PredictMed-CDSS: Artificial Intelligence-Based Decision Support System Predicting the Probability to Develop Neuromuscular Hip Dysplasia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Measurements
- Neurologic status was categorized based on the topography of spastic disorder (hemiplegia, diplegia, tri/quadriplegia), the presence of hypertonia in the upper or lower limbs, the presence of dystonia, and the severity of epilepsy.
- Spasticity was measured using the Bohannon and Smith modified Ashworth Scale and the Modified Tardieu Scale [14]. Dystonia is a neurological hyperkinetic movement disorder where continuous or repetitive muscle contractions lead to twisting and repetitive movements or unusual fixed postures. The presence of dystonia was classified as either present or absent through a clinical evaluation [19,20].
- Pediatric neurologists assessed the severity of epilepsy as either “well controlled” or “intractable” based on the guidelines set by the International League Against Epilepsy. Intractable epilepsy is defined as continued seizures despite treatment with a minimum of two antiepileptic medications [6] (Table 1).
- The clinical assessment of the hip primarily focused on internal rotation and hip abduction. The Melbourne Cerebral Palsy Hip Classification Scale (MCPHCS) was used to classify hip morphology. The modified Harris Hip Score (MHHS) was utilized to evaluate hip function, gait, and pain. All patients underwent at least one pelvic X-ray, with the most recent one being reviewed by a pediatric orthopedic surgeon in cases where multiple X-rays were available [1,2,3].
2.4. Data Analysis
2.4.1. Univariate Analysis
- OR = 1, No association between the variables. The predictor variable does not affect the odds of the outcome.
- OR > 1, Positive association between variables. An increase in the predictor variable is positively associated with higher odds of the outcome.
- OR < 1, Negative association between the variables. An increase in the predictor variable is (negatively) associated with lower odds of the outcome.
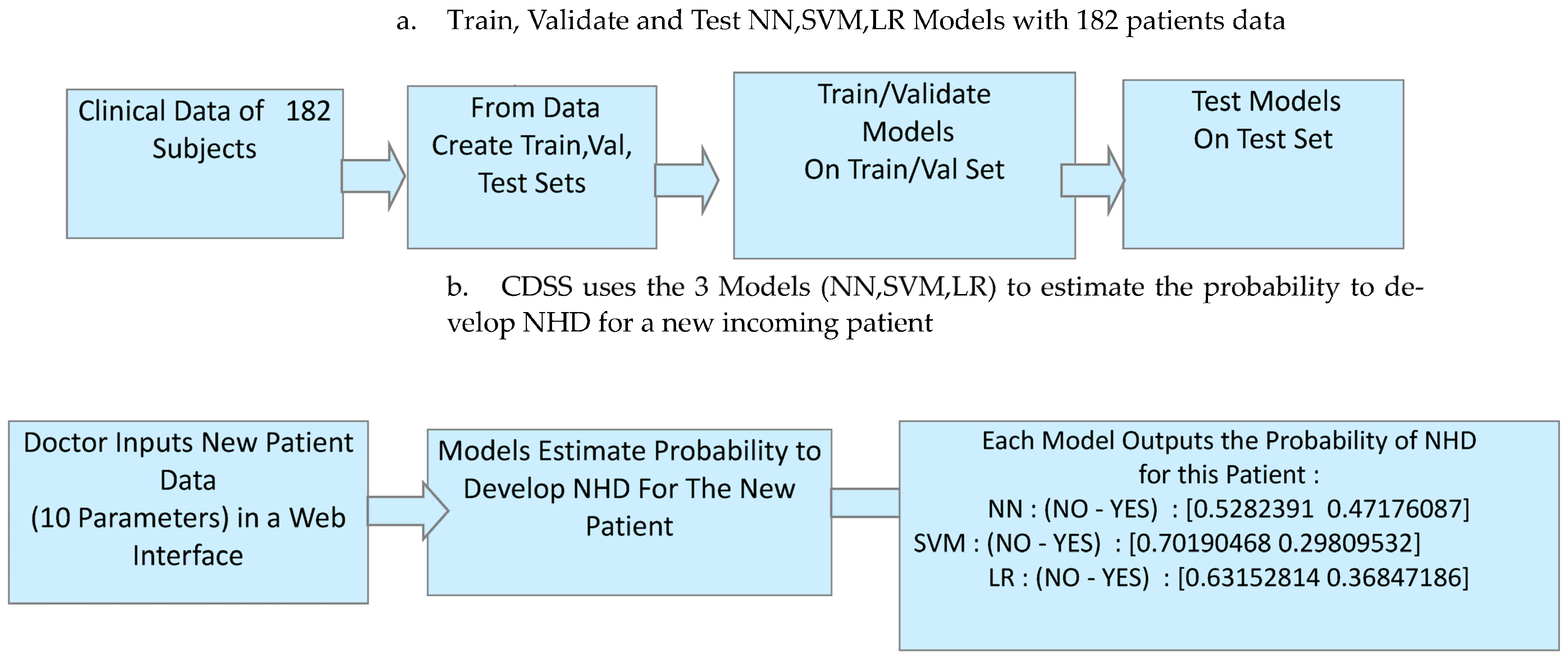
2.4.2. The PredictMed-Clinical Decision Support System
System Architecture
Predictive Metrics
Neural Network (NN) Model Architecture
Support Vector Machine (SVM) Model Architecture
Logistic Regression (LR) Model Architecture
2.5. Institutional Review Board Statement
3. Results
3.1. Statistical Analysis
- Previous history of orthopedic surgery (p = 0.0017);
- Truncal tone disorder (p = 0.0049);
- Poor motor function (p = 0.063).
