Malignant Giant Cell Tumor of Bone: A Study of Clinical, Pathological, and Prognostic Profile from One Single Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Inclusion Criteria
2.2. Definition of Variables
2.3. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Comparison of the Patients with PMGCTB and SMGCTB
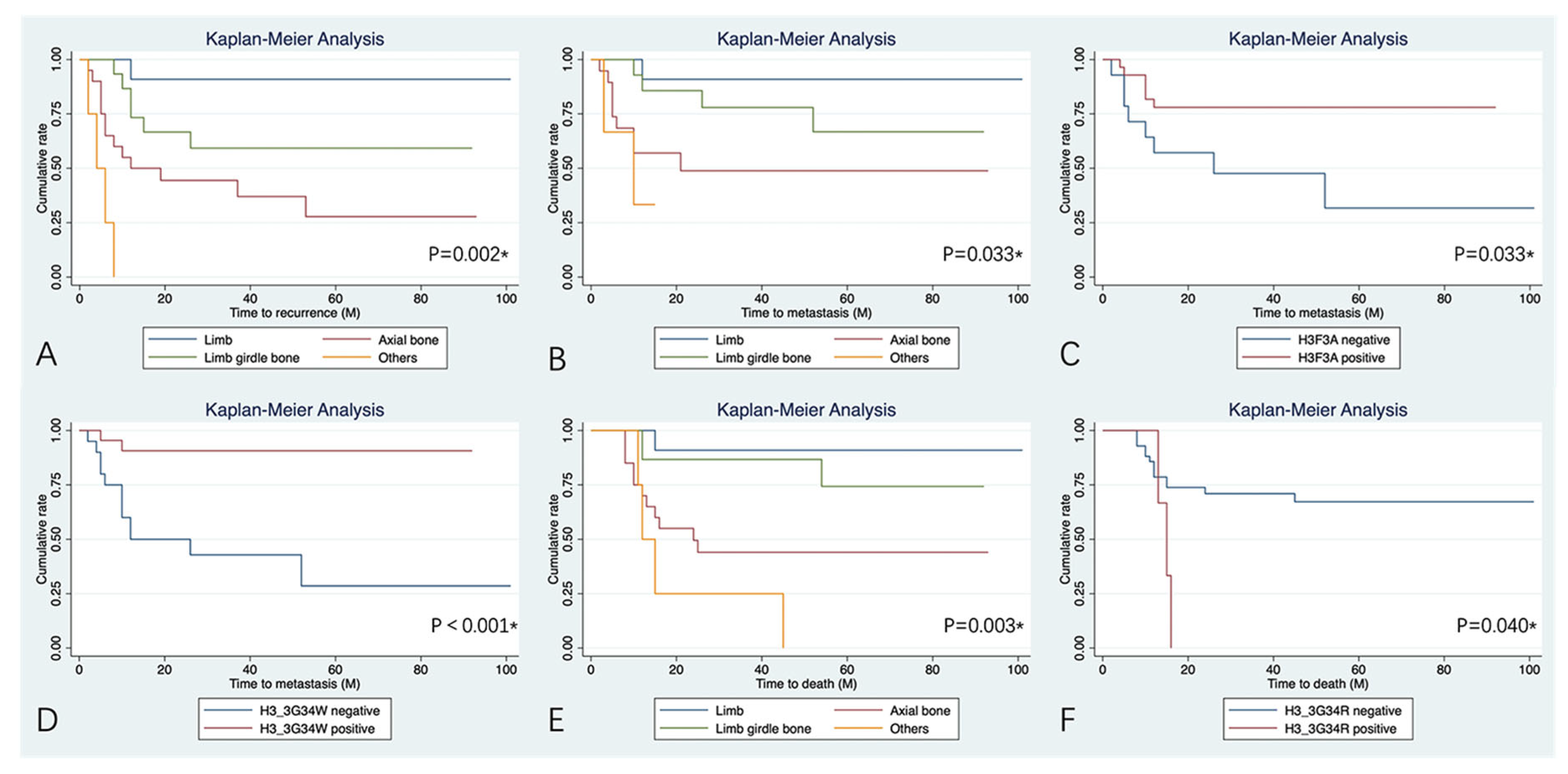
3.3. Clinical Outcomes and Potential Risk Factors for Prognosis
4. Discussion
4.1. Clinical, Demographic, and Prognostic Differences
4.2. Prognosis Influenced by the Tumor Location
4.3. H3F3A Mutation Status and Pathogenic Variants
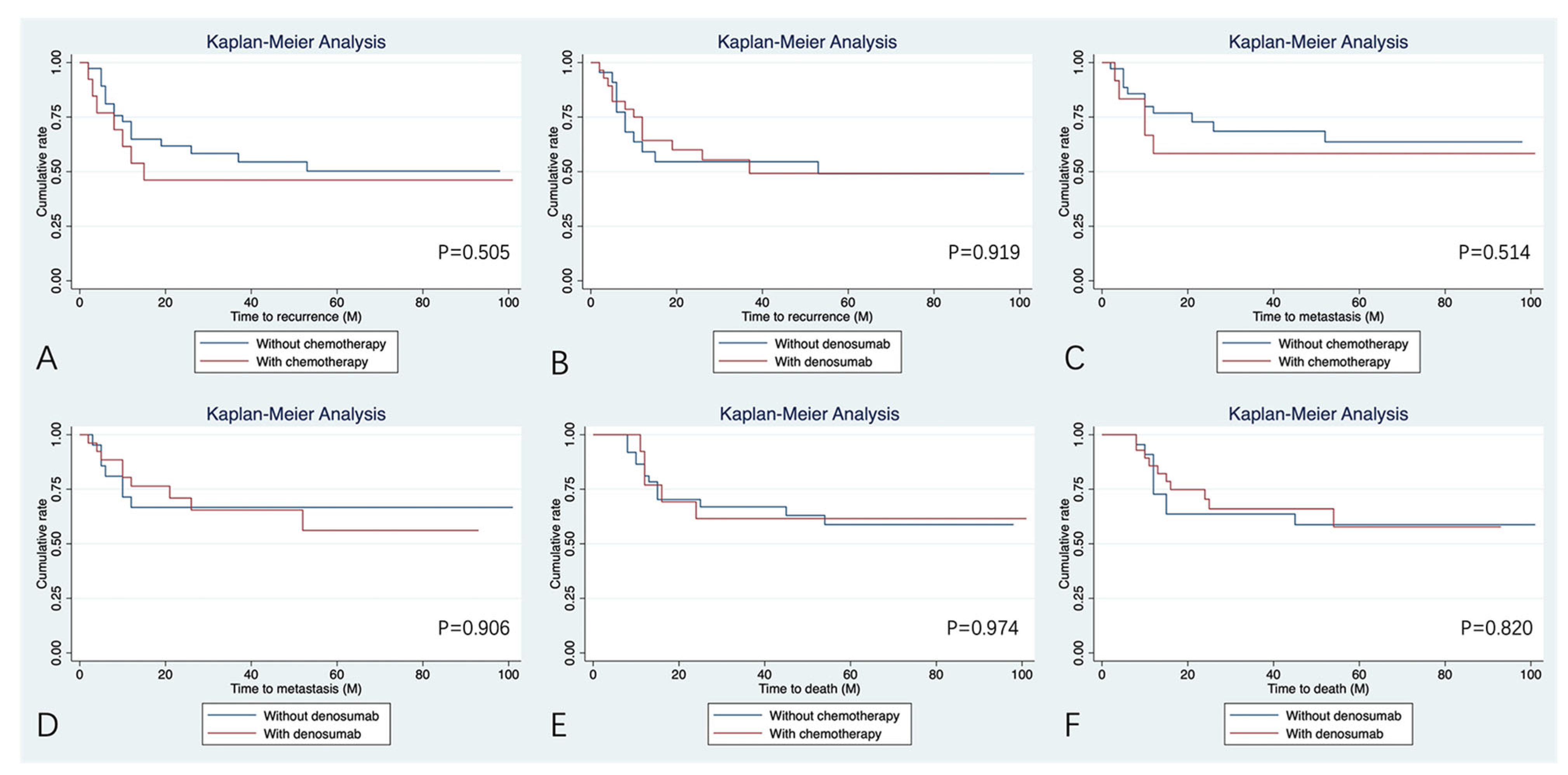
4.4. Treatment Benefit with Chemotherapy and Denosumab
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noh, B.J.; Park, Y.K. Giant cell tumor of bone: Updated molecular pathogenesis and tumor biology. Hum. Pathol. 2018, 81, 1–8. [Google Scholar] [CrossRef]
- Werner, M. Giant cell tumour of bone: Morphological, biological and histogenetical aspects. Int. Orthop. 2006, 30, 484–489. [Google Scholar] [CrossRef]
- Chakarun, C.J.; Forrester, D.M.; Gottsegen, C.J.; Patel, D.B.; White, E.A.; Matcuk, G.R. Giant cell tumor of bone: Review, mimics, and new developments in treatment. Radiographics 2013, 33, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, R.E.; Wunder, J.S.; Isler, M.H.; Bell, R.S.; Schachar, N.; Masri, B.A.; Moreau, G.; Davis, A.M.; Canadian Sarcoma, G. Giant cell tumor of long bone: A Canadian Sarcoma Group study. Clin. Orthop. Relat. Res 2002, 397, 248–258. [Google Scholar] [CrossRef]
- Klenke, F.M.; Wenger, D.E.; Inwards, C.Y.; Rose, P.S.; Sim, F.H. Giant cell tumor of bone: Risk factors for recurrence. Clin. Orthop. Relat. Res. 2011, 469, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, X.; Yan, T.; Ji, T.; Yang, R.; Guo, W. Risk factors for the local recurrence of giant cell tumours of the sacrum treated with nerve-sparing surgery. Bone Jt. J. 2020, 102-B, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sun, X.; Zang, J.; Qu, H. Intralesional excision versus wide resection for giant cell tumor involving the acetabulum: Which is better? Clin. Orthop. Relat. Res. 2012, 470, 1213–1220. [Google Scholar] [CrossRef]
- Skubitz, K.M. Giant cell tumor of bone: Current treatment options. Curr. Treat. Options Oncol. 2014, 15, 507–518. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S.; Presneau, N.; Scheipl, S.; Pillay, N.; Van Loo, P.; Wedge, D.C.; Cooke, S.L.; Gundem, G.; Davies, H.; et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat. Genet. 2013, 45, 1479–1482. [Google Scholar] [CrossRef]
- Rock, M.G.; Sim, F.H.; Unni, K.K.; Witrak, G.A.; Frassica, F.J.; Schray, M.F.; Beabout, J.W.; Dahlin, D.C. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J. Bone Jt. Surg. Am. 1986, 68, 1073–1079. [Google Scholar] [CrossRef]
