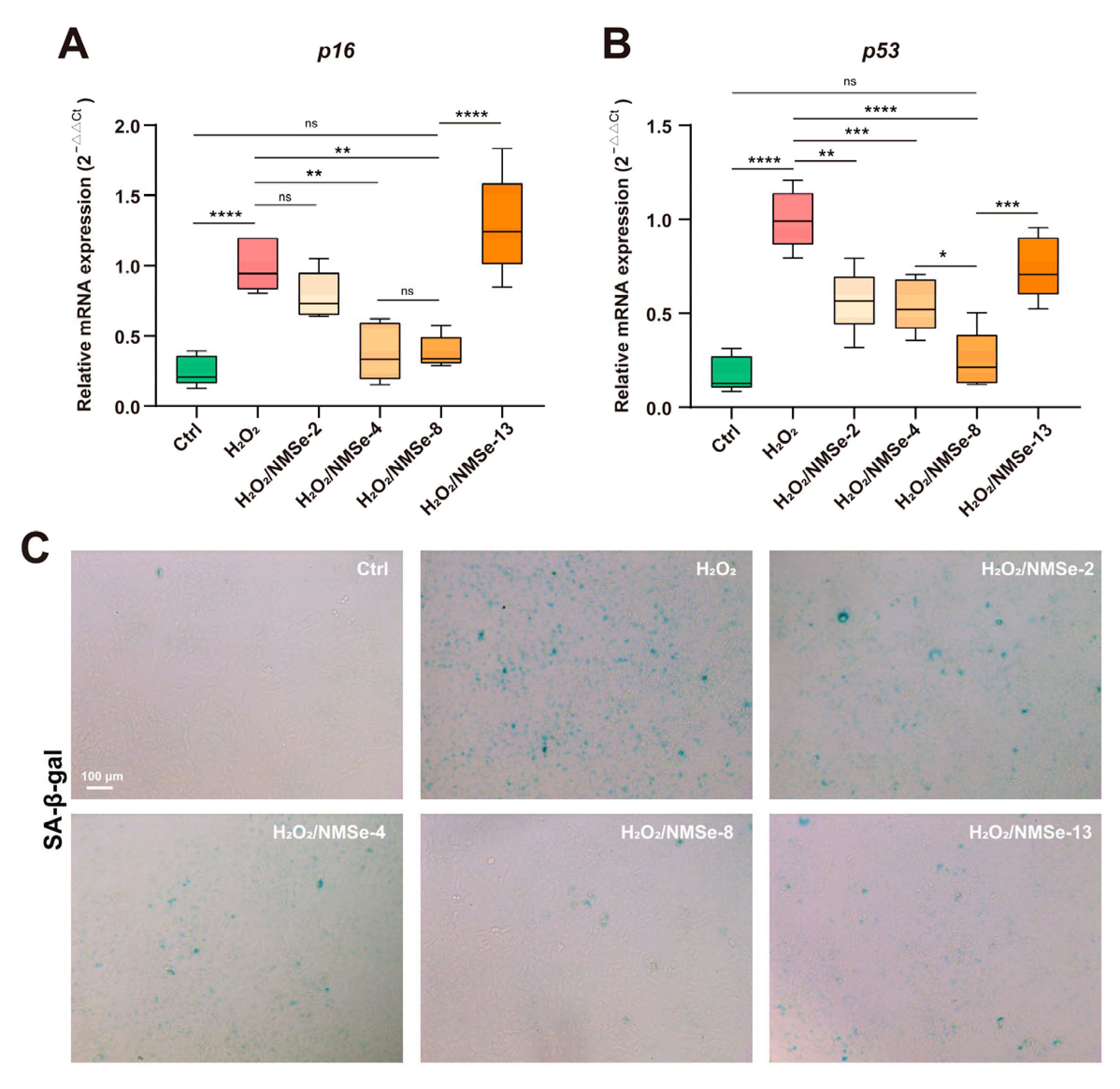
3.4. NMSe Nano-Micelles Delay BMSCs Senescence in Oxidative Microenvironment
3.5. NMSe Nano-Micelles Maintain the Osteogenic Ability of BMSCs in Oxidative Microenvironment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| BMSCs | Bone marrow mesenchymal stem cells |
| Se | Selenium |
| mPEG | Polyethylene glycol monomethyl ether |
| TMC | Trimethylenecarbonate |
| MSeSe | Diethylene disselenate carbonate dimer |
| PSe | Polymer of mPEG-b-P(TMC-co-MSeSe) |
| NMSe | Se-containing nano-micelles |
| PBS | Phosphate-buffered saline |
| CM | Culture medium |
| Alp | Alkaline phosphatase |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
References
- Cai, Y.; Song, W.; Li, J.; Jing, Y.; Liang, C.; Zhang, L.; Zhang, X.; Zhang, W.; Liu, B.; An, Y.; et al. The landscape of aging. Sci. China Life Sci. 2022, 65, 2354–2454. [Google Scholar] [CrossRef]
- Vaupel, J.W. Biodemography of human ageing. Nature 2010, 464, 536–542. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Zhang, Y.; Ji, R.; Li, Z.; Zou, J.; Gao, B. Regulatory cellular and molecular networks in the bone microenvironment during aging. Life Med. 2024, 3, lnae019. [Google Scholar] [CrossRef]
