Healing Kinetics of Sinus Lift Augmentation Using Biphasic Calcium Phosphate Granules: A Case Series in Humans
Abstract
1. Introduction
2. Materials and Methods
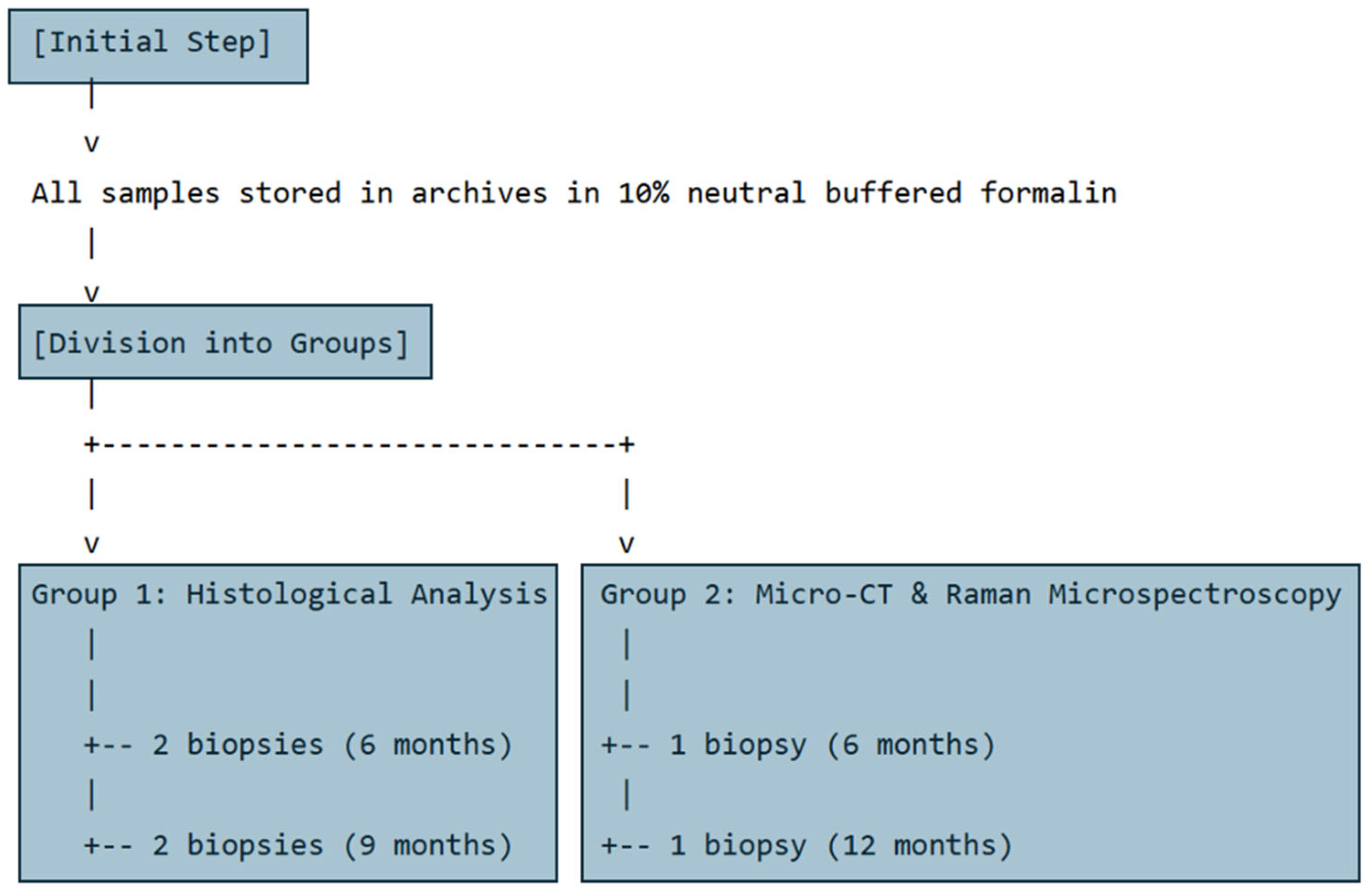
2.1. Sampling
2.2. Patient Selection
2.3. Ethical Approval
2.4. Sample Processing and Analysis
2.5. Optical Microscopy
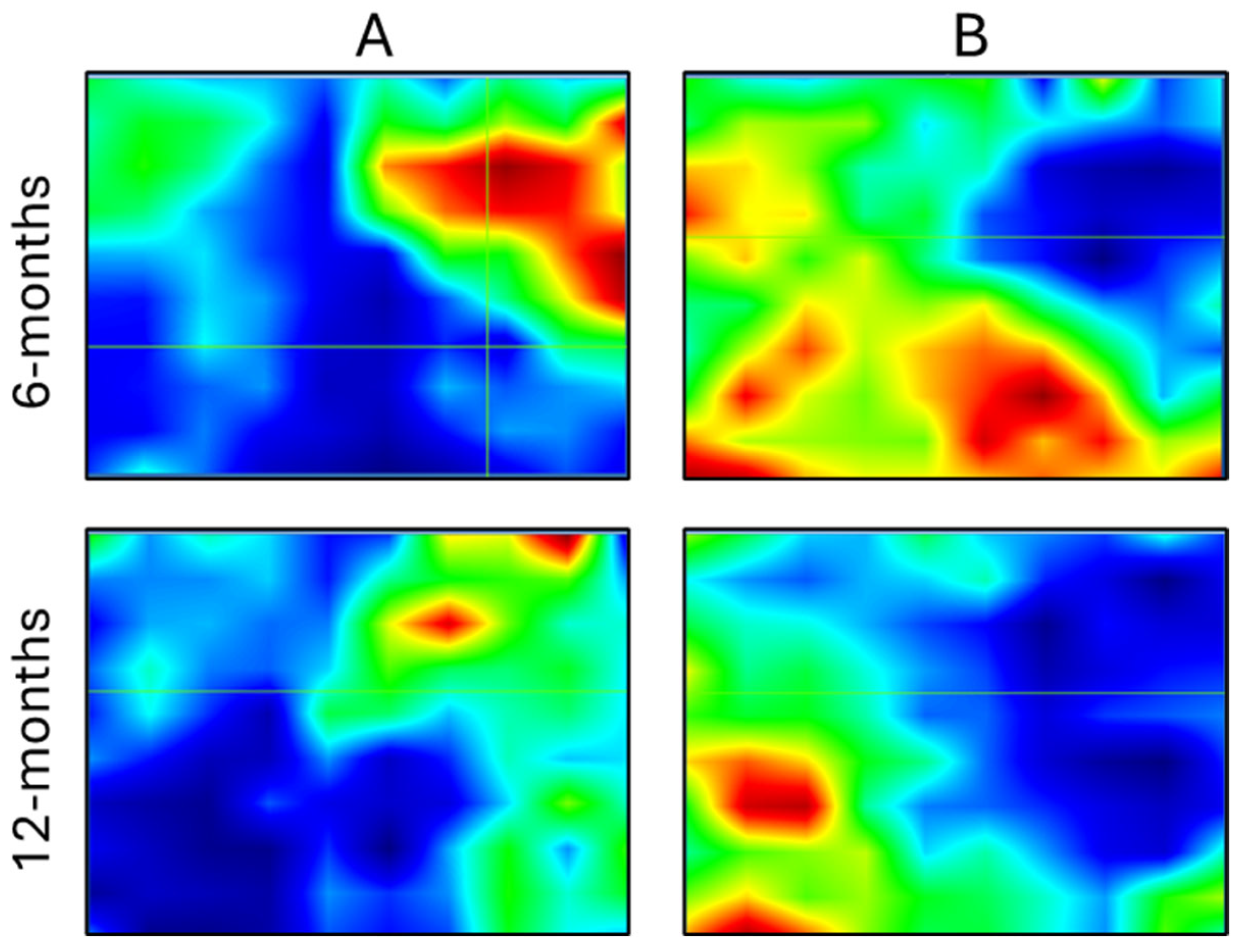
2.6. Micro-Computed Tomography
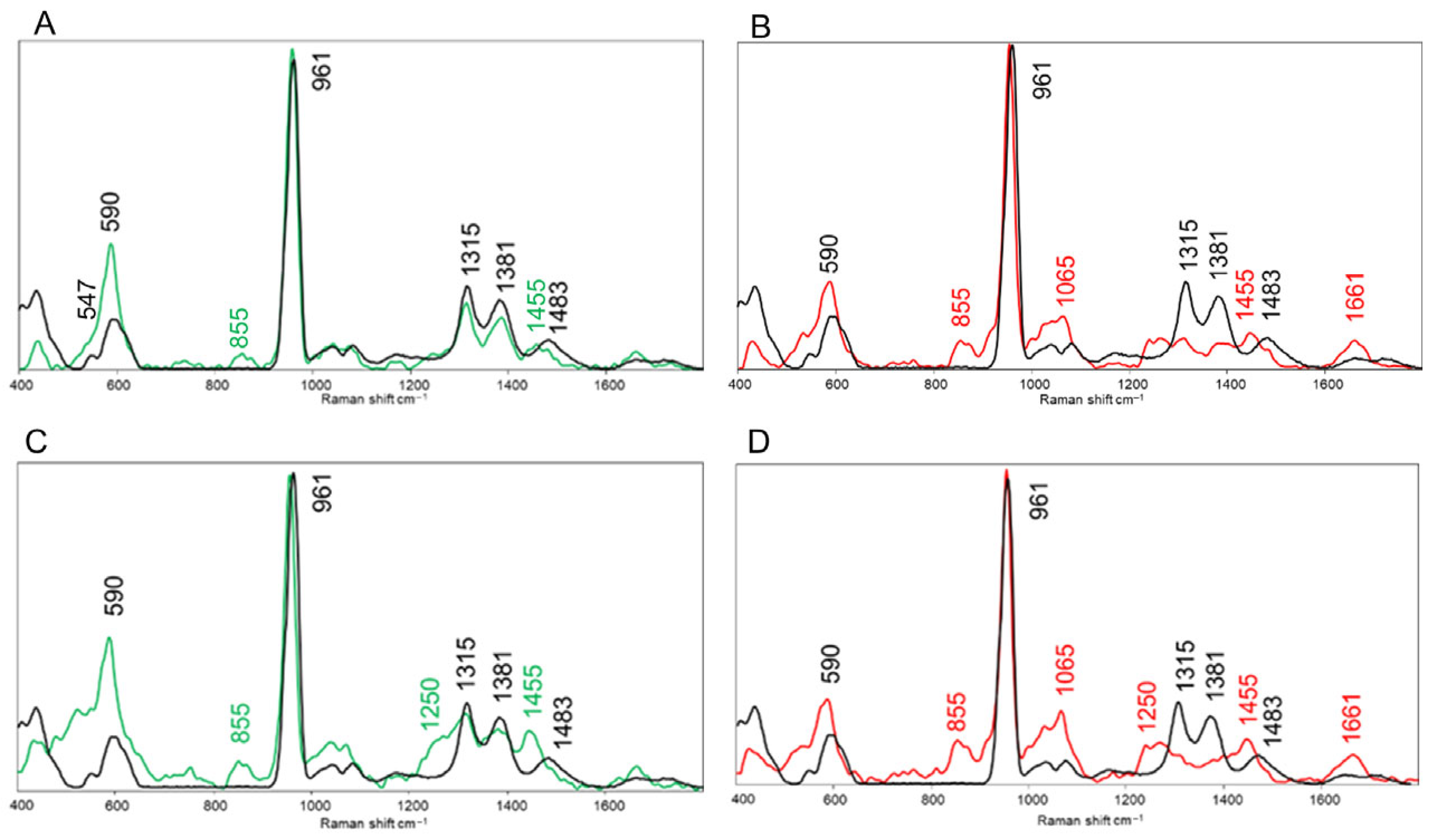
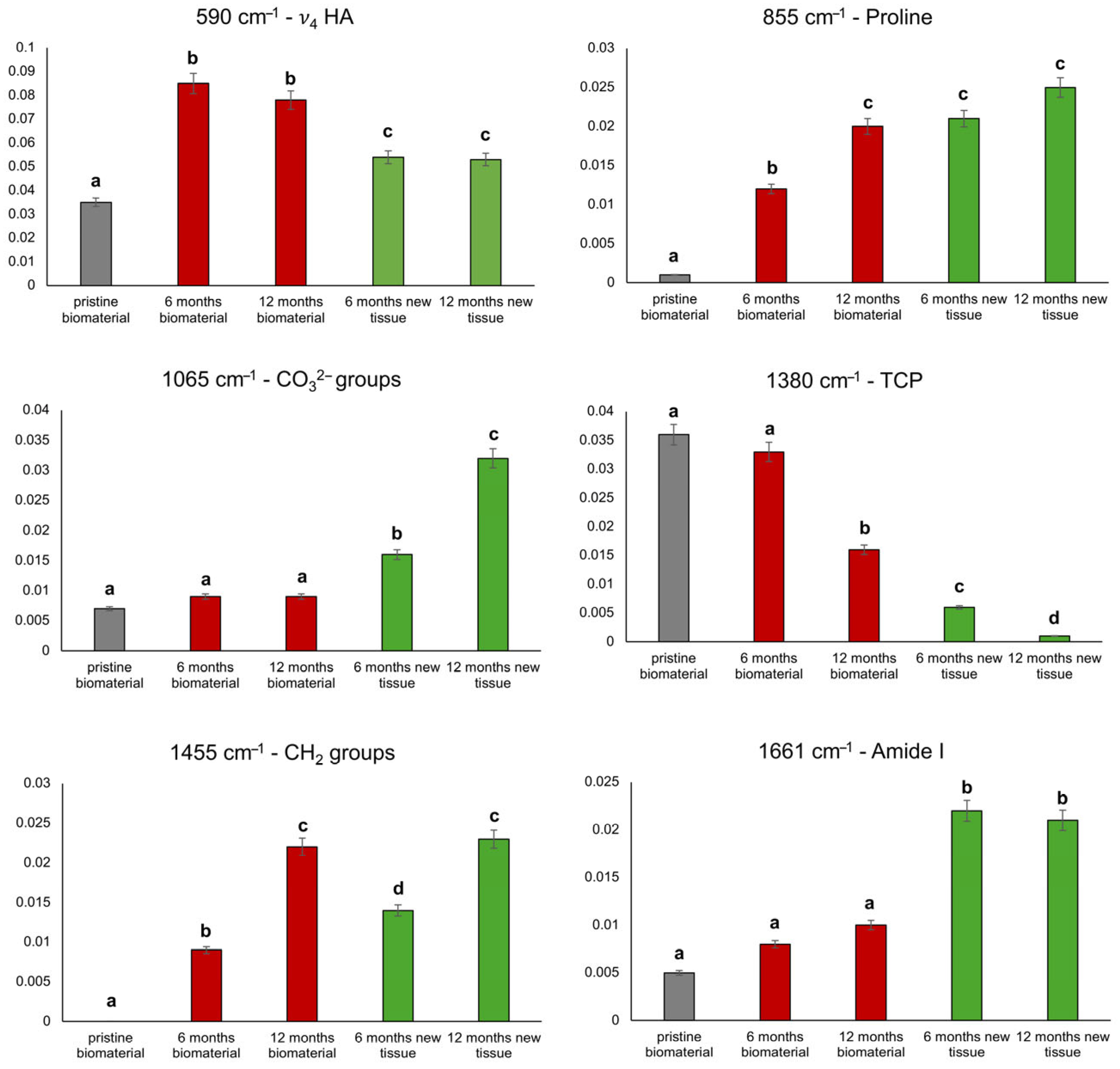
2.7. Raman Microspectroscopy
2.8. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| β-TCP | Beta tricalcium phosphate |
| Micro-CT | Micro-computed tomography |
| BCP | Biphasic calcium phosphate |
| RMS | Raman microspectroscopy |
| Vol% | Ratio between mineralized volume and total volume |
| Tb.Th | Mean trabecular thickness |
| Tb.Sp | Mean trabecular spacing |
| Conn.D | Connectivity density |
| DA | Anisotropy degree |
| FD | Fractal dimension |
| CP | Crestal portion |
| MP | Middle portion |
| AP | Apical portion |
References
- Gul, S.S. Prevalence and Severity of Circumferential Alveolar Bone Loss Using CBCT Images: A Retrospective Study of 20,620 Surfaces of 5155 Teeth. Diagnostics 2024, 14, 507. [Google Scholar] [CrossRef]
- Saleh, M.H.A.; Sabri, H.; Di Pietro, N.; Comuzzi, L.; Geurs, N.C.; Bou Semaan, L.; Piattelli, A. Clinical Indications and Outcomes of Sinus Floor Augmentation With Bone Substitutes: An Evidence-Based Review. Clin. Implant. Dent. Relat. Res. 2025, 27, e13400. [Google Scholar] [CrossRef]
- King, E.M.; Schofield, J. Restoratively Driven Planning for Implants in the Posterior Maxilla—Part 1: Alveolar Bone Healing, Bone Assessment and Clinical Classifications. Br. Dent. J. 2023, 235, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, S.; Rickert, D.; Gutwald, R.; Nagursky, H.; Oshima, T.; Xavier, S.P.; Christmann, J.; Kurz, P.; Menne, D.; Vissink, A.; et al. Bone Marrow Concentrate and