- Caudell, J.J.; Ballo, M.T.; Zagars, G.K.; Lewis, V.O.; Weber, K.L.; Lin, P.P.; Marco, R.A.; El-Naggar, A.K.; Benjamin, R.S.; Yasko, A.W. Radiotherapy in the management of giant cell tumor of bone. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 158–165. [Google Scholar] [CrossRef]
- Ruka, W.; Rutkowski, P.; Morysiński, T.; Nowecki, Z.; Zdzienicki, M.; Makula, D.; Ptaszyński, K.; Bylina, E.; Grzesiakowska, U. The megavoltage radiation therapy in treatment of patients with advanced or difficult giant cell tumors of bone. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 494–498. [Google Scholar] [CrossRef]
- Hosalkar, H.S.; Jones, K.J.; King, J.J.; Lackman, R.D. Serial arterial embolization for large sacral giant-cell tumors: Mid- to long-term results. Spine 2007, 32, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.P.; Guzel, V.B.; Moura, M.F.; Wallace, S.; Benjamin, R.S.; Weber, K.L.; Morello, F.A.; Jr Gokaslan, Z.L.; Yasko, A.W. Long-term follow-up of patients with giant cell tumor of the sacrum treated with selective arterial embolization. Cancer 2002, 95, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Blay, J.Y.; Rutkowski, P.; Le Cesne, A.; Reichardt, P.; Gelderblom, H.; Grimer, R.J.; Choy, E.; Skubitz, K.; Seeger, L.; et al. Denosumab in patients with giant-cell tumour of bone: A multicentre, open-label, phase 2 study. Lancet Oncol. 2019, 20, 1719–1729. [Google Scholar] [CrossRef]
- Balke, M.; Campanacci, L.; Gebert, C.; Picci, P.; Gibbons, M.; Taylor, R.; Hogendoorn, P.; Kroep, J.; Wass, J.; Athanasou, N. Bisphosphonate treatment of aggressive primary, recurrent and metastatic Giant Cell Tumour of Bone. BMC Cancer 2010, 10, 462. [Google Scholar] [CrossRef]
- Tse, L.F.; Wong, K.C.; Kumta, S.M.; Huang, L.; Chow, T.C.; Griffith, J.F. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: A case-control study. Bone 2008, 42, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Henshaw, R.; Seeger, L.; Choy, E.; Blay, J.Y.; Ferrari, S.; Kroep, J.; Grimer, R.; Reichardt, P.; Rutkowski, P.; et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013, 14, 901–908. [Google Scholar] [CrossRef]
- Stewart, F.W.; Coley, B.L.; Farrow, J.H. Malignant giant cell tumor of bone. Am. J. Pathol. 1938, 14, 515–536.517. [Google Scholar]
- Bertoni, F.; Bacchini, P.; Staals, E.L. Malignancy in giant cell tumor of bone. Cancer 2003, 97, 2520–2529. [Google Scholar] [CrossRef]
- Liu, W.; Chan, C.M.; Gong, L.; Bui, M.M.; Han, G.; Letson, G.D.; Yang, Y.; Niu, X. Malignancy in giant cell tumor of bone in the extremities. J. Bone Oncol. 2021, 26, 100334. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, D.C.; Cupps, R.E.; Johnson, E.W., Jr. Giant-cell tumor: A study of 195 cases. Cancer 1970, 25, 1061–1070. [Google Scholar] [CrossRef]
