Enhanced Oilfield-Produced-Water Treatment Using Fe3+-Augmented Composite Bioreactor: Performance and Microbial Community Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Oilfield-Produced Water
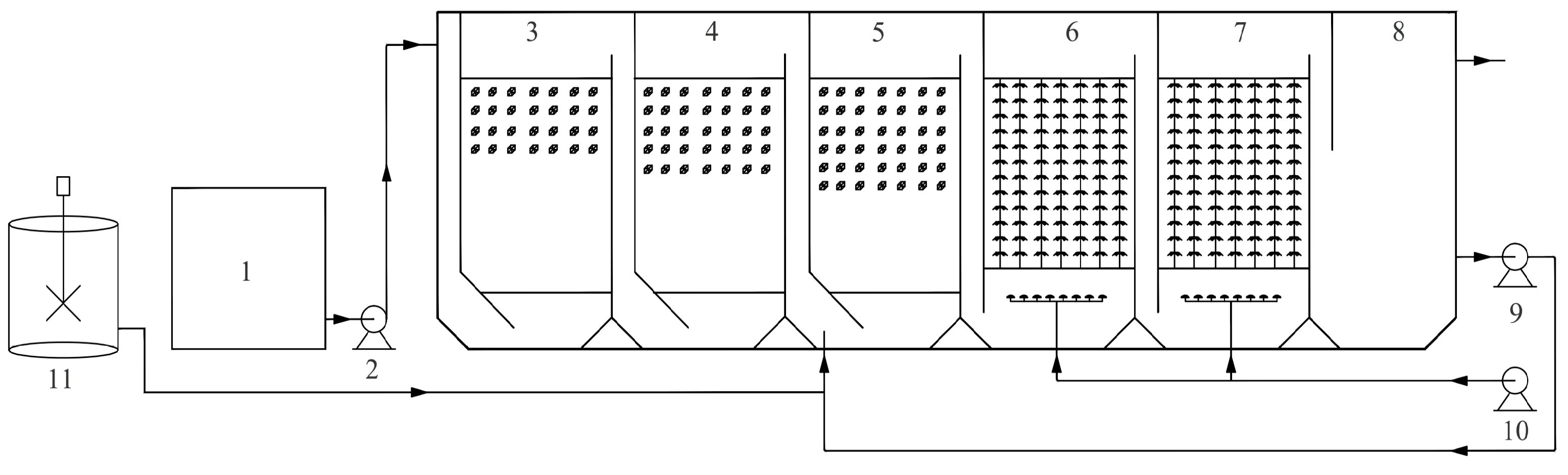
2.2. Experimental Setup and Operating Procedure
2.3. Analytical Methods
2.3.1. Physicochemical Analysis
2.3.2. Organic Composition Analysis
2.3.3. Microbial Community Analysis
2.3.4. Metagenomic Analysis
3. Results and Discussion
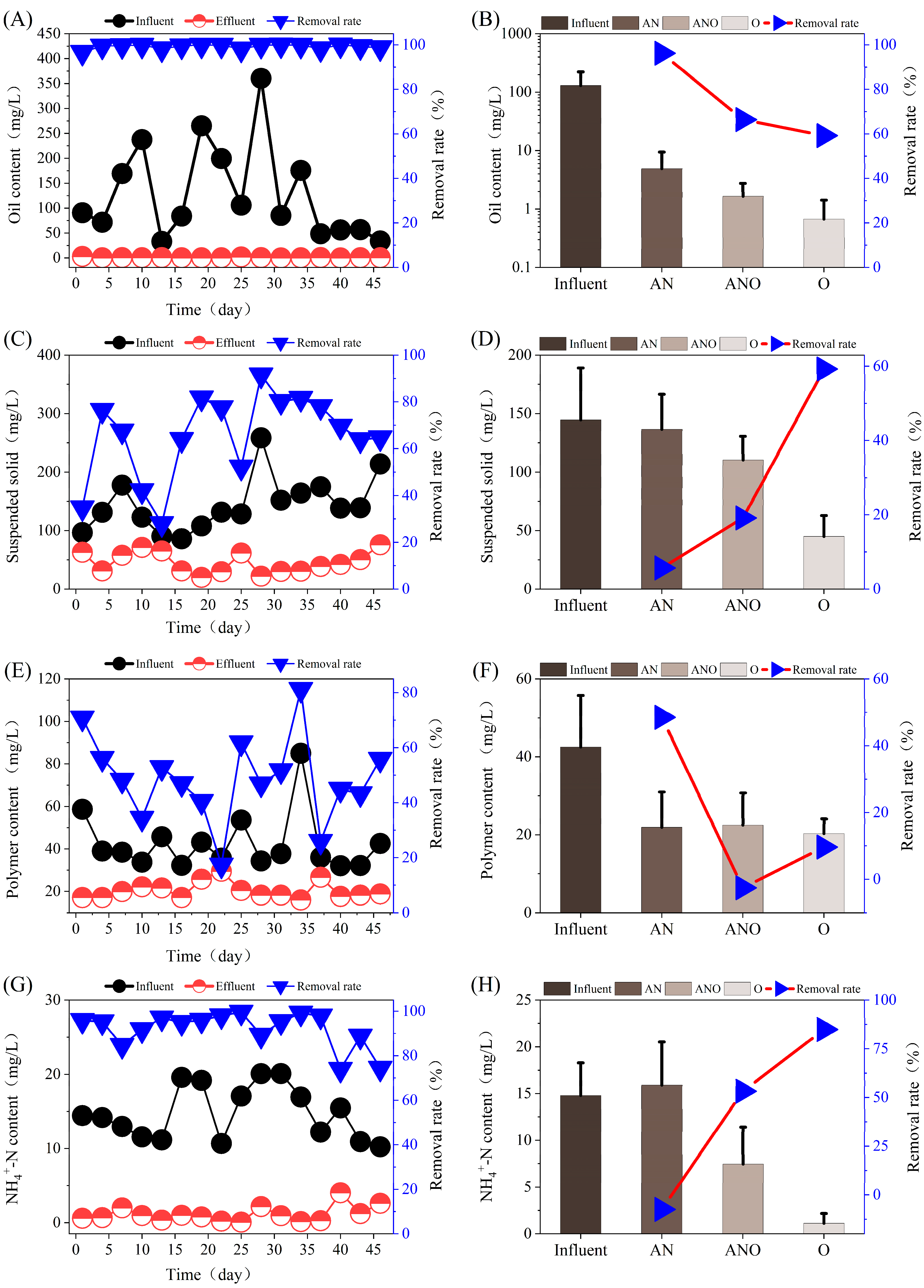
3.1. Pollutant Removal Performance of Fe3+-Augmented Bioreactor
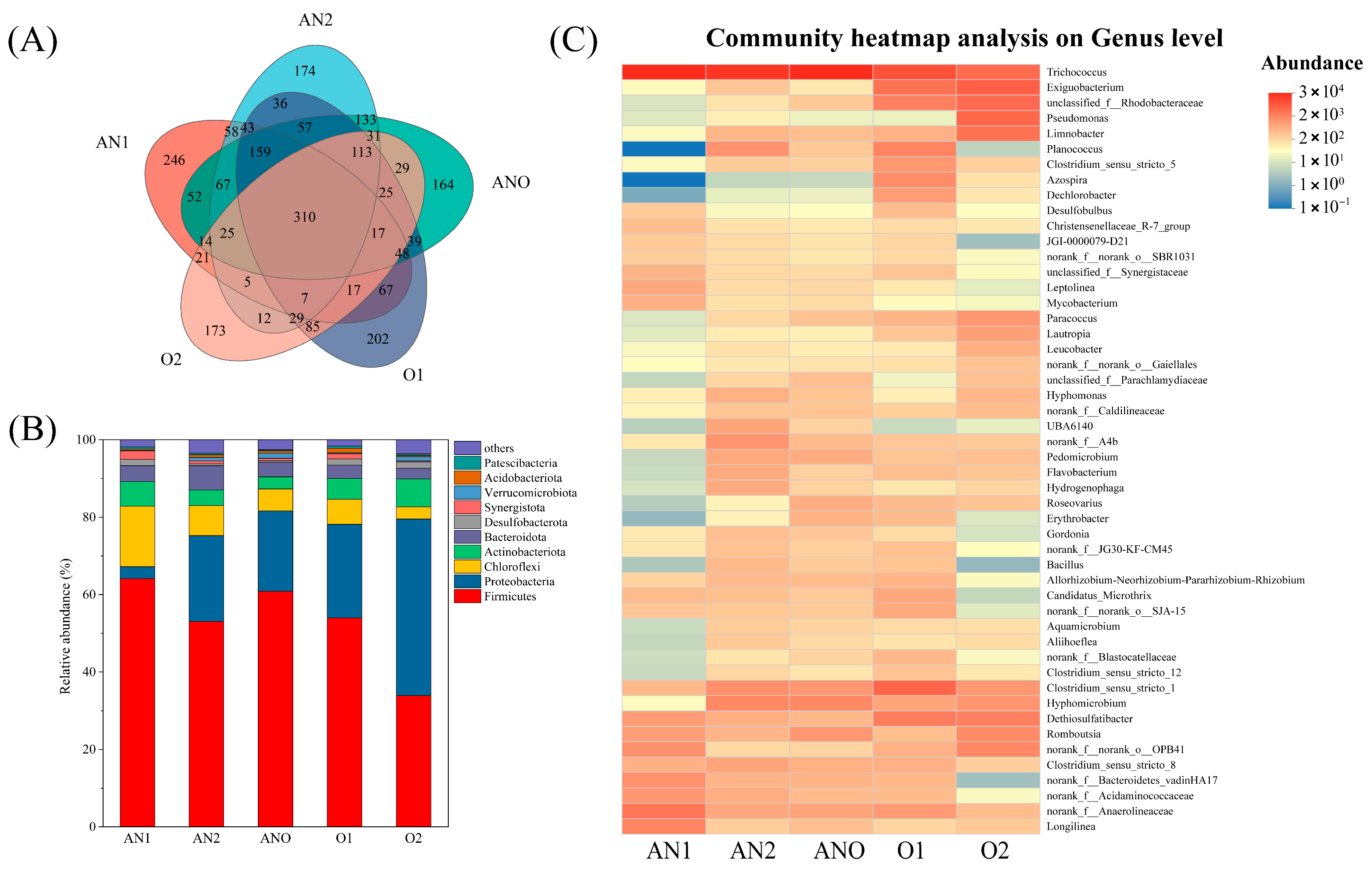
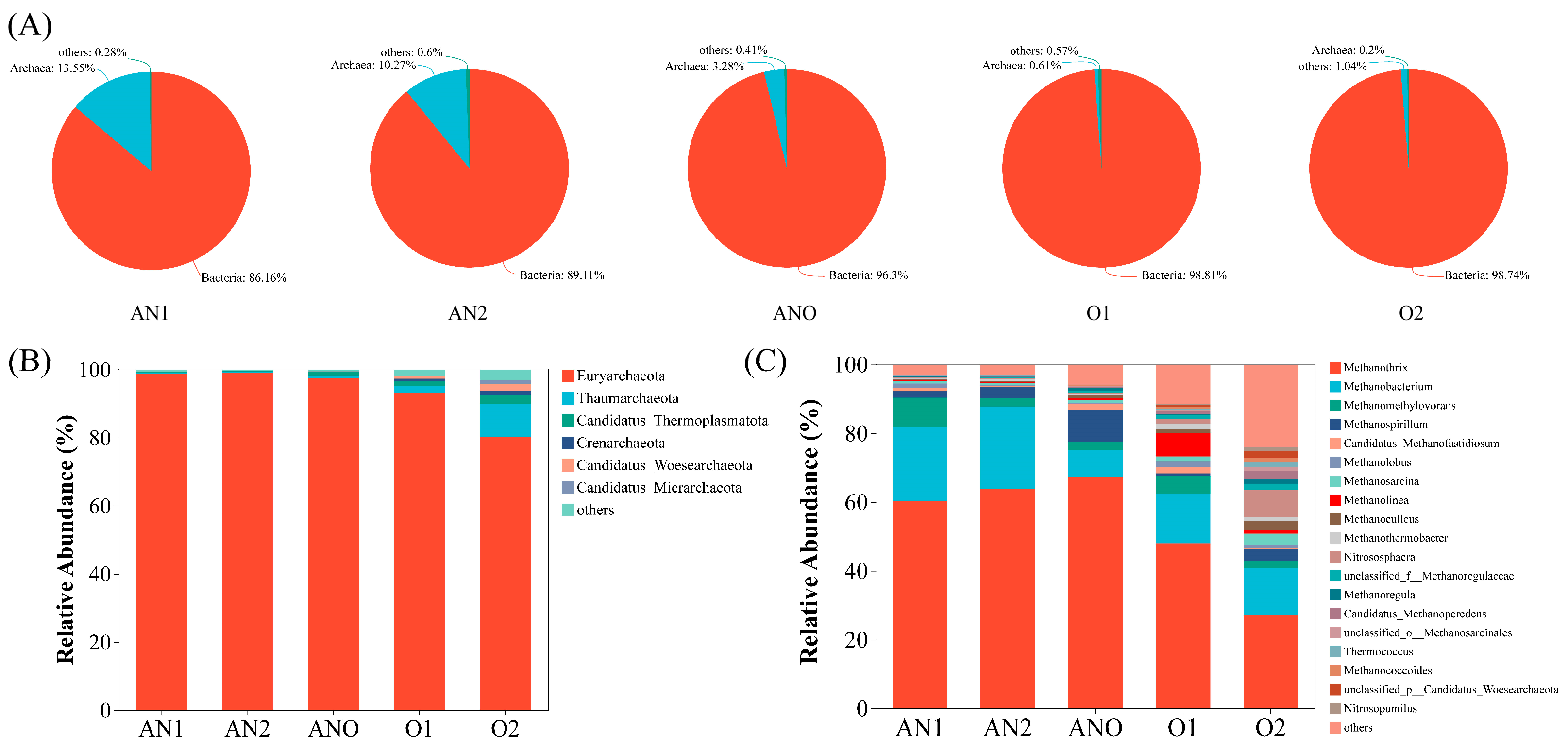
3.2. Microbial Community Structure
3.2.1. Bacterial Community Analysis
3.2.2. Archaeal Community Analysis
3.3. Metabolic Function Analysis
3.4. Redox Transformation Analysis of Fe3+/Fe2+
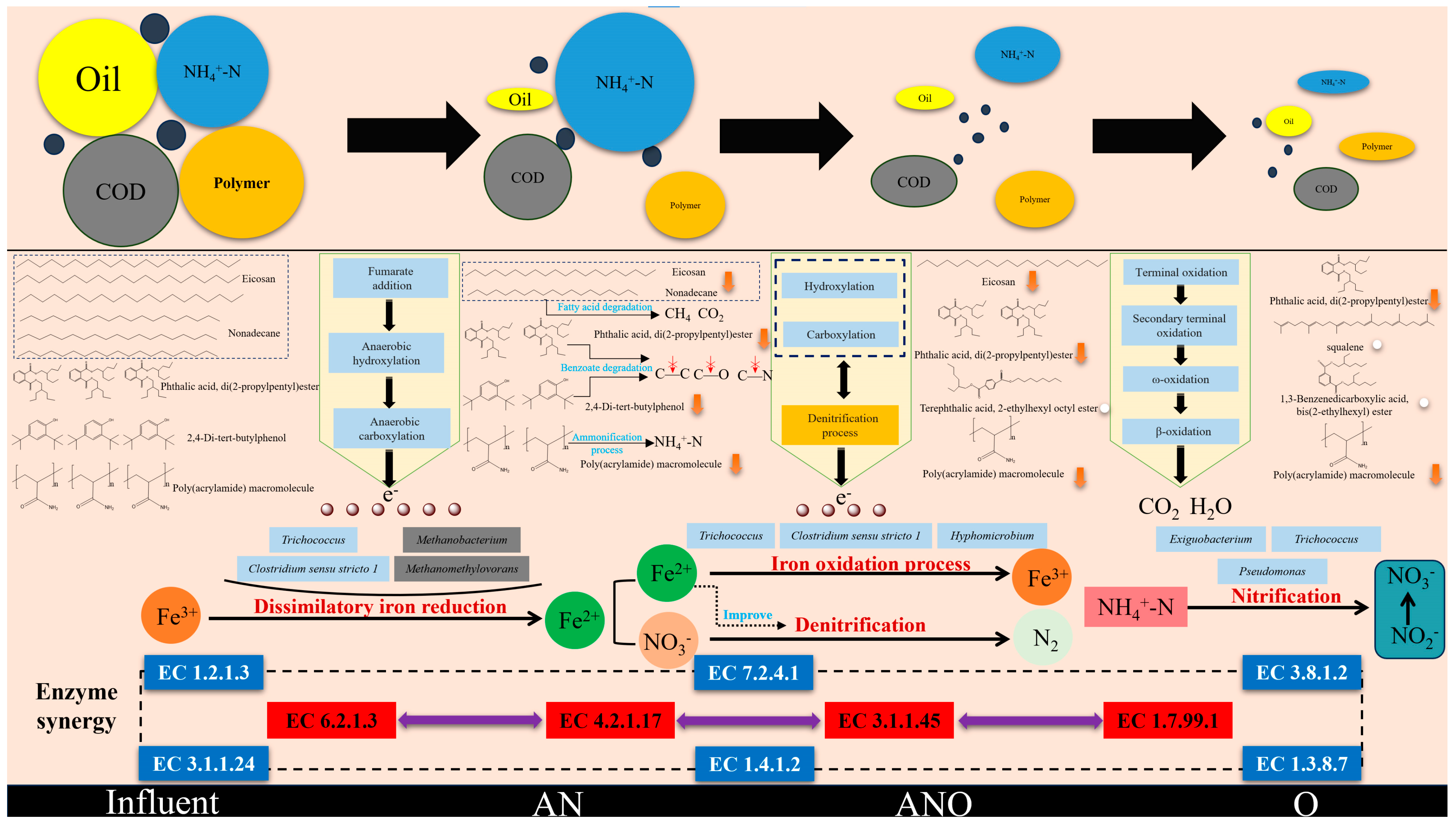
3.5. Mechanistic Insights into Fe3+-Augmented Synergistic Degradation of Organic Pollutants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Han, X.; Li, J.; Liu, R.; Wang, Q.; Huang, C.; Wang, X.; Zhang, L.; Lin, R. Review on Oil Displacement Technologies of Enhanced Oil Recovery: State-of-the-Art and Outlook. Energy Fuels 2023, 37, 2539–2568. [Google Scholar] [CrossRef]
- Cao, H.; Li, Y.; Gao, W.; Cao, J.; Sun, B.; Zhang, J. Experimental investigation on the effect of interfacial properties of chemical flooding for enhanced heavy oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132335. [Google Scholar] [CrossRef]