Bovine Bone Mineral for Sinus Floor Augmentation: A Controlled, Randomized, Single-Blinded Clinical and Histological Trial—Per-Protocol Analysis. Tissue Eng. Part A 2011, 17, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Slavin, B.V.; Wu, S.; Sturm, S.R.; Hwang, K.K.; Almada, R.; Mirsky, N.A.; Nayak, V.V.; Witek, L.; Coelho, P.G. An Evaluation of Novel AMP2-Coated Electrospun Composite Scaffolds for Intraoral Bone Regeneration: A Proof-of-Concept in Vivo Study. Front. Bioeng. Biotechnol. 2025, 13, 1443280. [Google Scholar] [CrossRef] [PubMed]
- Lima-Sánchez, B.; Baus-Domínguez, M.; Serrera-Figallo, M.-A.; Torres-Lagares, D. Advances in Synthetic Polymer Membranes for Guided Bone Regeneration in Dental Implants: A Scoping Review. J. Funct. Biomater. 2025, 16, 149. [Google Scholar] [CrossRef]
- Migliorini, F.; Cuozzo, F.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Autologous Bone Grafting in Trauma and Orthopaedic Surgery: An Evidence-Based Narrative Review. J. Clin. Med. 2021, 10, 4347. [Google Scholar] [CrossRef]
- Iezzi, G.; Piattelli, A.; Giuliani, A.; Mangano, C.; Manzon, L.; Degidi, M.; Iaculli, F.; Scarano, A.; Filippone, A.; Perrotti, V. Molecular, Cellular and Pharmaceutical Aspects of Bone Grafting Materials and Membranes During Maxillary Sinus-Lift Procedures. Part 1: A General Overview. Curr. Pharm. Biotechnol. 2017, 18, 19–32. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Chaudhari, S.; Khade, A.; Girase, V.; Dhatrak, P. A Systematic Review on Bone Grafts and Biomaterials Substitutes for Bone Regeneration. J. Phys. Conf. Ser. 2024, 2837, 012033. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, S.; Wang, H.; Chen, X.; Shuai, Y.; Wang, H.; Mao, Y.; He, F. Mesenchymal Stem Cells and Dental Implant Osseointegration during Aging: From Mechanisms to Therapy. Stem Cell Res. Ther. 2023, 14, 382. [Google Scholar] [CrossRef]