- Domovitov, S.V.; Healey, J.H. Primary malignant giant-cell tumor of bone has high survival rate. Ann. Surg. Oncol. 2010, 17, 694–701. [Google Scholar] [CrossRef]
- Anract, P.; De Pinieux, G.; Cottias, P.; Pouillart, P.; Forest, M.; Tomeno, B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: A review of 29 cases. Int. Orthop. 1998, 22, 19–26. [Google Scholar] [CrossRef]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Adv. Anat. Pathol. 2021, 28, 119–138. [Google Scholar] [CrossRef]
- Palmerini, E.; Picci, P.; Reichardt, P.; Downey, G. Malignancy in Giant Cell Tumor of Bone: A Review of the Literature. Technol. Cancer Res. Treat 2019, 18, 1533033819840000. [Google Scholar] [CrossRef]
- Papke, D.J.; Kovacs, S.K.; Odintsov, I.; Hornick, J.L.; Raskin, K.A.; Newman, E.T.; Lozano-Calderón, S.; Chebib, I.; Hung, Y.P.; Nielsen, G.P. Malignant Giant Cell Tumor of Bone: A Clinicopathologic Series of 28 Cases Highlighting Genetic Differences Compared With Conventional, Atypical, and Metastasizing Conventional Tumors. Am. J. Surg. Pathol. 2025, 49, 539–553. [Google Scholar] [CrossRef]
- Amary, F.; Berisha, F.; Ye, H.; Gupta, M.; Gutteridge, A.; Baumhoer, D.; Gibbons, R.; Tirabosco, R.; O’Donnell, P.; Flanagan, A.M. H3F3A (Histone 3.3) G34W Immunohistochemistry: A Reliable Marker Defining Benign and Malignant Giant Cell Tumor of Bone. Am. J. Surg. Pathol. 2017, 41, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Iwasaki, T.; Yamada, Y.; Matsumoto, Y.; Otsuka, H.; Yoshimoto, M.; Kohashi, K.; Taguchi, K.; Yokoyama, R.; Nakashima, Y.; et al. Diagnostic utility of histone H3.3 G34W, G34R, and G34V mutant-specific antibodies for giant cell tumors of bone. Hum. Pathol. 2018, 73, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, R.E. Giant cell tumor of bone. Orthop. Clin. N. Am. 2006, 37, 35–51. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Righi, A.; Mavrogenis, A.F.; Akahane, M.; Honoki, K.; Tanaka, Y.; Donati, D.M.; Errani, C. Late Local Recurrence of Bone Giant Cell Tumors Associated with an Increased Risk for Malignant Transformation. Cancers 2021, 13, 3644. [Google Scholar] [CrossRef]
- Tahir, I.; Andrei, V.; Pollock, R.; Saifuddin, A. Malignant giant cell tumour of bone: A review of clinical, pathological and imaging features. Skelet. Radiol. 2022, 51, 957–970. [Google Scholar] [CrossRef]
- Tang, X.; Guo, W.; Yang, R.; Yan, T.; Tang, S.; Li, D. Acetabular Reconstruction With Femoral Head Autograft After Intraarticular Resection of Periacetabular Tumors is Durable at Short-term Followup. Clin. Orthop. Relat. Res. 2017, 475, 3060–3070. [Google Scholar] [CrossRef]