- Xue, L.; Liu, P.; Zhang, Y. Status and Prospect of Improved Oil Recovery Technology of High Water Cut Reservoirs. Water 2023, 15, 1342. [Google Scholar] [CrossRef]
- Wu, M.; Zhai, M.; Li, X. Adsorptive removal of oil drops from ASP flooding-produced water by polyether polysiloxane-grafted ZIF-8. Powder Technol. 2021, 378, 76–84. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, X.; Gu, Z.; Tong, Y.; Meng, F.; Sun, L.; Liu, H.; Wang, Q. High-efficiency purification of alkali-surfactant-polymer flooding produced water by ultrasonication-ionic liquids combination: Performance and separation mechanism. Sep. Purif. Technol. 2025, 363, 132255. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhong, L.; Yuan, S.; Wang, Q.; Wei, C. Study on the emulsification characteristics of heavy oil during chemical flooding. Phys. Fluids 2023, 35, 53330. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Q.; Yang, X.; Zhang, X.; Chen, X.; Zhao, D. Effects of polymer, surfactant and solid particle on the stability of wastewater produced from surfactant/polymer flooding. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134419. [Google Scholar] [CrossRef]
- Saththasivam, J.; Ogunbiyi, O.; Lawler, J.; Al-Rewaily, R.; Liu, Z. Evaluating dissolved air flotation for oil/water separation using a hybridized coagulant of ferric chloride and chitosan. J. Water Process Eng. 2022, 47, 102836. [Google Scholar] [CrossRef]