- Ferraz, M.P. An Overview on the Big Players in Bone Tissue Engineering: Biomaterials, Scaffolds and Cells. Int. J. Mol. Sci. 2024, 25, 3836. [Google Scholar] [CrossRef]
- Gatto, M.L.; Cerqueni, G.; Furlani, M.; Riberti, N.; Tognoli, E.; Denti, L.; Leonardi, F.; Giuliani, A.; Mattioli-Belmonte, M.; Mengucci, P. Influence of Trabecular Geometry on Scaffold Mechanical Behavior and MG-63 Cell Viability. Materials 2023, 16, 2342. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A Review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- von Arx, T.; Cochran, D.L.; Hermann, J.S.; Schenk, R.K.; Higginbottom, F.L.; Buser, D. Lateral Ridge Augmentation and Implant Placement: An Experimental Study Evaluating Implant Osseointegration in Different Augmentation Materials in the Canine Mandible. Int. J. Oral Maxillofac. Implant. 2001, 16, 343–354. [Google Scholar]
- Buser, D.; Martin, W.; Belser, U.C. Optimizing Esthetics for Implant Restorations in the Anterior Maxilla: Anatomic and Surgical Considerations. Int. J. Oral Maxillofac. Implant. 2004, 19, 43–61. [Google Scholar]
- Bohner, M. Calcium Orthophosphates in Medicine: From Ceramics to Calcium Phosphate Cements. Injury 2000, 31, D37–D47. [Google Scholar] [CrossRef]
- Midura, R.J.; Vasanji, A.; Su, X.; Wang, A.; Midura, S.B.; Gorski, J.P. Calcospherulites11Calcospherulites (Calco: Calcium Salt+spherulite: Spherical Crystalline Body); Calcium-Containing, Spherical Bodies Have Also Been Referred to as Calcified Microspheres, Mineral Clusters, Crystal Ghost Aggregates, Calcification Nodules, Calcospherites, and Small Calcified Spheres. Isolated from the Mineralization Front of Bone Induce the Mineralization of Type I Collagen. Bone 2007, 41, 1005–1016. [Google Scholar] [CrossRef]
- Buser, D.; Hoffmann, B.; Bernard, J.; Lussi, A.; Mettler, D.; Schenk, R.K. Evaluation of Filling Materials in Membrane-protected Bone Defects. A Comparative Histomorphometric Study in the Mandible of Miniature Pigs. Clin. Oral Implant. Res. 1998, 9, 137–150. [Google Scholar] [CrossRef]