- Guo, W.; Sun, X.; Ji, T.; Tang, X. Outcome of surgical treatment of pelvic osteosarcoma. J. Surg. Oncol. 2012, 106, 406–410. [Google Scholar] [CrossRef]
- Guo, W.; Li, D.; Tang, X.; Yang, Y.; Ji, T. Reconstruction with modular hemipelvic prostheses for periacetabular tumor. Clin. Orthop. Relat. Res 2007, 461, 180–188. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, X.; Ji, T.; Yan, T.; Yang, R.; Yang, Y.; Wei, R.; Liang, H.; Guo, W. Is a Modular Pedicle-hemipelvic Endoprosthesis Durable at Short Term in Patients Undergoing Enneking Type I + II Tumor Resections With or Without Sacroiliac Involvement? Clin. Orthop. Relat. Res. 2018, 476, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.I.; Nakano, Y.; Honda-Kitahara, M.; Wakai, S.; Motoi, T.; Ogura, K.; Sano, N.; Shibata, T.; Okuma, T.; Iwata, S.; et al. Absence of H3F3A mutation in a subset of malignant giant cell tumor of bone. Mod. Pathol. 2019, 32, 1751–1761. [Google Scholar] [CrossRef]
- Nascimento, A.G.; Huvos, A.G.; Marcove, R.C. Primary malignant giant cell tumor of bone: A study of eight cases and review of the literature. Cancer 1979, 44, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Henshaw, R.; Skubitz, K.; Chawla, S.; Staddon, A.; Blay, J.-Y.; Roudier, M.; Smith, J.; Ye, Z.; Sohn, W.; et al. Denosumab in patients with giant-cell tumour of bone: An open-label, phase 2 study. Lancet Oncol. 2010, 11, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Hasenfratz, M.; Mellert, K.; Marienfeld, R.; von Baer, A.; Schultheiss, M.; Roitman, P.D.; Aponte-Tinao, L.A.; Lehner, B.; Möller, P.; Mechtersheimer, G.; et al. Profiling of three H3F3A-mutated and denosumab-treated giant cell tumors of bone points to diverging pathways during progression and malignant transformation. Sci. Rep. 2021, 11, 5709. [Google Scholar] [CrossRef]
- van Langevelde, K.; Cleven, A.H.G.; Navas Cañete, A.; van der Heijden, L.; van de Sande, M.A.J.; Gelderblom, H.; Bovée, J.V.M.G. Malignant Transformation of Giant Cell Tumor of Bone and the Association with Denosumab Treatment: A Radiology and Pathology Perspective. Sarcoma 2022, 2022, 3425221. [Google Scholar] [CrossRef]
- Branstetter, D.G.; Nelson, S.D.; Manivel, J.C.; Blay, J.-Y.; Chawla, S.; Thomas, D.M.; Jun, S.; Jacobs, I. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin. Cancer Res. 2012, 18, 4415–4424. [Google Scholar] [CrossRef]
