The Use of Therapeutic Peptides in Combination with Full-Thickness Skin Columns to Improve Healing of Excisional Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Carboxymethylcellulose (CMC) Hydrogel
2.2. Peptides
2.3. Animals and Anesthesia
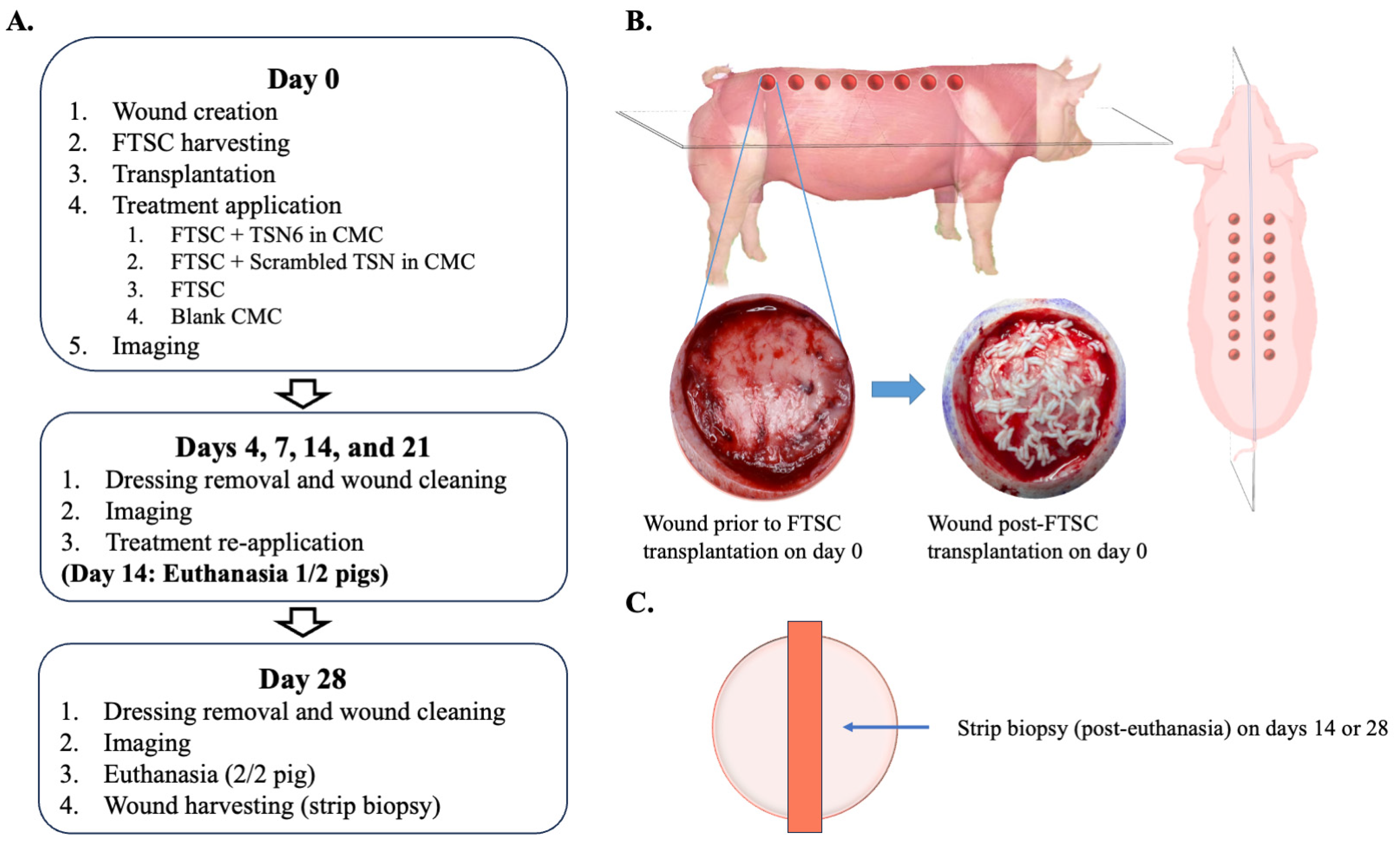
2.4. Study Design
2.5. Biopsies and Sample Processing
2.6. Outcome Measures
2.6.1. Histology
2.6.2. Macroscopic Imaging
2.7. Power and Statistical Analysis
3. Results
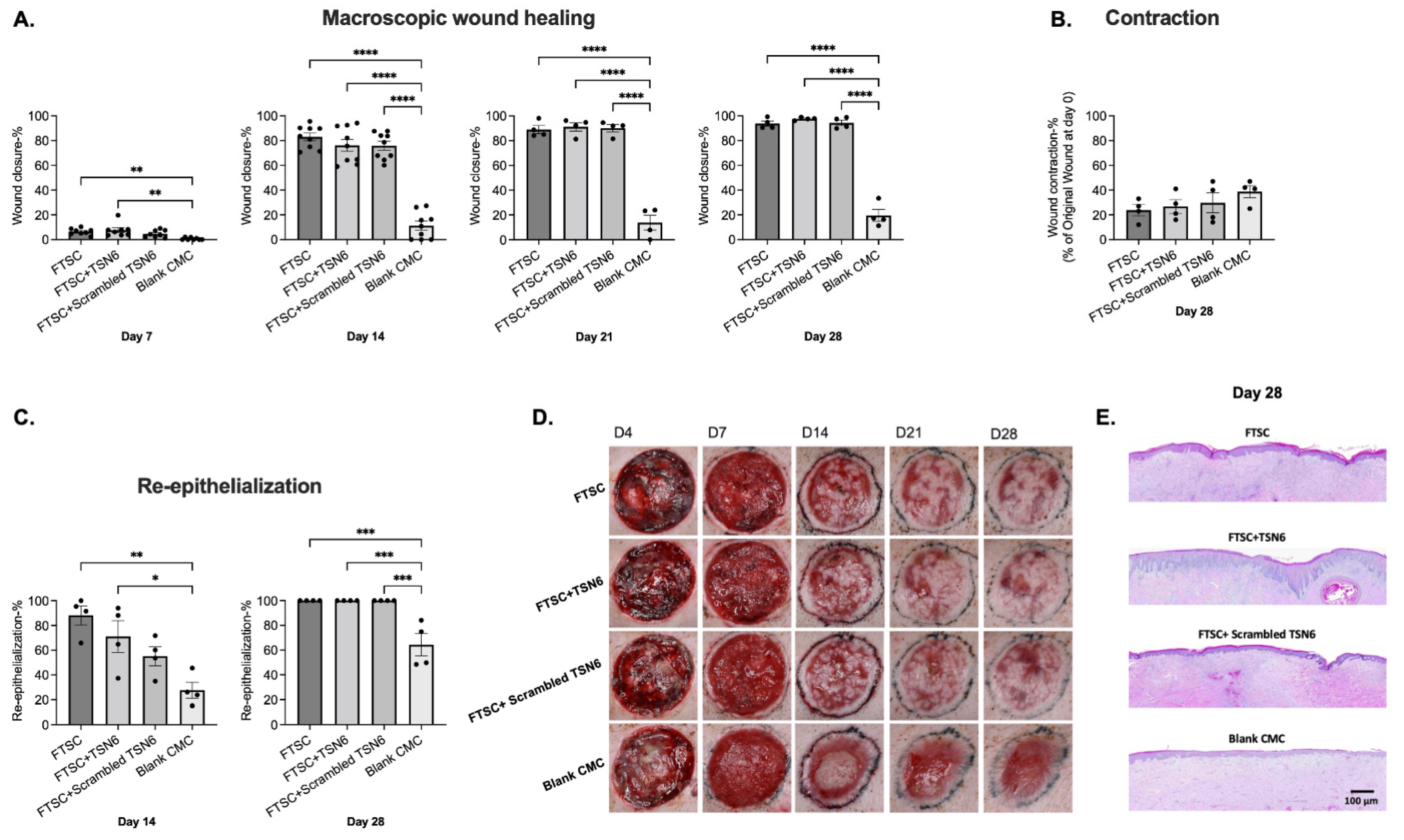
3.1. Wound Healing
3.2. Donor Site Healing
3.3. Quality of Healing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Analysis of variance | ANOVA |
| Animal Care and Use Review Office | ACURO |
| Carboxymethylcellulose | CMC |
| Extracellular matrix | ECM |
| Full-thickness skin column | FTSC |
| Hematoxylin and eosin | H&E |
| Institutional Animal Care and Use Committee | IACUC |
| Masson’s trichrome stain | MTS |
| Multimerin-1 | MMRN1 |
| Split-thickness skin grafting | STSG |
| Translin | TNS |
| University of Texas Health Science Center-San Antonio | UTHSCSA |
References
- Samuel, J.; Gharde, P.; Surya, D.; Durge, S.; Gopalan, V. A Comparative Review of Meshed Versus Unmeshed Grafts in Split-Thickness Skin Grafting: Clinical Implications and Outcomes. Cureus 2024, 16, e69606. [Google Scholar] [CrossRef]
- Kamolz, L.P.; Schintler, M.; Parvizi, D.; Selig, H.; Lumenta, D.B. The real expansion rate of meshers and micrografts: Things we should keep in mind. Ann. Burn. Fire Disasters 2013, 26, 26–29. [Google Scholar]
- Asuku, M.; Yu, T.C.; Yan, Q.; Böing, E.; Hahn, H.; Hovland, S.; Donelan, M.B. Split-thickness skin graft donor-site morbidity: A systematic literature review. Burns 2021, 47, 1525–1546. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, A.; Bilenchi, R.; Biagioli, M.; D’Aniello, C. Classification and Pathophysiology of Skin Grafts. Clin. Dermatol. 2005, 23, 332–337. [Google Scholar] [CrossRef]
- Dean, J.; Hoch, C.; Wollenberg, B.; Navidzadeh, J.; Maheta, B.; Mandava, A.; Knoedler, S.; Sherwani, K.; Baecher, H.; Schmitz, A.; et al. Advancements in bioengineered and autologous skin grafting techniques for skin reconstruction: A comprehensive review. Front. Bioeng. Biotechnol. 2025, 12, 1461328. [Google Scholar] [CrossRef]