- Weschenfelder, S.E.; Fonseca, M.J.C.; Borges, C.P. Treatment of produced water from polymer flooding in oil production by ceramic membranes. J. Pet. Sci. Eng. 2021, 196, 108021. [Google Scholar] [CrossRef]
- Liu, Y.; Bu, F.; Chen, S.; Jiang, M. Investigating effect of polymer concentrations on separation performance of hydrocyclone by sensitivity analysis. Energy Sci. Eng. 2021, 9, 1202–1215. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, C.; Ji, H.; Li, Z. Challenges of petroleum wastewater treatment and development trends of advanced treatment technologies: A review. J. Environ. Chem. Eng. 2024, 12, 113767. [Google Scholar] [CrossRef]
- Mansour, M.S.M.; Abdel-shafy, H.I.; Ibrahim, A.M. Petroleum wastewater: Environmental protection, treatment, and safe reuse: An overview. J. Environ. Manag. 2024, 351, 119827. [Google Scholar] [CrossRef] [PubMed]
- Lusinier, N.; Seyssiecq, I.; Sambusiti, C.; Jacob, M.; Lesage, N.; Roche, N. A comparative study of conventional activated sludge and fixed bed hybrid biological reactor for oilfield produced water treatment: Influence of hydraulic retention time. Chem. Eng. J. 2021, 420, 127611. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, C.; Zhou, S.; Bu, K.; Li, P.; Lin, X.; Jiang, L.; Zhang, C. Performance and microbial community analysis of a bio-contact oxidation reactor during the treatment of low-COD and high-salinity oilfield produced water. Bioresour. Technol. 2021, 335, 125267. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Nie, M.; Diwu, Z.; Wang, L.; Yan, H.; Lin, Y.; Zhang, B.; Wang, Y. Biological treatment of high salinity and low pH produced water in oilfield with immobilized cells of P. aeruginosa NY3 in a pilot-scale. J. Hazard. Mater. 2020, 381, 121232. [Google Scholar] [CrossRef] [PubMed]
- Ghafoori, S.; Omar, M.; Koutahzadeh, N.; Zendehboudi, S.; Malhas, R.N.; Mohamed, M.; Al-Zubaidi, S.; Redha, K.; Baraki, F.; Mehrvar, M. New advancements, challenges, and future needs on treatment of oilfield produced water: A state-of-the-art review. Sep. Purif. Technol. 2022, 289, 120652. [Google Scholar] [CrossRef]
- Peng, Y.; He, S.; Wu, F. Biochemical processes mediated by iron-based materials in water treatement: Enhancing nitrogen and phosphorus removal in low C/N ratio wastewater. Sci. Total Environ. 2021, 775, 145137. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Tang, J.; Zhao, M.; Huang, Z.; Shi, W.; Ruan, W. The enhanced biological methanation performance in trickle-bed reactor by different continuous hydrogen supply from nano-zero valent iron corrosion. J. Water Process Eng. 2022, 50, 103292. [Google Scholar] [CrossRef]
- Li, Y.; Kong, W.; Liu, H.; Hong, Y.; Huang, T. Enhanced degradation of phenolic compounds in coal gasification wastewater by activated carbon-Fe3O4 nanoparticles coupled with anaerobic co-metabolism. Biochem. Eng. J. 2022, 189, 108717. [Google Scholar] [CrossRef]