- Puri, A.; Gulia, A.; Hegde, P.; Verma, V.; Rekhi, B. Neoadjuvant denosumab: Its role and results in operable cases of giant cell tumour of bone. Bone Jt. J. 2019, 101-B, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Liu, X.; Yang, Y.; Guo, W.; Yang, R.; Tang, X.; Yan, T.; Li, Y.; Tang, S.; Li, D.; et al. Ultra-Short Course of Neo-Adjuvant Denosumab for Nerve-Sparing Surgery for Giant Cell Tumor of Bone in Sacrum. Spine 2022, 47, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Seeger, L.L.; Gambarotti, M.; Righi, A.; Reichardt, P.; Bukata, S.; Blay, J.-Y.; Dai, T.; Jandial, D.; Picci, P. Malignancy in giant cell tumor of bone: Analysis of an open-label phase 2 study of denosumab. BMC Cancer 2021, 21, 89. [Google Scholar] [CrossRef] [PubMed]
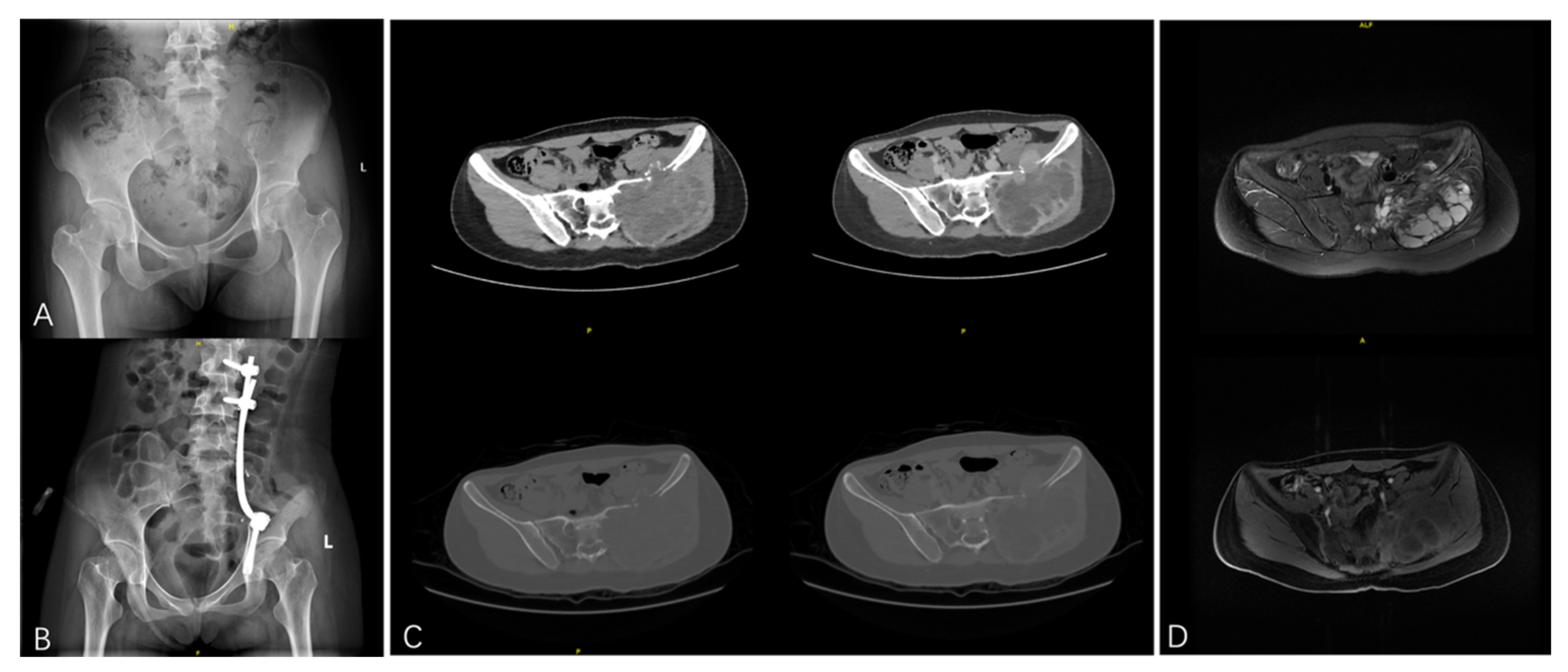
| Characteristics | Value |
|---|---|
| Gender (N, %) | |
| Male | 25 (50.0%) |
| Female | 25 (50.0%) |
| Age (Year, Mean ± SD) | 45.7 ± 14.9 |
| Location (N, %) | |
| Limb | 11 (22.0%) |
| Limb girdle bone | 20 (40.0%) |
| Axial bone | 15 (30.0%) |
| Others | 4 (8.0%) |
| Treatment history (N, %) | |
| Newly | 24 (48.0%) |
| Recurrent | 26 (52.0%) |
| Type (N, %) | |
| Primary | 24 (48.0%) |
| Secondary | 26 (52.0%) |
| Secondary to (N, %) | n = 26 |
| Surgery | 19 (73.1%) |
| Radiotherapy | 7 (26.9%) |
| Surgery history (N, %) | n = 19 |
| Curettage | 11 (57.9%) |
| Resection | 8 (42.1%) |
| Pathology (N, %) | |
| OS | 18 (36.0%) |
| CS | 4 (8.0%) |
| FS | 6 (12.0%) |
| UPS | 19 (38.0%) |
| MFH | 3 (6.0%) |
| H3F3A mutation status (N, %) | n = 45 |
| Positive | 30 (66.7%) |
| Negative | 15 (33.3%) |
| H3.3G34W (N, %) | n = 45 |
| Positive | 24 (53.3%) |
| Negative | 21 (46.7%) |
| H3.3G34V (N, %) | n = 45 |
| Positive | 4 (8.9%) |
| Negative | 41 (91.1%) |
| H3.3G34R (N, %) | n = 45 |
| Positive | 3 (6.7%) |
| Negative | 42 (93.3%) |
| Initial metastasis (N, %) | |
| Yes | 3 (6.0%) |
| No | 47 (94.0%) |
| Denosumab (N, %) | |
| Yes | 28 (56.0%) |
| No | 22 (44.0%) |
| Chemotherapy (N, %) | |
| Yes | 13 (26.0%) |
| No | 37 (74.0%) |
| Follow-up (Mons, Mean ± SD) | 41.6 ± 29.3 |
| Characteristics | PMGCTB (N = 24) | SMGCTB (N = 26) | p-Value |
|---|---|---|---|
| Gender (N, %) | |||
| Male | 11 (45.8%) | 14 (53.9%) | 0.571 |
| Female | 13 (54.2%) | 12 (46.1%) | |
| Age (Year, Mean ± SD) | 48.0 ± 16.9 | 43.5 ± 12.8 | 0.287 |
| Location (N, %) | 0.040 * | ||
| Limb | 6 (25.0%) | 5 (19.2%) | |
| Limb girdle bone | 11 (45.9%) | 4 (15.4%) | |
| Axial bone | 5 (20.8%) | 15 (57.7%) | |
| Others | 2 (8.3%) | 2 (7.7%) | |
| Treatment history (N, %) | <0.001 * | ||
| Newly | 22 (91.7%) | 2 (7.7%) | |
| Recurrent | 2 (8.3%) | 24 (92.3%) | |
| Pathology (N, %) | 0.171 | ||
| OS | 6 (25.0%) | 12 (46.2%) | |
| CS | 1 (4.2%) | 3 (11.5%) | |
| FS | 3 (12.5%) | 3 (11.5%) | |
| UPS | 11 (45.8%) | 8 (30.8%) | |
| MFH | 3 (12.5%) | 0 (0) | |
| H3F3A mutation status (N, %) | n = 22 | n = 23 | 0.020 * |
| Positive | 11 (50.0%) | 19 (82.6%) | |