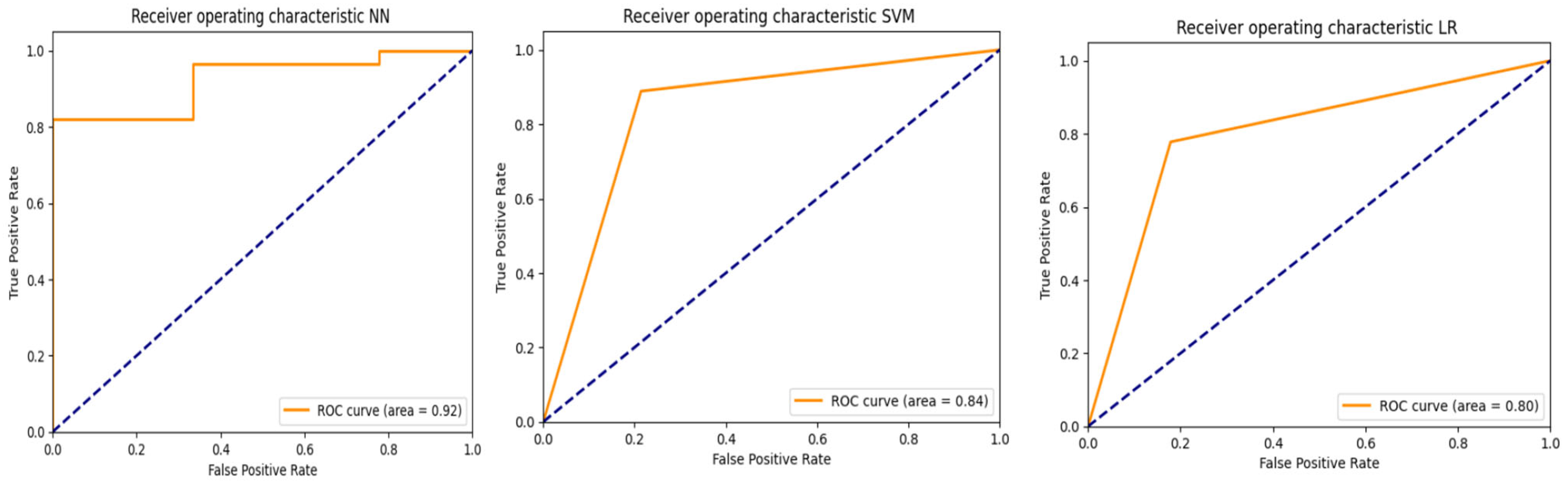
3.2. PredictMed-CDSS Performance Metrics
4. Discussion
- Physical therapy: Use targeted exercises to improve mobility. For instance, increased conservative measures such as abduction posture and exercises should also be proposed. Stretching exercises can help to maintain muscle flexibility and reduce stiffness. This therapy is important at all ages and its frequency can be modulated according to the risk of developing NHD.
- Orthotic devices: Use braces or supports to stabilize the hip joint and prevent further dislocation, with particular emphasis on adduction, especially in case of adductor spasticity or pelvic obliquity.
- Botulinum toxin injections: Administered to manage spasticity to target specific muscles causing abnormal tone and reduce involuntary contractions, as adductors, but also internal or external rotators and/or flexors.
- Early and less-invasive surgical interventions, as abductor muscle tenotomy, obturator nerve neurotomy, and hemi-epiphysiodesis of femoral neck to decrease coxa valga. These are often proposed during multisite surgeries to improve muscular balance and global posture, with the aim of avoiding less conservative surgery.
- Neurosurgical treatment of generalized spasticity: Intrathecal baclofen therapy (ITB) delivers baclofen directly to the spinal fluid, especially in quadriplegic adolescents, or selective dorsal rhizotomy, especially in diplegic younger children. Although they are not specific for hip dysplasia, releasing spasticity could decrease its risk.
- Non-conservative surgery: Total hip replacement or proximal femoral resection (head and neck) to manage pain, especially in long lasting subluxation/dislocation and damaged cephalic cartilage.