- Brunet, A.; Goodell, M.A.; Rando, T.A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 2022, 24, 45–62. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2020, 22, 75–95. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2014, 22, 377–388. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, J.; Feng, Z.; Guo, S.; Wang, M.; Wang, Z.; Li, Z.; Li, H.; Sui, L. N-acetylcysteine regulates dental follicle stem cell osteogenesis and alveolar bone repair via ROS scavenging. Stem Cell Res. Ther. 2022, 13, 466. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C—Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Ali, D.; Chen, L.; Kowal, J.M.; Okla, M.; Manikandan, M.; AlShehri, M.; AlMana, Y.; AlObaidan, R.; AlOtaibi, N.; Hamam, R.; et al. Resveratrol inhibits adipocyte differentiation and cellular senescence of human bone marrow stromal stem cells. Bone 2020, 133, 115252. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef]
- Dou, C.; Cao, Z.; Ding, N.; Hou, T.; Luo, F.; Kang, F.; Yang, X.; Jiang, H.; Xie, Z.; Hu, M.; et al. Cordycepin Prevents Bone Loss through Inhibiting Osteoclastogenesis by Scavenging ROS Generation. Nutrients 2016, 8, 231. [Google Scholar] [CrossRef]
- Kim, Y.E.; Kim, J. ROS-Scavenging Therapeutic Hydrogels for Modulation of the Inflammatory Response. ACS Appl. Mater. Interfaces 2021, 14, 23002–23021. [Google Scholar] [CrossRef]
- Li, J.; Han, F.; Ma, J.; Wang, H.; Pan, J.; Yang, G.; Zhao, H.; Zhao, J.; Liu, J.; Liu, Z.; et al. Targeting Endogenous Hydrogen Peroxide at Bone Defects Promotes Bone Repair. Adv. Funct. Mater. 2021, 32, 2111208. [Google Scholar] [CrossRef]
- Qian, F.; Han, Y.; Han, Z.; Zhang, D.; Zhang, L.; Zhao, G.; Li, S.; Jin, G.; Yu, R.; Liu, H. In Situ implantable, post-trauma microenvironment-responsive, ROS Depletion Hydrogels for the treatment of Traumatic brain injury. Biomaterials 2021, 270, 120675. [Google Scholar] [CrossRef]
- Yu, S.; Wang, C.; Yu, J.; Wang, J.; Lu, Y.; Zhang, Y.; Zhang, X.; Hu, Q.; Sun, W.; He, C.; et al. Injectable Bioresponsive Gel Depot for Enhanced Immune Checkpoint Blockade. Adv. Mater. 2018, 30, e1801527. [Google Scholar] [CrossRef]
- Li, S.; Zhu, C.; Zhou, X.; Chen, L.; Bo, X.; Shen, Y.; Guan, X.; Han, X.; Shan, D.; Sun, L.; et al. Engineering ROS-Responsive Bioscaffolds for Disrupting Myeloid Cell-Driven Immunosuppressive Niche to Enhance PD-L1 Blockade-Based Postablative Immunotherapy. Adv. Sci. 2022, 9, e2104619. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhuang, W.; Yu, T.; He, H.; Ma, B.; Chen, L.; Yang, L.; Li, G.; Wang, Y. ROS and GSH Dual-Responsive GEM Prodrug Micelles for ROS-Triggered Fluorescence Turn on Bioimaging and Cancer Therapy. Adv. Mater. Interfaces 2020, 7, 2000294. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, B.; Deng, Y.; Li, T.; Tian, Q.; Yuan, Z.; Ma, L.; Cheng, C.; Guo, Q.; Qiu, L. Biocatalytic and Antioxidant Nanostructures for ROS Scavenging and Biotherapeutics. Adv. Funct. Mater. 2021, 31, 2101804. [Google Scholar] [CrossRef]
- Hou, W.; Xu, H. Incorporating Selenium into Heterocycles and Natural Products—From Chemical Properties to Pharmacological Activities. J. Med. Chem. 2022, 65, 4436–4456. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, Y.; Yan, B.; Du, Z.; Lang, M. A Versatile Strategy to Main Chain Sulfur/Selenium-Functionalized Polycarbonates by Macro-Ring Closure of Diols and Subsequent Ring-Opening Polymerization. Chem. A Eur. J. 2017, 24, 789–792. [Google Scholar] [CrossRef]
- Wilson, A.; Shehadeh, L.A.; Yu, H.; Webster, K.A. Age-related molecular genetic changes of murine bone marrow mesenchymal stem cells. BMC Genom. 2010, 11, 229. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, W.-J.; Shin, H.-R.; Yoon, H.-I.; Moon, J.-I.; Lee, E.; Lim, J.-M.; Cho, Y.-D.; Lee, M.-H.; Kim, H.-G.; et al. ROS-induced PADI2 downregulation accelerates cellular senescence via the stimulation of SASP production and NFκB activation. Cell. Mol. Life Sci. 2022, 79, 155. [Google Scholar] [CrossRef]
- Umapathy, S.; Pan, I.; Issac, P.K.; Kumar, M.S.K.; Giri, J.; Guru, A.; Arockiaraj, J. Selenium Nanoparticles as Neuroprotective Agents: Insights into Molecular Mechanisms for Parkinson’s Disease Treatment. Mol. Neurobiol. 2024, 62, 6655–6682. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Gao, S.; Li, T.; Guo, Y.; Sun, C.; Xianyu, B.; Xu, H. Selenium-Containing Nanoparticles Combine the NK Cells Mediated Immunotherapy with Radiotherapy and Chemotherapy. Adv. Mater. 2020, 32, e1907568. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Sun, C.; Ma, Y.; Chen, X.; Wang, Y.; Chen, K.; Xie, F.; Zhang, Y.; Yuan, Y.; Liu, C. Rejuvenating Aged Bone Repair through Multihierarchy Reactive Oxygen Species-Regulated Hydrogel. Adv. Mater. 2023, 36, e2306552. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