- Tang, Y.; Dou, J.; Lu, Z.; Xu, J.; He, Y. Accelerating Fe2+/Fe3+ cycle via biochar to improve catalytic degradation efficiency of the Fe3+/persulfate oxidation. Environ. Pollut. 2023, 316, 120669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ran, Y.; Gong, Y.; Hong, C.; Xing, Y.; Sun, Y.; Wang, H.; Ling, W.; Wang, Y.; Feng, W.; et al. Study on the degradation performance of coking wastewater using an in-situ enhanced Fe2+/Fe3+ cycle dual-cathode Electro-Fenton system. J. Environ. Chem. Eng. 2024, 12, 114591. [Google Scholar] [CrossRef]
- Liang, D.; Yu, Z.; Wang, Y.; Zhang, Y.; Wang, R.; Hao, J.; Feng, Y. Differences in the efficiency and mechanisms of different iron-based materials driving synchronous nitrogen and phosphorus removal. Environ. Res. 2025, 268, 120706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yuan, R.; Wang, S.; Chen, H.; Zhou, B.; Cui, Z.; Zhang, C. Iron-based materials for nitrogen and phosphorus removal from wastewater: A review. J. Water Process Eng. 2024, 59, 104952. [Google Scholar] [CrossRef]
- Li, X.; Yuan, Y.; Huang, Y. Enhancing the nitrogen removal efficiency of a new autotrophic biological nitrogen-removal process based on the iron cycle: Feasibility, progress, and existing problems. J. Clean. Prod. 2021, 317, 128499. [Google Scholar] [CrossRef]
- Lu, X.; Zhong, Z.; Yan, R.; Zan, F.; Lou, W.; Liu, J.; Wu, X.; Zhang, B. La–Fe magnetic bentonite stimulated denitrifying phosphorus removal from low C/N wastewater in the A2/O process: Performance, microbial community, and potential mechanism. J. Clean. Prod. 2022, 373, 133746. [Google Scholar] [CrossRef]