5. Limitations, Conclusions, and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terjesen, T. The natural history of hip development in cerebral palsy. Dev. Med. Child Neurol. 2012, 54, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, G.; Lauge-Pedersen, H.; Wagner, P. Characteristics of children with hip displacement in cerebral palsy. BMC Musculoskelet. Disord. 2007, 8, 101. [Google Scholar] [CrossRef]
- Sorhage, A.; Stott, N.S. Hip surveillance in cerebral palsy: Review of clinical practice in a tertiary children's hospital using electronic health record linkage. J. Paediatr. Child Health 2025, 61, 94–99. [Google Scholar] [CrossRef]
- Kunhimangalam, R.; Ovallath, S.; Joseph, P.K. A clinical decision support system with an integrated EMR for diagnosis of peripheral neuropathy. J. Med. Syst. 2014, 38, 38. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelli, C.; Altamura, P.; Vieira, E.R.; Bertoncelli, D.; Solla, F. Predicting Hip Dysplasia in Teenagers with Cerebral Palsy in order to Optimize Prevention and Rehabilitation. A Longitudinal Descriptive Study. Dev. Neurorehabilit. 2020, 24, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelli, C.M.; Altamura, P.; Vieira, E.R.; Iyengar, S.S.; Solla, F.; Bertoncelli, D. PredictMed: A logistic regression–based model to predict health conditions in cerebral palsy. Health Inform. J. 2020, 26, 2105–2118. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, M.; Hägglund, G.; Riad, J.; Rodby-Bousquet, E.; Wagner, P. Prediction of hip displacement in children with cerebral palsy: Development of the CPUP hip score. Bone Jt. J. 2015, 97-B, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.-T.; Le, M.-B.; Le, L.H.; Andersen, J.; Lou, E. Assessment of hip displacement in children with cerebral palsy using machine learning approach. Med. Biol. Eng. Comput. 2021, 59, 1877–1887. [Google Scholar] [CrossRef]
- Bright, T.J.; Wong, A.; Dhurjati, R.; Bristow, E.; Bastian, L.; Coeytaux, R.R.; Samsa, G.; Hasselblad, V.; Williams, J.W.; Musty, M.D.; et al. Effect of clinical decision-support systems: A systematic review. Ann. Intern. Med. 2012, 157, 29–43. [Google Scholar] [CrossRef]
- Mitchell, T.M. Artificial neural networks. Mach. Learn. 1997, 45, 127. [Google Scholar]
- Jakkula, V. Tutorial on Support Vector Machine (SVM); School of EECS, Washington State University: Pullman, WA, USA, 2006. [Google Scholar]
- Menard, S. Applied Logistic Regression Analysis; Sage: Thousand Oaks, CA, USA, 2002; Volume 106. [Google Scholar]
- Vasey, B.; Nagendran, M.; Campbell, B.; Clifton, D.A.; Collins, G.S.; Denaxas, S.; Denniston, A.K.; Faes, L.; Geerts, B.; Ibrahim, M.; et al. Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. Nat. Med. 2022, 28, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelli, C.M.; Latalski, M.; Bertoncelli, D.; Bagui, S.; Bagui, S.C.; Gautier, D.; Solla, F. Prediction Model for Identifying Computational Phenotypes of Children with Cerebral Palsy Needing Neurotoxin Treatments. Toxins 2022, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelli, C.M.; Bertoncelli, D.; Elbaum, L.; Latalski, M.; Altamura, P.; Musoff, C.; Rampal, V.; Solla, F. Validation of a Clinical Prediction Model for the Development of Neuromuscular Scoliosis: A Multinational Study. Pediatr. Neurol. 2018, 79, 14–20. [Google Scholar] [CrossRef]
- Sæther, R.; Jørgensen, L. Intra- and inter-observer reliability of the trunk impairment scale for children with cerebral palsy. Res. Dev. Disabil. 2011, 32, 727–739. [Google Scholar] [CrossRef]
- Dequeker, G.; Van Campenhout, A.; Feys, H.; Molenaers, G. Evolution of self-care and functional mobility after single-event multilevel surgery in children and adolescents with spastic diplegic cerebral palsy. Dev. Med. Child Neurol. 2018, 60, 505–512. [Google Scholar] [CrossRef]
- Dingemans, S.A.; Kleipool, S.C.; Mulders, M.A.M.; Winkelhagen, J.; Schep, N.W.L.; Goslings, J.C.; Schepers, T. Normative data for the lower extremity functional scale (LEFS). Acta Orthop. 2017, 88, 422–426. [Google Scholar] [CrossRef]