- Jensen, S.S.; Broggini, N.; Hjørting-Hansen, E.; Schenk, R.; Buser, D. Bone Healing and Graft Resorption of Autograft, Anorganic Bovine Bone and Β-tricalcium Phosphate. A Histologic and Histomorphometric Study in the Mandibles of Minipigs. Clin. Oral Implant. Res. 2006, 17, 237–243. [Google Scholar] [CrossRef]
- Bonardi, J.P.; Pereira, R.d.S.; Mourão, C.F.; Coelho Mendes, B.; Lowenstein, A.; Montemezzi, P.; Giubilato, F.; Okamoto, R.; Hochuli-Vieira, E. Clinical Assessment of Biphasic Calcium Phosphate in Granules and Paste Forms in Human Maxillary Sinus Bone Augmentation: A Randomized, Split-Mouth Clinical Trial. Materials 2023, 16, 1059. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic Calcium Phosphate Bioceramics: Preparation, Properties and Applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef]
- Mangano, C.; Perrotti, V.; Shibli, J.A.; Mangano, F.; Ricci, L.; Piattelli, A.; Iezzi, G. Maxillary Sinus Grafting with Biphasic Calcium Phosphate Ceramics: Clinical and Histologic Evaluation in Man. Int. J. Oral Maxillofac. Implant. 2013, 28, 51–56. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Jiménez-Guerra, A.; Ortiz-Garcia, I.; Garrido, N.M.; Moreno-Muñoz, J.; Núñez-Márquez, E.; Rondón-Romero, J.L.; Cabanillas-Balsera, D.; López-López, J.; Monsalve-Guil, L. Implant Treatment by Guided Surgery Supporting Overdentures in Edentulous Mandible Patients. Int. J. Environ. Res. Public Health 2021, 18, 11836. [Google Scholar] [CrossRef]
- Mangano, C.; Giuliani, A.; De Tullio, I.; Raspanti, M.; Piattelli, A.; Iezzi, G. Case Report: Histological and Histomorphometrical Results of a 3-D Printed Biphasic Calcium Phosphate Ceramic 7 Years After Insertion in a Human Maxillary Alveolar Ridge. Front. Bioeng. Biotechnol. 2021, 9, 614325. [Google Scholar] [CrossRef] [PubMed]