- Wan, L.; Liu, H.; Wang, X. Anaerobic ammonium oxidation coupled to Fe(III) reduction: Discovery, mechanism and application prospects in wastewater treatment. Sci. Total Environ. 2022, 818, 151687. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.-T.; Lai, W.-X.; Zhang, Y.-B.; Liu, Y.-W. Feammox process driven anaerobic ammonium removal of wastewater treatment under supplementing Fe(III) compounds. Sci. Total Environ. 2022, 804, 149965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, M.; You, S.; Ma, D.; Zhao, J.; Chen, Z. Effect of Fe3+ on the sludge properties and microbial community structure in a lab-scale A2O process. Sci. Total Environ. 2021, 780, 146505. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, B.; Li, J.; Zhang, X. Fe3+ addition for enhancing the formation and stability of aerobic granular sludge to treat low-strength wastewater. J. Water Process Eng. 2025, 71, 107144. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2018.
- Li, C.; Li, J.; Wang, N.; Zhao, Q.; Wang, P. Status of the treatment of produced water containing polymer in oilfields: A review. J. Environ. Chem. Eng. 2021, 9, 105303. [Google Scholar] [CrossRef]
- Wang, N.; Sun, X.; Zhao, Q.; Wang, P. Treatment of polymer-flooding wastewater by a modified coal fly ash-catalysed Fenton-like process with microwave pre-enhancement: System parameters, kinetics, and proposed mechanism. Chem. Eng. J. 2021, 406, 126734. [Google Scholar] [CrossRef]