- Rafieezadeh, D.; Abbaspour, M. Safety and effectiveness of micropigmentation skin grafting using the Meek method. Int. J. Burn. Trauma 2024, 14, 107–114. [Google Scholar] [CrossRef]
- Milner, S.M. Cultured Epithelial Autograft. Eplasty 2023, 23, QA14. [Google Scholar] [PubMed]
- Fuchs, C.; Pham, L.; Henderson, J.; Stalnaker, K.J.; Anderson, R.R.; Tam, J. Multi-faceted enhancement of full-thickness skin wound healing by treatment with autologous micro skin tissue columns. Sci. Rep. 2021, 11, 1688. [Google Scholar] [CrossRef]
- Tam, J.; Wang, Y.; Vuong, L.N.; Fisher, J.M.; Farinelli, W.A.; Anderson, R.R. Reconstitution of full-thickness skin by microcolumn grafting. J. Tissue Eng. Regen. Med. 2017, 11, 2796–2805. [Google Scholar] [CrossRef]
- Jaller, J.A.; Herskovitz, I.; Borda, L.J.; Mervis, J.; Darwin, E.; Hirt, P.A.; Lev-Tov, H.; Kirsner, R.S. Evaluation of Donor Site Pain After Fractional Autologous Full-Thickness Skin Grafting. Adv. Wound Care 2018, 7, 309–314. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Purohit, K.; Reddy, N.; Sunna, A. Exploring the Potential of Bioactive Peptides: From Natural Sources to Therapeutics. Int. J. Mol. Sci. 2024, 25, 1391. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef]
- Duarte-Mata, D.I.; Salinas-Carmona, M.C. Antimicrobial peptides’ immune modulation role in intracellular bacterial infection. Front. Immunol. 2023, 14, 1119574. [Google Scholar] [CrossRef]
- Adnan, S.B.; Maarof, M.; Fauzi, M.B.; Md Fadilah, N.I. Antimicrobial Peptides in Wound Healing and Skin Regeneration: Dual Roles in Immunity and Microbial Defense. Int. J. Mol. Sci. 2025, 26, 5920. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Wolf, L.; Deckenback, J.; Hamblin, M.R.; Herman, I.M. Human platelet-rich plasma- and extracellular matrix-derived peptides promote impaired cutaneous wound healing in vivo. PloS ONE 2012, 7, e32146. [Google Scholar] [CrossRef]