- Bertoncelli, C.M.; Solla, F.; Loughenbury, P.R.; Tsirikos, A.I.; Bertoncelli, D.; Rampal, V. Risk Factors for Developing Scoliosis in Cerebral Palsy: A Cross-Sectional Descriptive Study. J. Child Neurol. 2017, 32, 657–662. [Google Scholar] [CrossRef]
- Hareb, F.; Rampal, V.; Bertoncelli, C.M.; Rosello, O.; Solla, F. Botulinum toxin in children with cerebral palsy: An update. Neuropediatrics 2019, 51, 001–005. [Google Scholar] [CrossRef]
- Baratloo, A.; Hosseini, M.; Negida, A.; El Ashal, G. Part 1: Simple Definition and Calculation of Accuracy, Sensitivity and Specificity. Emergency 2015, 3, 48–49. [Google Scholar] [PubMed] [PubMed Central]
- Yang, S.; Berdine, G. The receiver operating characteristic (ROC) curve. Southwest Respir. Crit. Care Chron. 2017, 5, 34–36. [Google Scholar] [CrossRef]
- Prechelt, L. Early Stopping-But When? In Neural Networks: Tricks of the Trade; Springer: Berlin/Heidelberg, Germany, 2002; pp. 55–69. [Google Scholar]
- Cortes, C.; Mehryar, M.; Afshin, R. L2 regularization for learning kernels. arXiv 2012. [Google Scholar] [CrossRef]
- Das, S.; Radovich, E.; Karki, S.; Calvert, C.; Shakya, R.; Penn-Kekana, L.; Shrestha, A.; Karmacharya, B.M.; McCarthy, O.L.; Campbell, O.M.R. Impact of digital antenatal care intervention on paper-based antenatal care recordkeeping: A before-and-after study in primary healthcare facilities in Nepal. BMJ Open 2025, 15, e086255. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Yiwen, W.; Yonghui, Y. Development and Validation of a Prognostic Model for Independent Walking in Children with Cerebral Palsy Based on Machine Learning. Arch. Phys. Med. Rehabilitation 2025. online ahead of print. [Google Scholar] [CrossRef]
- Nikhil, K. Deep Learning with Python: A Hands-on Introduction; Apress: Berkeley, CA, USA, 2017; pp. 97–111. ISBN 978-1-4842-2765-7. [Google Scholar]
- Bo, P.; Nijkamp, E.; Wu, Y.N. Deep learning with tensorflow: A review. J. Educ. Behav. Stat. 2020, 45, 227–248. [Google Scholar]
- Lau, M.M.; Lim, K.H. Review of Adaptive Activation Function in Deep Neural Network. In Proceedings of the 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Sarawak, Malaysia, 3–6 December 2018. [Google Scholar]
- Srivastava, N.; Hinton, G.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R. Dropout: A simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 2014, 15, 1929–1958. [Google Scholar]
- Kingma, D.P.; Ba, J.A. A method for stochastic optimization. arXiv 2014. [Google Scholar] [CrossRef]
- StClair, R.; Cross Entropy Loss and Uses in Machine Learning. 13 June 2023. Available online: https://www.aiplusinfo.com/blog/cross-entropy-loss-and-uses-in-machine-learning/ (accessed on 29 July 2025).
- Johnson, J.M.; Khoshgoftaar, T.M. Survey on deep learning with class imbalance. J. Big Data 2019, 6, 27. [Google Scholar] [CrossRef]
- You, K.; Long, M.; Wang, J.; Jordan, M.I. How does learning rate decay help modern neural networks? arXiv 2019. [Google Scholar] [CrossRef]
- Yuan, R.; Janzen, I.; Devnath, L.; Khattra, S.; Myers, R.; Lam, S.; MacAulay, C. MA19.11 Predicting Future Lung Cancer Risk with Low-Dose Screening CT Using an Artificial Intelligence Model. J. Thorac. Oncol. 2023, 18, S174. [Google Scholar] [CrossRef]
- Valdivia, J.T.A.; Rojas, V.G.; Astudillo, C.A. Deep learning-based classification of hemiplegia and diplegia in cerebral palsy using postural control analysis. Sci. Rep. 2025, 15, 8811. [Google Scholar] [CrossRef]
- Rojas-Dominguez, A.; Padierna, L.C.; Valadez, J.M.C.; Puga-Soberanes, H.J.; Fraire, H.J. Optimal hyper-parameter tuning of SVM classifiers with application to medical diagnosis. IEEE Access 2017, 6, 7164–7176. [Google Scholar] [CrossRef]
- Jin, Z.; Hei, T.G.; Jin, K. Application of Support Vector Machines for Categorizing Biological and Medical Data. Conference on Computer Graphics, Artificial Intelligence, and Data Processing. Available online: https://api.semanticscholar.org/CorpusID:268781951 (accessed on 29 July 2025).