| Negative | 11 (50.0%) | 4 (17.4%) | |
| H3.3G34W (N, %) | n = 22 | n = 23 | 0.300 |
| Positive | 10 (45.4%) | 14 (60.9%) | |
| Negative | 12 (54.6%) | 9 (39.1%) | |
| H3.3G34V (N, %) | n = 22 | n = 23 | 0.400 |
| Positive | 0 (0) | 4 (17.4%) | |
| Negative | 22 (100%) | 19 (82.6%) | |
| H3.3G34R (N, %) | n = 22 | n = 23 | 0.577 |
| Positive | 1 (4.5%) | 2 (8.7%) | |
| Negative | 21 (95.5%) | 21 (91.3%) | |
| Initial metastasis (N, %) | 0.600 | ||
| Yes | 1 (4.2%) | 2 (7.7%) | |
| No | 23 (95.8%) | 24 (92.3%) | |
| Denosumab (N, %) | 0.050 * | ||
| Yes | 10 (41.7%) | 18 (69.2%) | |
| No | 12 (58.3%) | 8 (30.8%) | |
| Chemotherapy (N, %) | 0.075 | ||
| Yes | 9 (37.5%) | 4 (15.4%) | |
| No | 15 (62.5%) | 22 (84.6%) | |
| Follow-up (Mons, Med/IQR) | 38.5 (16–64) | 37.5 (13–68) | 0.484 |
| Recurrence (N, %) | 0.153 | ||
| Yes | 9 (37.5%) | 15 (57.7%) | |
| No | 15 (62.5%) | 11 (42.3%) | |
| TTR (Mons, Med/IQR) | 8 (3–8) | 10 (5–19) | 0.150 |
| 5-year RFS (95%CI) | 62.5% (40.3–78.4%) | 36.6% (16.8–56.8%) | 0.261 |
| Metastasis (N, %) | n = 23 | n = 24 | 0.260 |
| Yes | 6 (26.1%) | 10 (41.7%) | |
| No | 17 (73.9%) | 14 (58.3%) | |
| TTM (Mons, Med/IQR) | 5 (3–10) | 10 (5–21) | 0.496 |
| 5-year MFS (95%CI) | 73.9% (50.1–87.3%) | 50.0% (25.2–70.6%) | 0.332 |
| Survival (N, %) | 0.216 | ||
| Yes | 17 (70.8%) | 14 (53.8%) | |
| No | 7 (29.2%) | 12 (46.2%) | |
| TTD (Mons, Med/IQR) | 12 (10–16) | 12.5 (9.5–20) | 1.000 |
| 5-year OAS (95%CI) | 70.0% (47.0–84.5%) | 48.1% (25.5–67.5%) | 0.227 |
| Characteristics | SMGCTB (N = 26) | p-Value | |
|---|---|---|---|
| PS (N = 19) | PR (N = 7) | ||
| Gender (N, %) | 0.838 | ||
| Male | 10 (52.6%) | 4 (57.1%) | |
| Female | 9 (47.4%) | 3 (42.9%) | |
| Age (Year, Mean ± SD) | 41.8 ± 14.2 | 48.0 ± 7.1 | 0.287 |
| Location (N, %) | 0.160 | ||
| Limb | 5 (26.3%) | 0 (0) | |
| Limb girdle bone | 9 (47.4%) | 6 (85.7%) | |
| Axial bone | 4 (21.0%) | 0 (0) | |
| Others | 1 (5.3%) | 1 (14.3%) | |
| Pathology (N, %) | 0.983 | ||
| OS | 9 (47.4%) | 3 (42.9%) | |
| CS | 2 (10.5%) | 1 (14.3%) | |
| FS | 2 (10.5%) | 1 (14.3%) | |
| UPS | 6 (31.6%) | 2 (28.5%) | |
| MFH | 0 (0) | 0 (0) | |
| LI (Mons, Med/IQR) | 31 (7–144) | 90 (36–131) | 0.140 |
| H3F3A mutation status (N, %) | n = 17 | n = 6 | 0.539 |
| Positive | 13 (76.5%) | 6 (100%) | |
| Negative | 4 (23.5%) | 0 (0) | |
| H3.3G34W (N, %) | n = 17 | n = 6 | 0.340 |
| Positive | 9 (52.9%) | 5 (83.3%) | |
| Negative | 8 (47.1%) | 1 (16.7%) | |
| H3.3G34V (N, %) | n = 17 | n = 6 | 0.957 |
| Positive | 3 (17.7%) | 1 (16.7%) | |
| Negative | 14 (82.3%) | 5 (83.3%) | |
| H3.3G34R (N, %) | n = 17 | n = 6 | 0.420 |
| Positive | 1 (5.9%) | 1 (16.7%) | |
| Negative | 16 (94.1%) | 5 (83.3%) | |