- Gomes, A.; Teixeira, C.; Ferraz, R.; Prudêncio, C.; Gomes, P. Wound-Healing Peptides for Treatment of Chronic Diabetic Foot Ulcers and Other Infected Skin Injuries. Molecules 2017, 22, 1743. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Geevarghese, A.; Herman, I.M. Bioactive peptides derived from vascular endothelial cell extracellular matrices promote microvascular morphogenesis and wound healing in vitro. Wound Repair Regen. 2011, 19, 59–70. [Google Scholar] [CrossRef]
- Sheets, A.R.; Demidova-Rice, T.N.; Shi, L.; Ronfard, V.; Grover, K.V.; Herman, I.M. Identification and Characterization of Novel Matrix-Derived Bioactive Peptides: A Role for Collagenase from Santyl® Ointment in Post-Debridement Wound Healing? PLoS ONE 2016, 11, e0159598. [Google Scholar] [CrossRef]
- Vuola, J.; Lindford, A. Skin Grafting. Adv. Wound Care 2025, 14, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Md Fadilah, N.I.; Shahabudin, N.A.; Mohd Razif, R.A.; Sanyal, A.; Ghosh, A.; Baharin, K.I.; Ahmad, H.; Maarof, M.; Motta, A.; Fauzi, M.B. Discovery of bioactive peptides as therapeutic agents for skin wound repair. J. Tissue Eng. 2024, 15, 20417314241280359. [Google Scholar] [CrossRef]
- Lim, C.; Lim, J.; Choi, S. Wound-Induced Hair Follicle Neogenesis as a Promising Approach for Hair Regeneration. Mol. Cells 2023, 46, 573–578. [Google Scholar] [CrossRef]
- Tam, J.; Purschke, M.; Fuchs, C.; Wang, Y.; Anderson, R.R. Skin Microcolumns as a Source of Paracrine Signaling Factors. Adv. Wound Care 2020, 9, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Rettinger, C.L.; Fletcher, J.L.; Carlsson, A.H.; Chan, R.K. Accelerated epithelialization and improved wound healing metrics in porcine full-thickness wounds transplanted with full-thickness skin micrografts. Wound Repair Regen. 2017, 25, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Xavier, S.; Roy, J.; Li, S.; Klover, P.J.; Thangapazham, R.L.; Wang, J.A.; Aduba, D.C., Jr.; Raiciulescu, S.; Sperling, L.C.; Herman, I.M.; et al. A Matrix-Derived Bioactive Peptide Enhances Epidermal Thickness and Hair Follicle Neogenesis in Grafted Dermal-Epidermal Composites. Wound Repair Regen. 2025, 33, e70036. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Rittié, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal. 2016, 10, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef]