- Jolly, K. Machine Learning with Scikit-Learn Quick Start Guide: Classification, Regression, and Clustering Techniques in Python; Packt Publishing Ltd.: Birmingham, UK, 2018. [Google Scholar]
- Bertoncelli, C.M.; Bertoncelli, D.; Bagui, S.S.; Bagui, S.C.; Costantini, S.; Solla, F. Identifying Postural Instability in Children with Cerebral Palsy Using a Predictive Model: A Longitudinal Multicenter Study. Diagnostics 2023, 13, 2126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Găman, M.A.; Dugăeşescu, M.; Popescu, D.C. Applications of Artificial Intelligence in Acute Promyelocytic Leukemia: An Avenue of Opportunities? A Systematic Review. J. Clin. Med. 2025, 14, 1670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devnath, L.; Luo, S.; Summons, P.; Wang, D.; Shaukat, K.; Hameed, I.A.; Alrayes, F.S. Deep Ensemble Learning for the Automatic Detection of Pneumoconiosis in Coal Worker’s Chest X-ray Radiography. J. Clin. Med. 2022, 11, 5342. [Google Scholar] [CrossRef]
- Yuan, C.; Li, H.; Zhang, J.; Gao, C.; Wang, Z.; Sun, M.; Jiang, Y.; Wang, H. Prediction model for growth trends of subpleural subsolid nodules using CT radiomics and radiological features. Sci. Rep. 2025, 15, 24800. [Google Scholar] [CrossRef]
- Dibbern, K.N.; Krzak, M.G.; Olivas, A.; Albert, M.V.; Krzak, J.J.; Kruger, K.M. Scoping Review of Machine Learning Techniques in Marker-Based Clinical Gait Analysis. Bioengineering 2025, 12, 591. [Google Scholar] [CrossRef]
- Picciolini, O.; Le Metayer, M.; Consonni, D.; Cozzaglio, M.; Porro, M.; Gasparroni, V.; Panou, A.; Mosca, F.; Portinaro, N.M. Can we prevent hip dislocation in children with cerebral palsy? Effects of postural management. Eur. J. Phys. Rehabil. Med. 2016, 52, 682–690. [Google Scholar]
- Kim, H.R.; Kim, H.S. Optimal cutoffs of cardiometabolic risk for postmenopausal Korean women. Asian Nurs. Res. 2017, 11, 107–112. [Google Scholar] [CrossRef]
- Shore, B.J.; Shrader, M.W.; Narayanan, U.; Miller, F.; Graham, H.K.; Mulpuri, K. Hip Surveillance for Children With Cerebral Palsy: A Survey of the POSNA Membership. J. Pediatr. Orthop. 2017, 37, e409–e414. [Google Scholar] [CrossRef]
- Kentish, M.; Wynter, M.; Snape, N.; Boyd, R. Five-year outcome of state-wide hip surveillance of children and adolescents with cerebral palsy. J. Pediatr. Rehabilitation Med. 2011, 4, 205–217. [Google Scholar] [CrossRef]