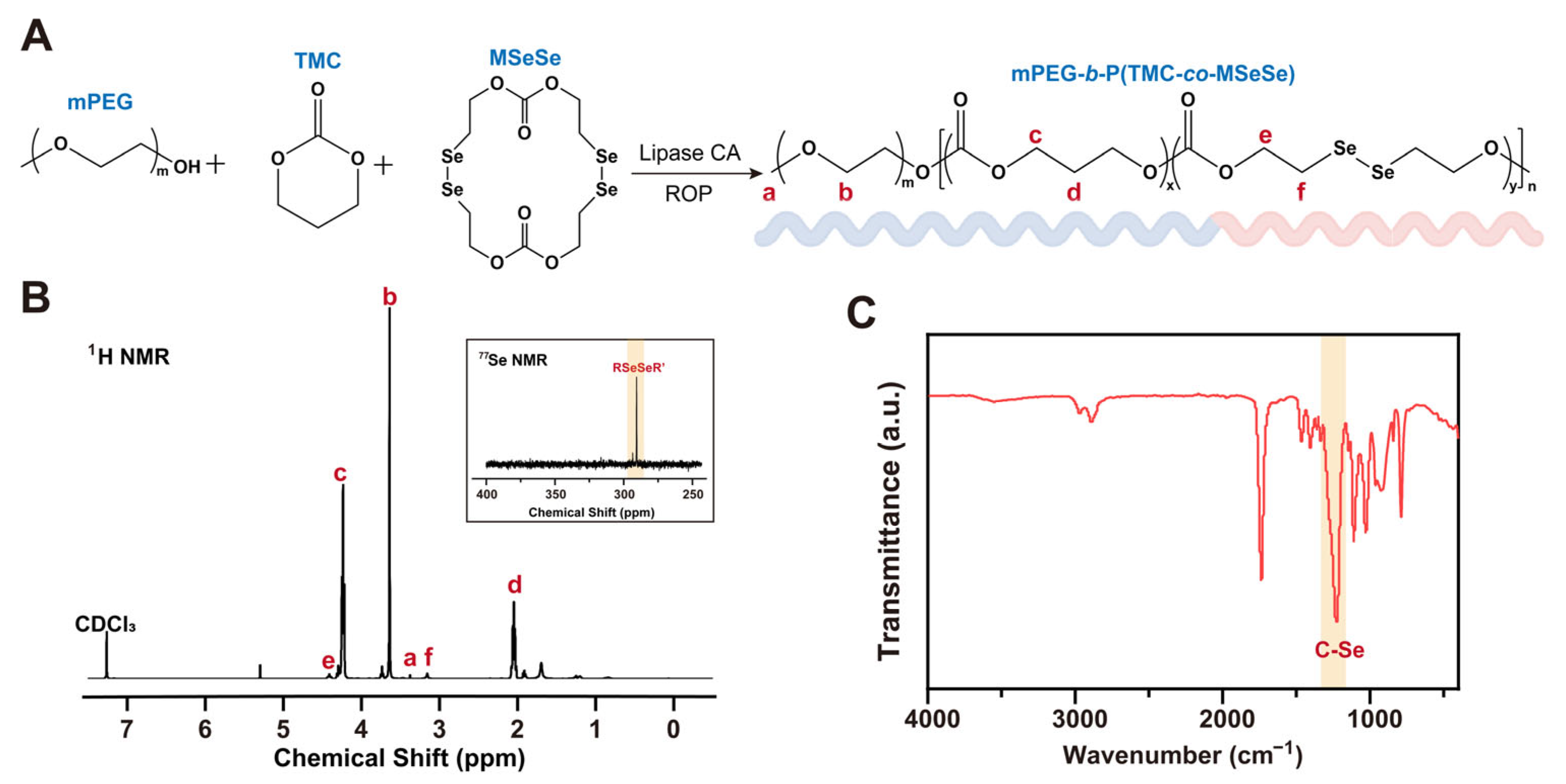
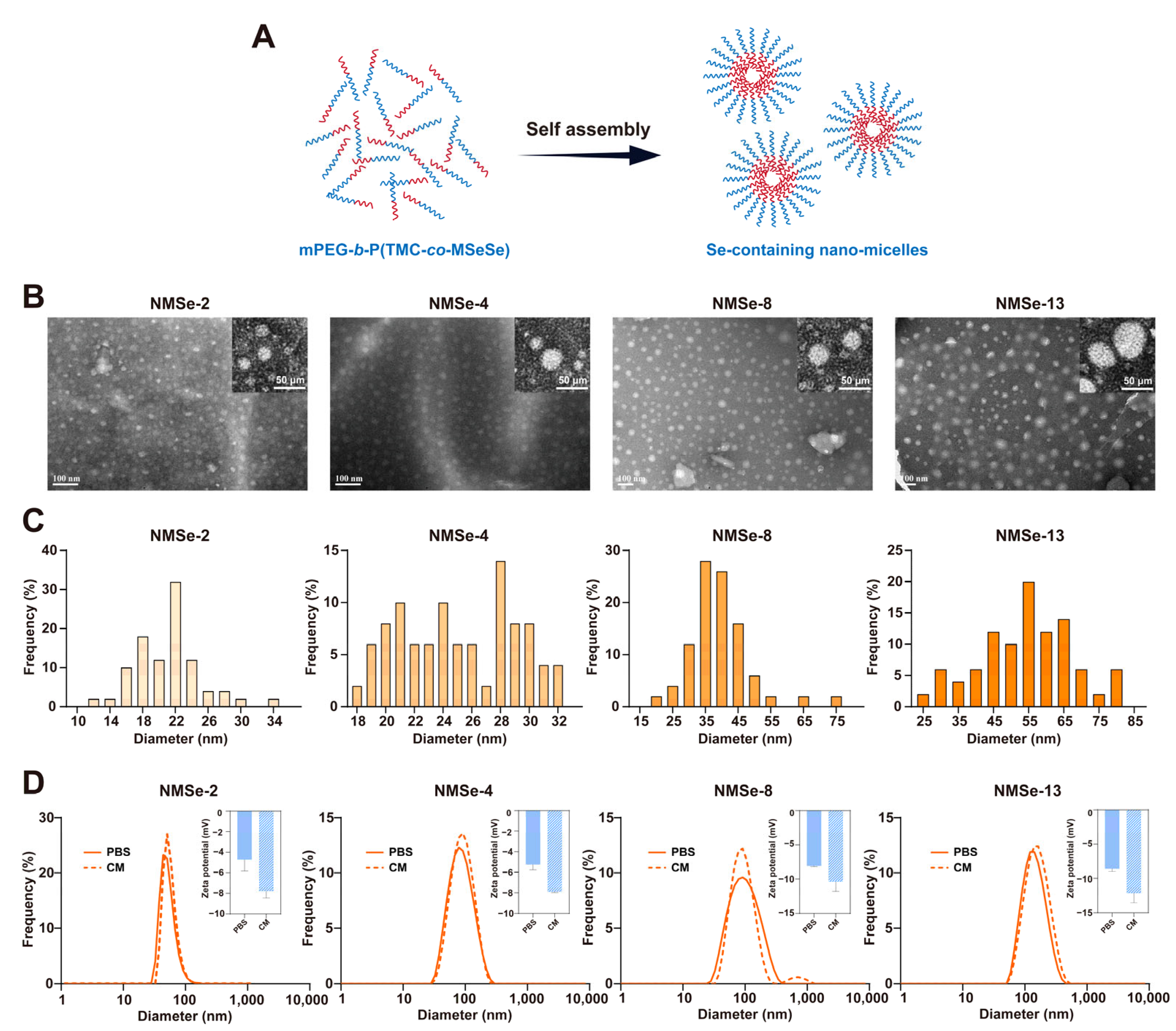
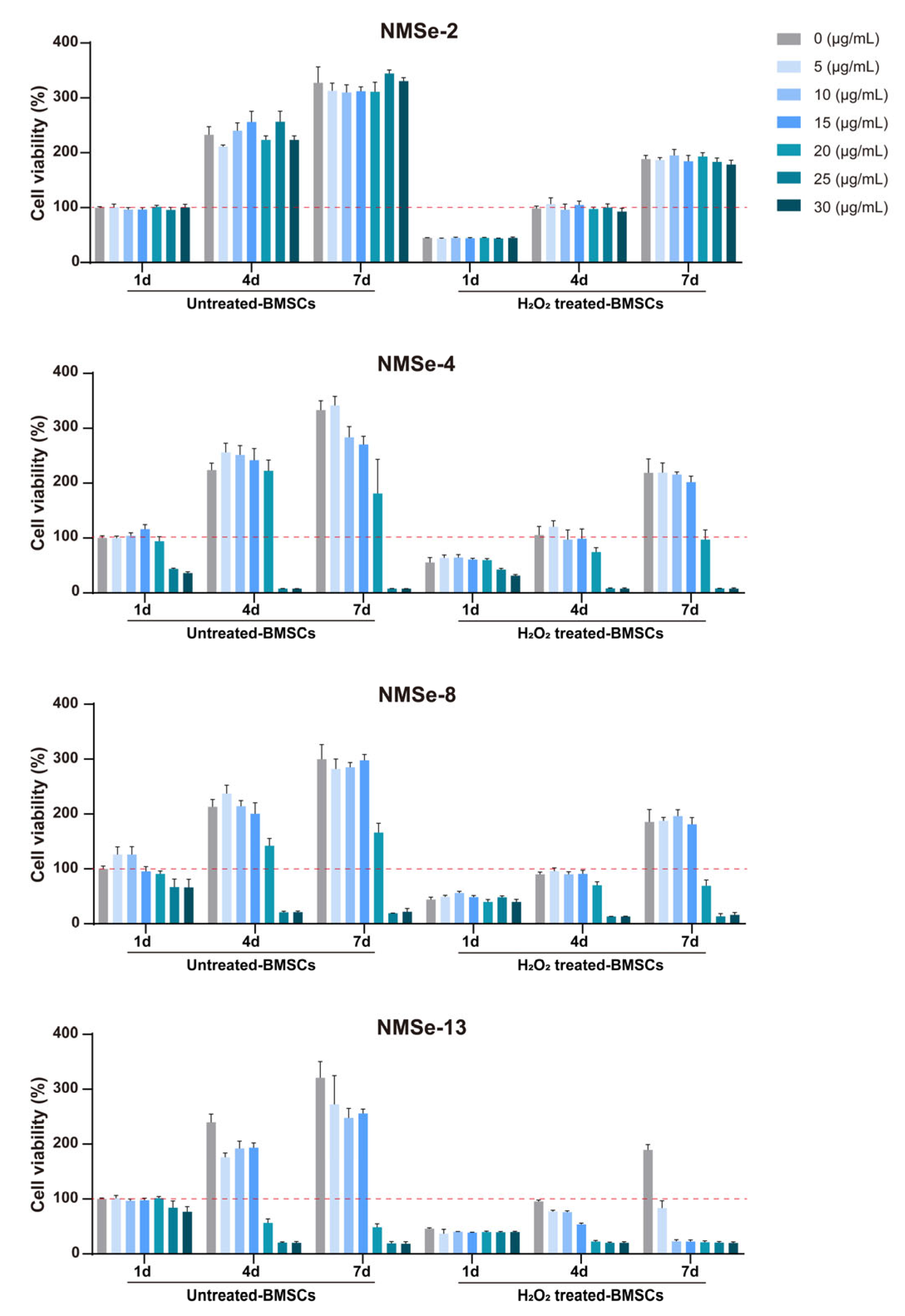
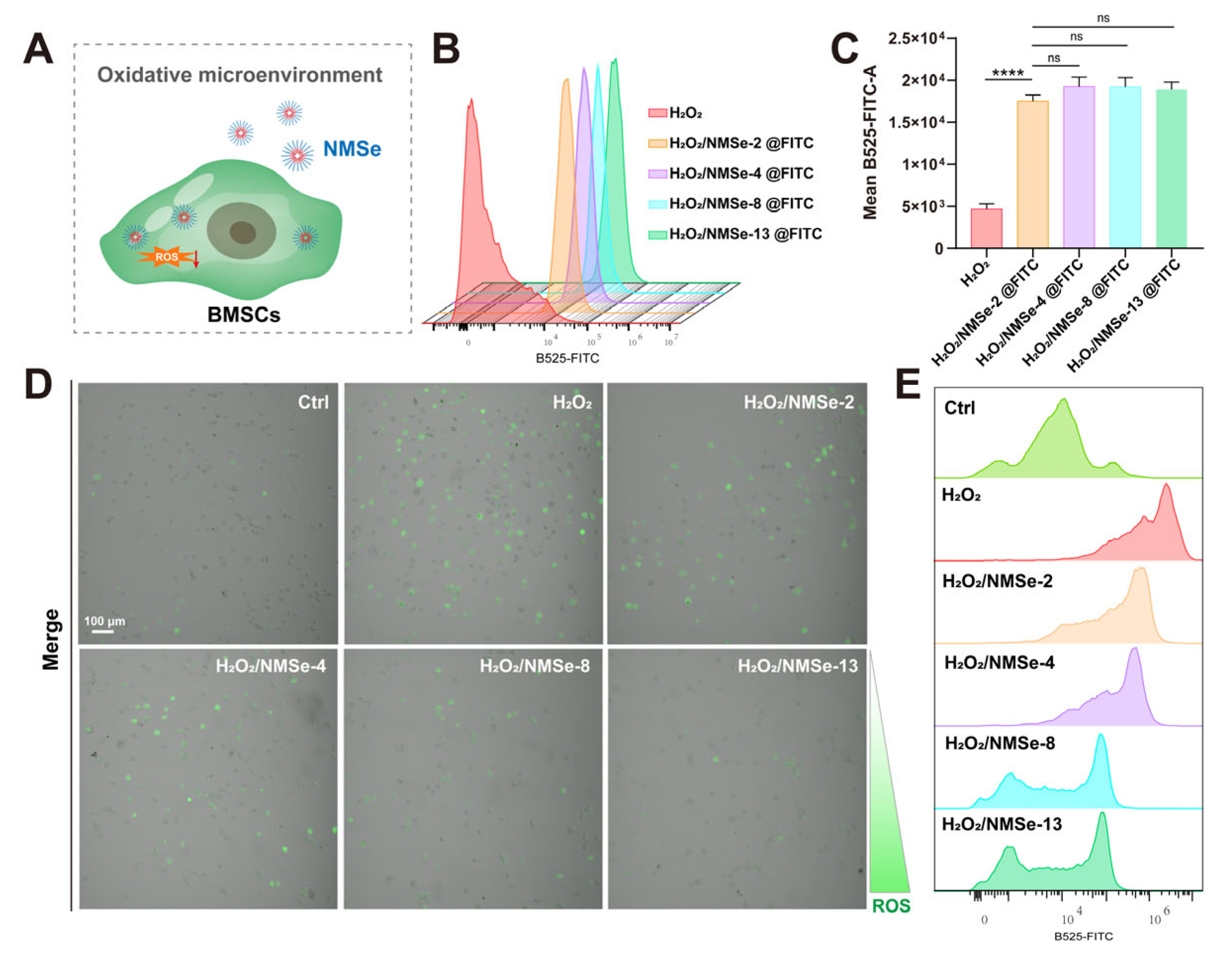

| mPEG5000 (mg) | TMC (mg) | MSeSe (mg) | Nomenclature of Polymers |
|---|---|---|---|
| 25 | 195 | 5 | PSe5 |
| 25 | 190 | 10 | PSe10 |
| 25 | 180 | 20 | PSe20 |
| 25 | 170 | 30 | PSe30 |
| Target Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| Gapdh | TGACCACAGTCCATGCCATC | GACGGACACATTGGGGGTAG |
| p16 | CTCCTTGGCTTCACTTCTGG | CTCCCTCCCTCTGCTAACCT |
| p53 | CTACTTCCCAGCAGGGTGTC | CAGACCAAGAGGTTGGGTCG |
| Col1 | TGGATGGCTGCACGAGT | TTGGGATGGAGGGAGTTTA |
| Runx2 | ATCCAGCCACCTTCACTTACACC | GGGACCATTGGGAACTGATAGG |
| Ocn | GCCCTGACTGCATTCTGCCTCT | TCACCACCTTACTGCCCTCCTG |
| Polymer | Mn a (KDa) | Mn b (KDa) | PDI | Se Content in Nano-Micelles (Wt %) | Nomenclature of Nano-Micelles |
|---|---|---|---|---|---|
| PSe5 | 20.9 | 15.7 | 1.28 | 1.9 | NMSe-2 |
| PSe10 | 21.0 | 15.9 | 1.35 | 4.4 | NMSe-4 |
| PSe20 | 21.7 | 16.4 | 1.36 | 8.1 | NMSe-8 |