- Chen, L.; Mirza, R.; Kwon, Y.; DiPietro, L.A.; Koh, T.J. The murine excisional wound model: Contraction revisited. Wound Repair Regen. 2015, 23, 874–877. [Google Scholar] [CrossRef]
- Aarabi, S.; Bhatt, K.A.; Shi, Y.; Paterno, J.; Chang, E.I.; Loh, S.A.; Holmes, J.W.; Longaker, M.T.; Yee, H.; Gurtner, G.C. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007, 21, 3250–3261. [Google Scholar] [CrossRef]
- Lin, H.; Zheng, Z.; Yuan, J.; Zhang, C.; Cao, W.; Qin, X. Collagen Peptides Derived from Sipunculus nudus Accelerate Wound Healing. Molecules 2021, 26, 1385. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Z.; Tian, P.; Han, Q.; Zhang, J.; Zhang, A.M.; Song, Y. Wound healing mechanism of antimicrobial peptide cathelicidin-DM. Front. Bioeng. Biotechnol. 2022, 10, 977159. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, J.R.; Martin, Y. Strategies Demonstrating Efficacy in Reducing Wound Contraction In Vivo. Adv. Wound Care. 2013, 2, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.A.; MacNeil, S. The mechanism of skin graft contraction: An update on current research and potential future therapies. Burns 2008, 34, 153–163. [Google Scholar] [CrossRef]
- Keenan, C.S.; Cooper, L.; Nuutila, K.; Chapa, J.; Christy, S.; Chan, R.K.; Carlsson, A.H. Full-thickness skin columns: A method to reduce healing time and donor site morbidity in deep partial-thickness burns. Wound Repair Regen. 2023, 31, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.A.; Chu, P.Y.; Ma, W.L.; Cheng, W.C.; Chan, S.T.; Yang, J.C.; Wu, Y.C. Enzyme-Digested Peptides Derived from Lates calcarifer Enhance Wound Healing after Surgical Incision in a Murine Model. Mar. Drugs 2021, 19, 154. [Google Scholar] [CrossRef]
- Braza, M.E.; Marietta, M.; Fahrenkopf, M.P. Split-Thickness Skin Grafts. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 14 February 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551561/ (accessed on 15 July 2025).