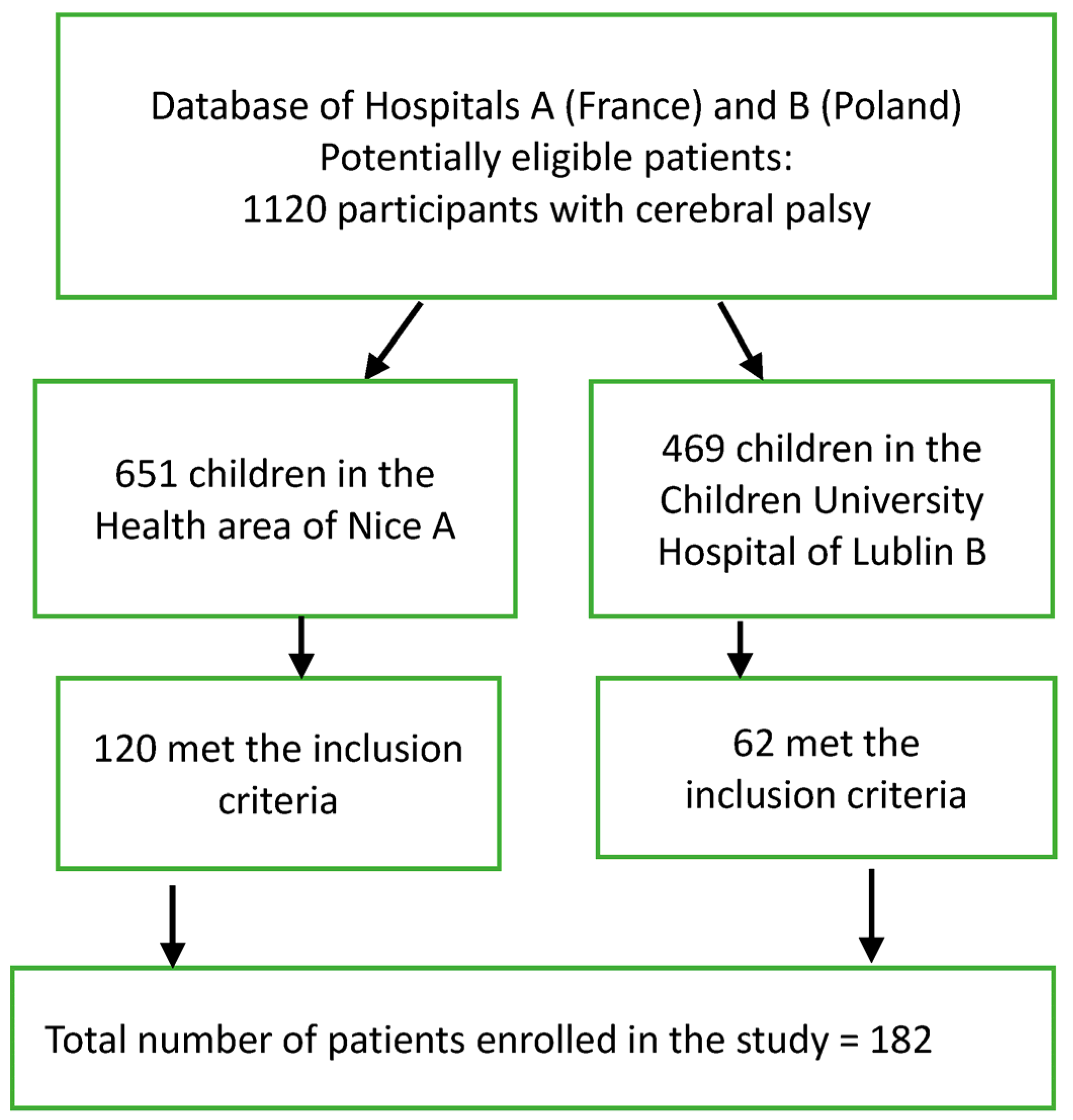
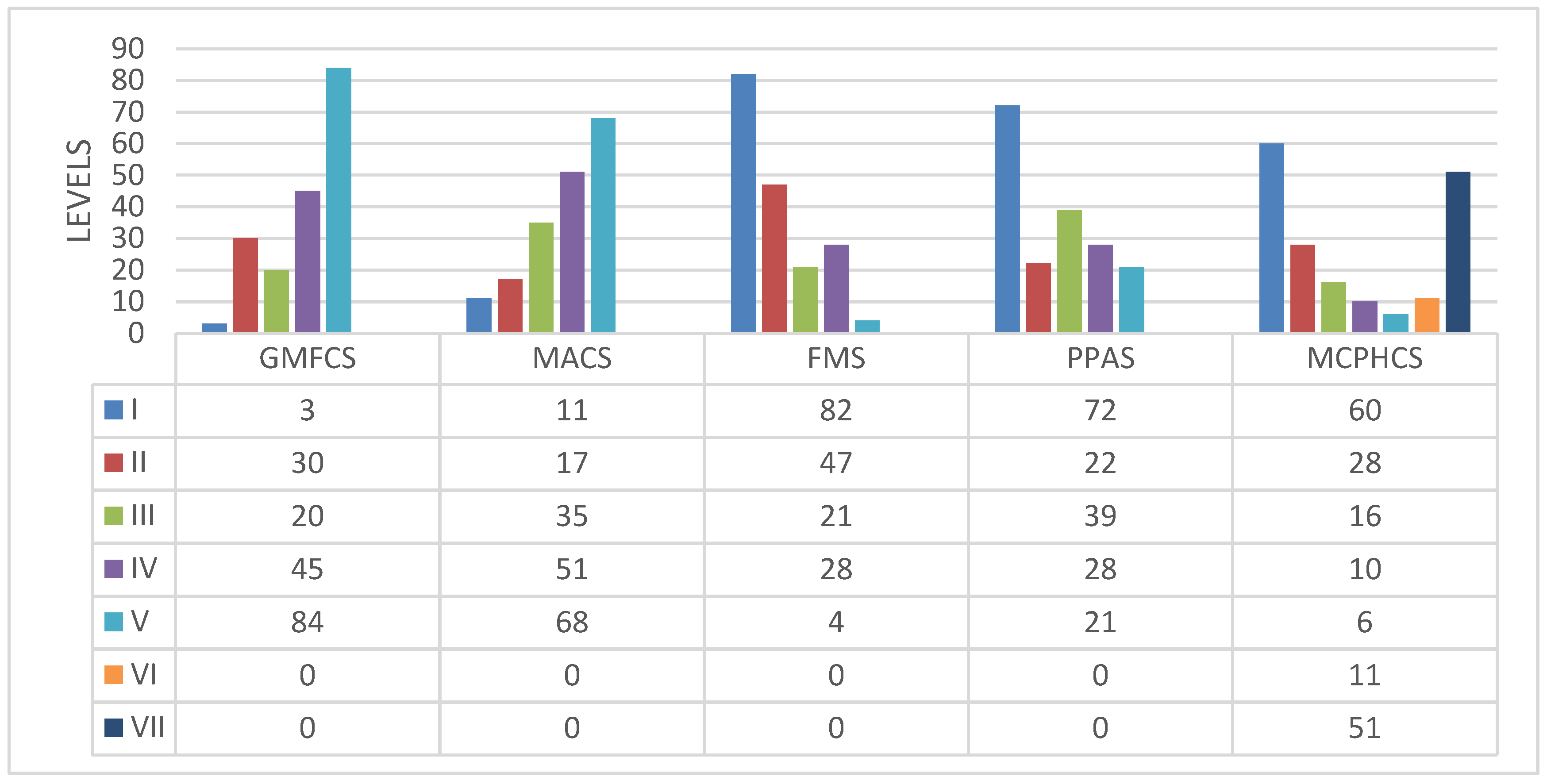


| Pediatric Hospital A | Children Hospital B | Multicenter | |||
|---|---|---|---|---|---|
| Patients Profile | Neuromuscular Hip Dysplasia | Neuromuscular Hip Dysplasia | Total (%) | ||
| Yes (%) | No (%) | Total (%) | Yes (%) | No (%) | |
| Patients n. (%) | 33 (27) | 87 (73) | 120 (100) | 28 (45) | 34 (65) |
| Male | 22 (30) | 52 (70) | 74 (100) | 12 (39) | 18 (61) |
| Female | 11 (24) | 35 (76) | 46 (100) | 16 (50) | 16 (50) |
| Average age (mean, SD) | 16.3 (1.8) | 16.7 (1.8) | 16.5 (1.8) | 15.8 (1.8) | 16.0 (1.8) |
| Antenatal causes | 27 (36) | 48 (64) | 75 (100) | 9 (33) | 17 (67) |
| Perinatal causes | 14 (45) | 17 (55) | 31 (100) | 18 (54) | 15 (46) |
| Postnatal causes | 3 (21) | 11 (79) | 14 (100) | 1 (33) | 2 (67) |
| Spasticity n. (%) | 29 (32) | 62 (68) | 91 (100) | 28 (45) | 34 (65) |
| Hemiplegia | 2 (20) | 8 (80) | 10 (100) | 1 (9) | 10 (91) |
| Diplegia | 1 (5) | 18 (95) | 19 (100) | 9 (30) | 21 (70) |
| Tri/quadriplegia | 26 (42) | 36 (58) | 62 (100) | 18 (89) | 3 (11) |
| Dystonia n. (%) | 3 (20) | 11 (80) | 14 (100) | 8 (58) | 6 (42) |
| Severe scoliosis (%) | 17 (40) | 25 (60) | 42 (100) | 24 (62) | 15 (38) |
| Standing ability (%) | 3 (5) | 49 (95) | 52 (100) | 4 (11) | 26 (89) |
| Truncal tone disorder (%) | 27 (40) | 40 (60) | 67 (100) | 13 (75) | 5 (25) |
| Well-controlled epilepsy n. (%) | 18 (35) | 34 (65) | 52 (100) | 12 (38) | 19 (62) |
| Intractable epilepsy | 5 (15) | 27 (85) | 32 (100) | 11 (100) | 0 (0) |
| No epilepsy | 6 (17) | 29 (83) | 35 (100) | 5 (25) | 15 (75) |
| Pediatric Hospital A | Children Hospital B | Multicenter Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Independent Variables | Hip Dysplasia | p Value | Hip Dysplasia | p Value | OR | 95% CIs | Z Statistic | |||
| Yes | No | Yes | No | |||||||
| Standing position | Yes | 3 | 49 | >0.0001 | 3 | 25 | >0.0001 | 0.06 | 0.026–0.166 | 5.32 |
| No | 31 | 37 | 25 | 9 | ||||||
| Independent walking | Yes | 0 | 42 | >0.0001 | 1 | 28 | >0.0001 | 0.02 | 0.001–0.081 | 4.41 |
| No | 37 | 41 | 26 | 7 | ||||||
| Spasticity | Yes | 31 | 60 | 0.0038 | 28 | 1 | >0.0001 | 29.01 | 6.67–124.14 | 4.61 |
| No | 2 | 27 | 0 | 33 | ||||||
| MACS ≤ 4 vs. MACS 3 | Yes | 30 | 60 | 0.0038 | 24 | 3 | >0.0001 | 8.42 | 3.37–21.04 | 4.47 |