| Initial metastasis (N, %) | 0.372 | ||
| Yes | 2 (10.5%) | 0 (0) | |
| No | 17 (89.5%) | 7 (100%) | |
| Denosumab (N, %) | 0.883 | ||
| Yes | 13 (68.4%) | 5 (71.4%) | |
| No | 6 (31.6%) | 2 (28.6%) | |
| Chemotherapy (N, %) | 0.925 | ||
| Yes | 3 (15.8%) | 1 (14.3%) | |
| No | 16 (84.2%) | 6 (85.7%) | |
| Follow-up (Mons, Med/IQR) | 39 (12–79) | 25 (13–45) | 0.623 |
| Recurrence (N, %) | 0.390 | ||
| Yes | 10 (52.6%) | 5 (71.4%) | |
| No | 9 (47.4%) | 2 (28.6%) | |
| TTR (Mons, Med/IQR) | 11 (5–12) | 10 (6–19) | 0.711 |
| 5-year RFS (95%CI) | 45.6% (22.3–66.3%) | 38.1% (6.1–71.6%) | 0.376 |
| Metastasis (N, %) | n = 17 | n = 7 | 0.939 |
| Yes | 7 (41.2%) | 3 (42.9%) | |
| No | 10 (58.8%) | 4 (57.1%) | |
| TTM (Mons, Med/IQR) | 10 (5–26) | 10 (10–21) | 0.726 |
| 5-year MFS (95%CI) | 52.3% (23.3–74.9%) | 44.4% (6.6–78.5%) | 0.755 |
| Survival (N, %) | 0.495 | ||
| Yes | 11 (57.9%) | 3 (42.9%) | |
| No | 8 (42.1%) | 4 (57.1%) | |
| TTD (Mons, Med/IQR) | 12 (9.5–15) | 19 (10.5–35) | 0.492 |
| 5-year OAS (95%CI) | 54.1% (27.4–74.7%) | 26.8% (1.3–67.0%) | 0.510 |
| 5-Year RFS (95%CI) | p-Value | 5-Year MFS (95%CI) | p-Value | 5-Year OAS (95%CI) | p-Value | |
|---|---|---|---|---|---|---|
| H3.3G34W | 0.163 | 0.002 * | 0.096 | |||
| Positive | 61.1% (35.3–79.2%) | 90.7% (67.6–97.6%) | 76.4% (51.0–89.8%) | |||
| Negative | 37.5% (17.7–57.4%) | 28.6% (6.7–55.9%) | 47.1% (25.1–66.4%) | |||
| H3.3G34V | 0.544 | 0.224 | 0.158 | |||
| Positive | 25.0% (8.9–66.5%) | 25.0% (8.9–66.5%) | 25.0% (8.9–66.5%) | |||
| Negative | 52.5% (34.7–67.5%) | 66.7% (46.4–80.7%) | 66.5% (48.9–79.2%) | |||
| H3.3G34R | 0.482 | 0.199 | 0.049 * | |||
| Positive | 33.3% (9.0–77.4%) | 33.3% (9.0–77.4%) | 33.3% (9.0–77.4%) | |||
| Negative | 53.5% (35.8–68.3%) | 65.0% (45.3–79.2%) | 67.2% (49.9–79.7%) |
| Variables | RFS | MFS (N = 47) | OAS | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Gender | 0.333 | 0.759 | 0.683 | |||
| Male | Ref | Ref | Ref | |||
| Female | 0.67 (0.30–1.51) | 0.86 (0.32–2.29) | 0.83 (0.34–2.04) | |||
| Age | 1.01 (0.98–1.04) | 0.379 | 1.01 (0.98–1.05) | 0.524 | 1.01 (0.98–1.05) | 0.407 |
| Location | 0.002 * | 0.033 * | 0.003 * | |||
| Limb | Ref | Ref | Ref | |||
| Limb girdle bone | 4.79 (0.58–39.76) | 0.147 | 3.07 (0.34–27.49) | 0.316 | 2.12 (0.22–20.43) | 0.515 |
| Axial bone | 11.06 (1.44–84.76) | 0.021 * | 7.75 (0.98–61.45) | 0.052 | 8.20 (1.06–63.61) | 0.044 * |
| Others | 56.73 (5.87–548.51) | <0.001 * | 15.31 (1.35–173.37) | 0.028 * | 17.90 (1.98–162.14) | 0.010 * |
| Treatment history | 0.228 | 0.300 | 0.246 | |||
| Newly | Ref | Ref | Ref | |||
| Recurrent | 1.65 (0.72–3.77) | 1.69 (0.62–4.66) | 1.72 (0.68–4.37) | |||
| Type | 0.267 | 0.337 | 0.232 | |||