- Tatum, H. Maxillary and Sinus Implant Reconstructions. Dent. Clin. N. Am. 1986, 30, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Fazzalari, N.L.; Parkinson, I.H. Fractal Dimension and Architecture of Trabecular Bone. J. Pathol. 1996, 178, 100–105. [Google Scholar] [CrossRef]
- Domander, R.; Felder, A.A.; Doube, M. BoneJ2—Refactoring Established Research Software. Wellcome Open Res. 2021, 6, 37. [Google Scholar] [CrossRef]
- Cuscó, R.; Guitián, F.; de Aza, S.; Artús, L. Differentiation between Hydroxyapatite and β-Tricalcium Phosphate by Means of μ-Raman Spectroscopy. J. Eur. Ceram. Soc. 1998, 18, 1301–1305. [Google Scholar] [CrossRef]
- Bakan, F. A Systematic Study of the Effect of PH on the Initialization of Ca-Deficient Hydroxyapatite to β-TCP Nanoparticles. Materials 2019, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Montanez Supelano, N.D.; Estupiñan, H.A.; García, S.J.; Peña, D.Y. Fabrication and Characterization of Novel Biphasic Calcium Phosphate and Nanosized Hydroxyapatite Derived from Fish Otoliths in Different Composition Ratios. Chem. Eng. Trans. 2018, 64, 307–312. [Google Scholar]
- de Aza, P.N.; Santos, C.; Pazo, A.; de Aza, S.; Cuscó, R.; Artús, L. Vibrational Properties of Calcium Phosphate Compounds. 1. Raman Spectrum of β-Tricalcium Phosphate. Chem. Mater. 1997, 9, 912–915. [Google Scholar] [CrossRef]
- Pascaretti-Grizon, F.; Guillaume, B.; Terranova, L.; Arbez, B.; Libouban, H.; Chappard, D. Maxillary Sinus Lift with Beta-Tricalcium Phosphate (β-TCP) in Edentulous Patients: A Nanotomographic and Raman Study. Calcif. Tissue Int. 2017, 101, 280–290. [Google Scholar] [CrossRef]
- Huang, S.-H.; Hsu, T.-T.; Huang, T.-H.; Lin, C.-Y.; Shie, M.-Y. Fabrication and Characterization of Polycaprolactone and Tricalcium Phosphate Composites for Tissue Engineering Applications. J. Dent. Sci. 2017, 12, 33–43. [Google Scholar] [CrossRef]