| No | 2 | 28 | 4 | 31 | ||||||
| Scoliosis | Yes | 17 | 25 | 0.0311 | 24 | 15 | 0.0013 | 4.15 | 2.15–7.99 | 4.33 |
| No | 16 | 62 | 4 | 19 | ||||||
| Truncal tone disorder | Yes | 27 | 40 | 0.0004 | 13 | 5 | 0.0105 | 3.21 | 1.68–6.12 | 3.36 |
| No | 6 | 47 | 15 | 29 | ||||||
| GMFCS ≤ 4 vs. GMFCS 3 | Yes | 35 | 57 | 0.0661 | 27 | 25 | 0.0172 | 4.03 | 1.58–10.24 | 3.10 |
| No | 5 | 23 | 1 | 9 | ||||||
| Independent Variables | Z Test of Coefficients | |||
|---|---|---|---|---|
| Odds Ratio Estimate | Standard Error | Z Ratio | ||
| Intercept | −7.1046 | 0.0008 | 1.4281 | −4.9749 |
| Scoliosis (NS) | 0.5887 | 1.8016 | 0.4940 | 1.1918 |
| Truncal tone disorder (TT) | 0.8882 | 2.4307 | 0.3160 | 2.8109 |
| Spasticity (SP) | 0.4762 | 1.6099 | 0.3067 | 1.5523 |
| GMFCS score | 0.3973 | 1.4878 | 0.38747 | 1.0256 |
| MACS score | 0.5217 | 1.6848 | 0.2811 | 1.8558 |
| Epilepsy (E) | −0.5988 | 0.5494 | 0.3892 | −1.5385 |
| Etiology (ET) | 0.2914 | 1.3382 | 0.3525 | 0.8267 |
| Dystonia (D) | −0.3568 | 0.6999 | 0.5692 | −0.6268 |
| Sex (SE) | −0.5284 | 0.5895 | 0.4891 | −1.0805 |
| History of previous surgery (SU) | 1.6501 | 5.2075 | 0.52796 | 3.1256 |
| Classifier | Accuracy | Accuracy CI (95%) | Specificity | Specificity CI (95%) | Sensitivity | Sensitivity CI (95%) | AUROC | AUROC CI (95%) |
|---|---|---|---|---|---|---|---|---|
| NN | 0.84 | [0.69, 0.92] | 0.82 | [0.64, 0.92] | 0.89 | [0.57, 0.98] | 0.92 | [0.83, 1.01] |
| SVM | 0.81 | [0.66, 0.91] | 0.79 | [0.60, 0.90] | 0.89 | [0.57, 0.98] | 0.84 | [0.72, 0.96] |
| LR | 0.81 | [0.66, 0.91] | 0.82 | [0.64, 0.92] | 0.78 | [0.45, 0.94] | 0.8 | [0.67, 0.93] |
| Input of the patient data related to the risk of NHD will show as follows: 1. Neuromuscular scoliosis (NS); 2.Truncal tone (TT); 3. Spasticity (SP); 4. GMFCS(G): 5. 463 MACS(M); 6. Epilepsy (E); 7. Etiology (ET); 8. Dystonia (D); 9. Sex (SE); 10. Surgery (SU). | ||||||
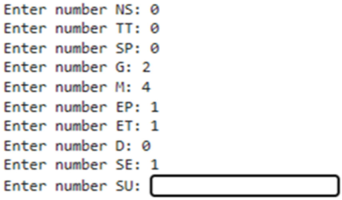 | ||||||
| Output (patient probability of having NHD) will show as follows: NN : probability of new patient of having NHD (NO–YES) : [0.96 0.04]; SVM : probability of new patient of having NHD (NO–YES) : [0.97 0.03]; LR : probability of new patient of having NHD (NO–YES) : [0.99 0.01]. Following Classifier-specific values for accuracy, sensitivity, and specificity, along with their 95% confidence intervals based on Wilson score intervals using confusion matrix data. Python libraries statsmodels were used for the calculation. | ||||||