- Alsaif, A.; Karam, M.; Hayre, A.; Abul, A.; Aldubaikhi, A.; Kahlar, N. Full thickness skin graft versus split thickness skin graft in paediatric patients with hand burns: Systematic review and meta-analysis. Burns 2023, 49, 1017–1027. [Google Scholar] [CrossRef]
- Moors, J.J.E.; Xu, Z.; Xie, K.; Rashad, A.; Vladu, O.; Egger, J.; Röhrig, R.; Hölzle, F.; Puladi, B. Full-thickness skin graft versus split-thickness skin graft for fasciocutaneous radial forearm free flap donor site closure: A systematic review and meta-analysis. Syst. Rev. 2025, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Elrod, J.; Moellmeier, D.; Schiestl, C.; Mohr, C.; Neuhaus, K. Comparative analysis of functional and aesthetic outcomes of retroauricular full thickness versus plantar glabrous split thickness skin grafts in pediatric palmar hand burns. Burns 2020, 46, 639–646. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlsson, A.H.; Herman, I.M.; Christy, S.; Larson, D.; Chan, R.K.; Darling, T.N.; Nuutila, K. The Use of Therapeutic Peptides in Combination with Full-Thickness Skin Columns to Improve Healing of Excisional Wounds. Bioengineering 2025, 12, 856. https://doi.org/10.3390/bioengineering12080856
Carlsson AH, Herman IM, Christy S, Larson D, Chan RK, Darling TN, Nuutila K. The Use of Therapeutic Peptides in Combination with Full-Thickness Skin Columns to Improve Healing of Excisional Wounds. Bioengineering. 2025; 12(8):856. https://doi.org/10.3390/bioengineering12080856
Chicago/Turabian StyleCarlsson, Anders H., Ira M. Herman, Sean Christy, David Larson, Rodney K. Chan, Thomas N. Darling, and Kristo Nuutila. 2025. "The Use of Therapeutic Peptides in Combination with Full-Thickness Skin Columns to Improve Healing of Excisional Wounds" Bioengineering 12, no. 8: 856. https://doi.org/10.3390/bioengineering12080856
APA StyleCarlsson, A. H., Herman, I. M., Christy, S., Larson, D., Chan, R. K., Darling, T. N., & Nuutila, K. (2025). The Use of Therapeutic Peptides in Combination with Full-Thickness Skin Columns to Improve Healing of Excisional Wounds. Bioengineering, 12(8), 856. https://doi.org/10.3390/bioengineering12080856