- Notarstefano, V.; Gioacchini, G.; Byrne, H.J.; Zacà, C.; Sereni, E.; Vaccari, L.; Borini, A.; Carnevali, O.; Giorgini, E. Vibrational Characterization of Granulosa Cells from Patients Affected by Unilateral Ovarian Endometriosis: New Insights from Infrared and Raman Microspectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 212, 206–214. [Google Scholar] [CrossRef]
- Orilisi, G.; Monterubbianesi, R.; Notarstefano, V.; Tosco, V.; Vitiello, F.; Giuliani, G.; Putignano, A.; Orsini, G. New Insights from Raman MicroSpectroscopy and Scanning Electron Microscopy on the Microstructure and Chemical Composition of Vestibular and Lingual Surfaces in Permanent and Deciduous Human Teeth. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 260, 119966. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Movasaghi, Z.; Rehman, S.; Rehman, I. ur Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2015, 50, 46–111. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, Y.; Ma, Y.; Xiao, L.; Ji, W.; Zhang, Y.; Wang, X. Current Application of Beta-Tricalcium Phosphate in Bone Repair and Its Mechanism to Regulate Osteogenesis. Front. Mater. 2021, 8, 698915. [Google Scholar] [CrossRef]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing Scaffold Pore Size for Tissue Engineering: Insights across Various Tissue Types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef]


| Case | Gender | Age | Healing Time | Patients’ Medical Records |
|---|---|---|---|---|
| Case 1 | F | 45 | 6 months | The patient had undergone an extraction of a tooth 1.6 approximately 1 year earlier due to a root fracture. The residual basal bone was insufficient for implant placement, necessitating maxillary sinus floor elevation. |
| Case 2 | M | 65 | 6 months | The patient presented with edentulism of the upper jaw, requiring implant rehabilitation through bilateral maxillary sinus floor elevation (bone harvested from the left side). |