| PSe30 | 22.2 | 16.5 | 1.42 | 13.4 | NMSe-13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Xie, F.; Sun, C.; Wang, X.; Zhang, F.; Zhang, Y.; Liu, C.; Yuan, Y. Selenium-Containing Nano-Micelles Delay the Cellular Senescence of BMSCs Under Oxidative Environment and Maintain Their Regenerative Capacity. Bioengineering 2025, 12, 920. https://doi.org/10.3390/bioengineering12090920
He Z, Xie F, Sun C, Wang X, Zhang F, Zhang Y, Liu C, Yuan Y. Selenium-Containing Nano-Micelles Delay the Cellular Senescence of BMSCs Under Oxidative Environment and Maintain Their Regenerative Capacity. Bioengineering. 2025; 12(9):920. https://doi.org/10.3390/bioengineering12090920
Chicago/Turabian StyleHe, Zirui, Fangru Xie, Chuanhao Sun, Xuan Wang, Fan Zhang, Yan Zhang, Changsheng Liu, and Yuan Yuan. 2025. "Selenium-Containing Nano-Micelles Delay the Cellular Senescence of BMSCs Under Oxidative Environment and Maintain Their Regenerative Capacity" Bioengineering 12, no. 9: 920. https://doi.org/10.3390/bioengineering12090920
APA StyleHe, Z., Xie, F., Sun, C., Wang, X., Zhang, F., Zhang, Y., Liu, C., & Yuan, Y. (2025). Selenium-Containing Nano-Micelles Delay the Cellular Senescence of BMSCs Under Oxidative Environment and Maintain Their Regenerative Capacity. Bioengineering, 12(9), 920. https://doi.org/10.3390/bioengineering12090920