| Classifier | Accuracy | Accuracy CI (95%) | Specificity | Specificity CI (95%) | Sensitivity | Sensitivity CI (95%) |
|---|---|---|---|---|---|---|
| NN | 0.84 | [0.69, 0.92] | 0.82 | [0.64, 0.92] | 0.89 | [0.57, 0.98] |
| SVM | 0.81 | [0.66, 0.91] | 0.79 | [0.60, 0.90] | 0.89 | [0.57, 0.98] |
| LR | 0.81 | [0.66, 0.91] | 0.82 | [0.64, 0.92] | 0.78 | [0.45, 0.94] |
| First Author | Year | Subjects | Type of Predictors | AUC | Accuracy | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|
| Hermanson [7] | 2015 | 145 | Clinical and radiological | 0.87 | Not available | Not available | Not available |
| Bertoncelli [6] | 2020 | 102 | Clinical | Not available | 77% | 98% | 43% |
| Pham [8] | 2021 | 122 | Radiological | Not available | 91% | 93% | 88% |
| Bertoncelli—Current series | 2025 | 182 | Clinical | 0.92 | 84% | 89% | 82% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoncelli, C.M.; Solla, F.; Latalski, M.; Bagui, S.; Bagui, S.C.; Costantini, S.; Bertoncelli, D. PredictMed-CDSS: Artificial Intelligence-Based Decision Support System Predicting the Probability to Develop Neuromuscular Hip Dysplasia. Bioengineering 2025, 12, 846. https://doi.org/10.3390/bioengineering12080846
Bertoncelli CM, Solla F, Latalski M, Bagui S, Bagui SC, Costantini S, Bertoncelli D. PredictMed-CDSS: Artificial Intelligence-Based Decision Support System Predicting the Probability to Develop Neuromuscular Hip Dysplasia. Bioengineering. 2025; 12(8):846. https://doi.org/10.3390/bioengineering12080846
Chicago/Turabian StyleBertoncelli, Carlo M., Federico Solla, Michal Latalski, Sikha Bagui, Subhash C. Bagui, Stefania Costantini, and Domenico Bertoncelli. 2025. "PredictMed-CDSS: Artificial Intelligence-Based Decision Support System Predicting the Probability to Develop Neuromuscular Hip Dysplasia" Bioengineering 12, no. 8: 846. https://doi.org/10.3390/bioengineering12080846
APA StyleBertoncelli, C. M., Solla, F., Latalski, M., Bagui, S., Bagui, S. C., Costantini, S., & Bertoncelli, D. (2025). PredictMed-CDSS: Artificial Intelligence-Based Decision Support System Predicting the Probability to Develop Neuromuscular Hip Dysplasia. Bioengineering, 12(8), 846. https://doi.org/10.3390/bioengineering12080846