| Primary | Ref | Ref | Ref | |||
| Secondary | 1.59 (0.69–3.63) | 1.63 (0.59–4.50) | 1.75 (0.69–4.44) | |||
| Pathology | 0.138 | 0.227 | 0.433 | |||
| OS | Ref | Ref | Ref | |||
| CS | 1.30 (0.26–6.46) | 0.748 | 2.45 (0.41–14.69) | 0.327 | 1.34 (0.27–6.69) | 0.718 |
| FS | 1.76 (0.50–6.29) | 0.378 | 2.49 (0.50–12.36) | 0.264 | 0.91 (0.18–4.55) | 0.913 |
| UPS | 2.15 (0.80–5.75) | 0.127 | 2.74 (0.72–10.37) | 0.139 | 1.37 (0.49–3.86) | 0.549 |
| MFH | NA | NA | NA | NA | NA | NA |
| H3F3A mutation status | 0.512 | 0.033 * | 0.663 | |||
| Positive | Ref | Ref | Ref | |||
| Negative | 1.35 (0.56–3.26) | 3.16 (1.10–9.14) | 1.26 (0.46–3.46) | |||
| H3.3G34W | 0.068 | <0.001 * | 0.057 | |||
| Positive | Ref | Ref | Ref | |||
| Negative | 2.25 (0.93–5.45) | 8.87 (1.97–40.05) | 2.80 (0.97–8.08) | |||
| H3.3G34V | 0.373 | 0.148 | 0.138 | |||
| Positive | Ref | Ref | Ref | |||
| Negative | 0.55 (0.16–1.88) | 0.35 (0.09–1.27) | 0.34 (0.09–1.22) | |||
| H3.3G34R | 0.061 | 0.157 | 0.040 * | |||
| Positive | Ref | Ref | Ref | |||
| Negative | 0.25 (0.07–0.87) | 0.28 (0.06–1.29) | 0.26 (0.07–0.94) | |||
| Initial metastasis | 0.331 | - | - | 0.218 | ||
| Yes | Ref | - | - | Ref | ||
| No | 0.45 (0.10–1.92) | - | - | 0.35 (0.08–1.52) | ||
| Denosumab | 0.919 | 0.906 | 0.820 | |||
| Yes | Ref | Ref | Ref | |||
| No | 1.04 (0.46–2.34) | 0.94 (0.35–2.54) | 1.11 (0.45–2.74) | |||
| Chemotherapy | 0.505 | 0.514 | 0.974 | |||
| Yes | Ref | Ref | Ref | |||
| No | 0.74 (0.30–1.78) | 0.70 (0.24–2.01) | 0.98 (0.35–2.74) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Sun, X.; Wang, J.; Liang, H.; Liu, X.; Yang, Y.; Tang, X.; Guo, W. Malignant Giant Cell Tumor of Bone: A Study of Clinical, Pathological, and Prognostic Profile from One Single Center. Bioengineering 2025, 12, 911. https://doi.org/10.3390/bioengineering12090911
Shi J, Sun X, Wang J, Liang H, Liu X, Yang Y, Tang X, Guo W. Malignant Giant Cell Tumor of Bone: A Study of Clinical, Pathological, and Prognostic Profile from One Single Center. Bioengineering. 2025; 12(9):911. https://doi.org/10.3390/bioengineering12090911
Chicago/Turabian StyleShi, Jingtian, Xin Sun, Jichuan Wang, Haijie Liang, Xingyu Liu, Yi Yang, Xiaodong Tang, and Wei Guo. 2025. "Malignant Giant Cell Tumor of Bone: A Study of Clinical, Pathological, and Prognostic Profile from One Single Center" Bioengineering 12, no. 9: 911. https://doi.org/10.3390/bioengineering12090911
APA StyleShi, J., Sun, X., Wang, J., Liang, H., Liu, X., Yang, Y., Tang, X., & Guo, W. (2025). Malignant Giant Cell Tumor of Bone: A Study of Clinical, Pathological, and Prognostic Profile from One Single Center. Bioengineering, 12(9), 911. https://doi.org/10.3390/bioengineering12090911