| Case 3 | F | 53 | 9 months | Tooth 1.4 had been missing for approximately 7 years, also requiring sinus floor elevation due to insufficient residual bone volume. |
| Case 4 | F | 60 | 9 months | There was edentulism in the molar region of the right maxilla, along with carious lesions affecting the premolars, which were subsequently extracted. |
| Case 5 | M | 65 | 6 months | The patient presented with edentulism of the upper jaw, requiring implant rehabilitation through bilateral maxillary sinus floor elevation (bone harvested from the right side). |
| Case 6 | M | 62 | 12 months | The right posterior maxillary region had been edentulous for approximately 5 years. To enable implant placement and restore function, sinus floor elevation was required. |
| New Bone (%) | Residual Particles (%) | Marrow Spaces (%) | |
|---|---|---|---|
| Case 1–6 months | 12.54 | 10.18 | 77.29 |
| Case 2–6 months | 18.27 | 29.88 | 51.85 |
| Mean ± SD | 15.40 ± 4.05 | 20.03 ± 13.93 | 64.56 ± 17.99 |
| Case 3–9 months | 28.06 | 14.62 | 57.32 |
| Case 4–9 months | 25.43 | 23.29 | 51.29 |
| Mean ± SD | 26.74 ± 1.86 | 18.95 ± 6.13 | 54.30 ± 4.27 |
| Bone (%) | Marrow Spaces (%) | |
|---|---|---|
| Case 1–6 months | 37.09 | 62.91 |
| Case 2–6 months | 31.10 | 68.89 |
| Mean ± SD | 34.09 ± 4.22 | 65.90 ± 4.22 |
| Case 3–9 months | 40.74 | 59.26 |
| Case 4–9 months | 50.52 | 49.48 |
| Mean ± SD | 45.63 ± 6.91 | 54.37 ± 6.91 |
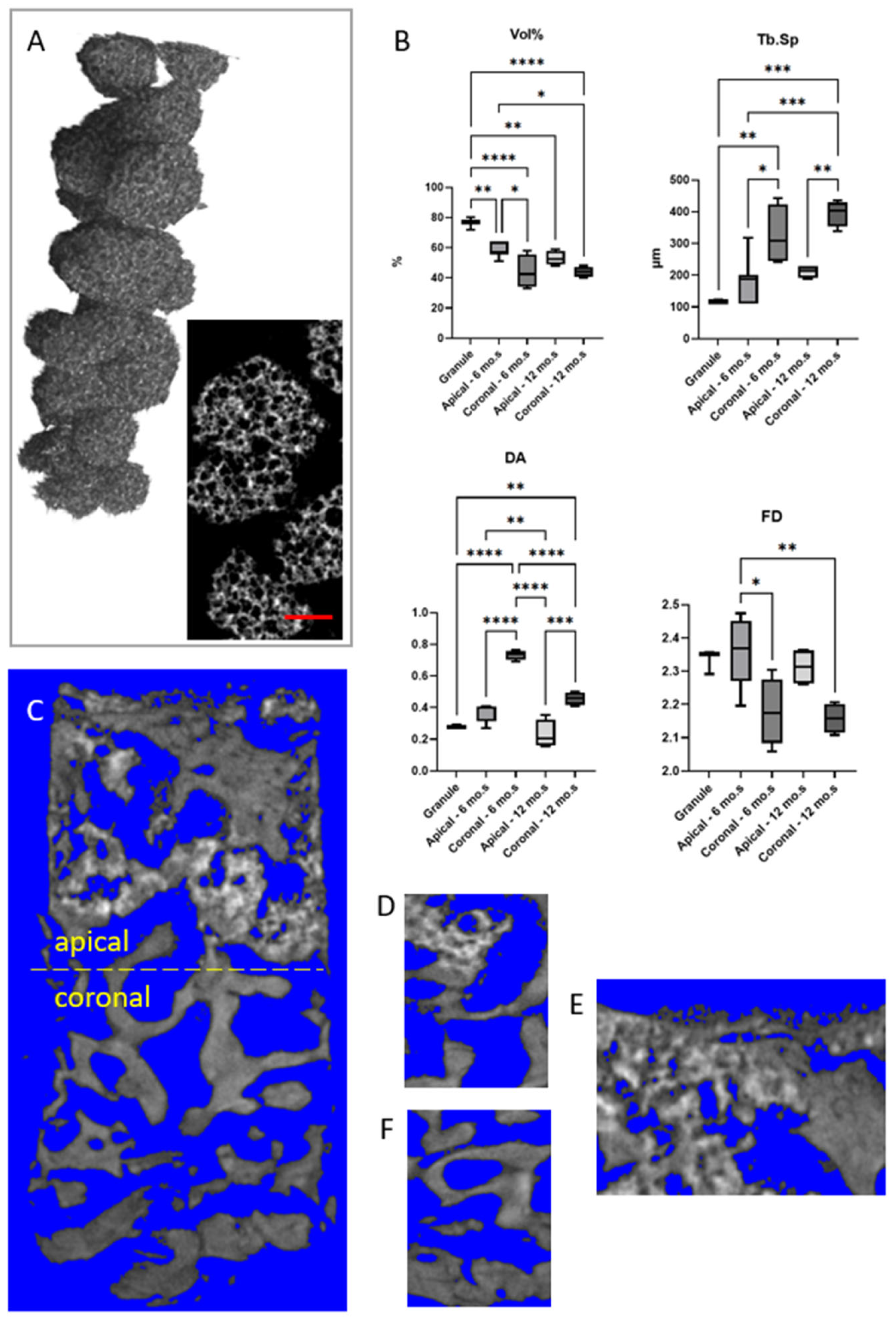
| Morphometric Parameter | Granule | Case 5 Apical (6 mo.) | Case 5 Coronal (6 mo.) | Case 6 Apical (12 mo.) | Case 6 Coronal (12 mo.) |
|---|---|---|---|---|---|
| Vol% [%] | 76 ± 4 (72 ÷ 80) | 58 ± 5 (51 ÷ 64) | 44 ± 11 (33 ÷ 58) | 53 ± 5 (48 ÷ 59) | 44 ± 3 (40 ÷ 48) |
| Tb.Th [µm] | 195 ± 13 (182 ÷ 208) | 211 ± 30 (182 ÷ 254) | 265 ± 81 (202 ÷ 371) | 218 ± 28 (182 ÷ 241) | 284 ± 37 (254 ÷ 338) |
| Tb.Sp [µm] | 119 ± 4 (117 ÷ 124) | 187 ± 70 (111 ÷ 319) | 325 ± 96 (241 ÷ 442) | 211 ± 20 (189 ÷ 228) | 395 ± 41 (338 ÷ 436) |
| Conn.D (×10−5) [px−3] | 0.75 ± 0.65 (0.12 ÷ 1.42) | 1.50 ± 1.02 (0.55 ÷ 3.11) | 0.51 ± 0.20 (0.36 ÷ 0.79) | 1.14 ± 0.37 (0.77 ÷ 1.70) | 0.39 ± 0.19 (0.18 ÷ 0.63) |
| DA | 0.28 ± 0.01 (0.28 ÷ 0.29) | 0.37 ± 0.05 (0.27 ÷ 0.41) | 0.73 ± 0.03 (0.69 ÷ 0.77) | 0.23 ± 0.09 (0.16 ÷ 0.36) | 0.46 ± 0.04 (0.41 ÷ 0.50) |
| FD | 2.33 ± 0.04 (2.29 ÷ 2.36) | 2.36 ± 0.10 (2.20 ÷ 2.47) | 2.18 ± 0.10 (2.06 ÷ 2.30) | 2.31 ± 0.06 (2.26 ÷ 2.36) | 2.16 ± 0.05 (2.11 ÷ 2.21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlani, M.; Notarstefano, V.; Riberti, N.; D’Amico, E.; Pierfelice, T.V.; Mangano, C.; Giorgini, E.; Iezzi, G.; Giuliani, A. Healing Kinetics of Sinus Lift Augmentation Using Biphasic Calcium Phosphate Granules: A Case Series in Humans. Bioengineering 2025, 12, 848. https://doi.org/10.3390/bioengineering12080848
Furlani M, Notarstefano V, Riberti N, D’Amico E, Pierfelice TV, Mangano C, Giorgini E, Iezzi G, Giuliani A. Healing Kinetics of Sinus Lift Augmentation Using Biphasic Calcium Phosphate Granules: A Case Series in Humans. Bioengineering. 2025; 12(8):848. https://doi.org/10.3390/bioengineering12080848
Chicago/Turabian StyleFurlani, Michele, Valentina Notarstefano, Nicole Riberti, Emira D’Amico, Tania Vanessa Pierfelice, Carlo Mangano, Elisabetta Giorgini, Giovanna Iezzi, and Alessandra Giuliani. 2025. "Healing Kinetics of Sinus Lift Augmentation Using Biphasic Calcium Phosphate Granules: A Case Series in Humans" Bioengineering 12, no. 8: 848. https://doi.org/10.3390/bioengineering12080848
APA StyleFurlani, M., Notarstefano, V., Riberti, N., D’Amico, E., Pierfelice, T. V., Mangano, C., Giorgini, E., Iezzi, G., & Giuliani, A. (2025). Healing Kinetics of Sinus Lift Augmentation Using Biphasic Calcium Phosphate Granules: A Case Series in Humans. Bioengineering, 12(8), 848. https://doi.org/10.3390/bioengineering12080848