- Pan, K.; Guo, T.; Liao, H.; Huang, Z.; Qian, Z.; Li, F.; Li, J. Adding iron shavings in activated sludge system to enhance removal of refractory organics and nitrogen for textile-dyeing wastewater. J. Environ. Chem. Eng. 2023, 11, 110999. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, D.; Lu, Q.; Zhao, Q.; Ma, J.; Liu, G.; Ouyang, J.; Luo, E.; Li, C.; Wei, L. A study on the efficiency and microbial community of anaerobic/anaerobic/anoxic/oxic membrane bioreactor for treating saline alkali-surfactant-polymer flooding wastewater. J. Water Process Eng. 2025, 71, 107305. [Google Scholar] [CrossRef]
- Qian, G.; Liu, P.; Wei, L.; Mackey, H.; Hao, T. Can a compact biological system be used for real hydraulic fracturing wastewater treatment? Sci. Total Environ. 2022, 816, 151524. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Xiang, Y.; Cooper, P.; Cassol, G.S.; Luo, Y.; Zeng, Q.; Shang, C.; Ren, Z.J.; Chen, G. Evaluating UV254 absorbance reductions in landfill leachate for municipal sewage co-treatment through timed UV/electrooxidation. J. Hazard. Mater. 2023, 445, 130624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, J.; Zhang, S.; Yoza, B.A.; Li, Q.X.; Zhan, Y.; Ye, H.; Zhao, P.; Chen, C. Characteristics of bacterial populations in an industrial scale petrochemical wastewater treatment plant: Composition, function and their association with environmental factors. Environ. Res. 2020, 189, 109939. [Google Scholar] [CrossRef] [PubMed]
- Parab, A.S.; Ghose, M.; Manohar, C.S.; Gauns, M.U.; Paul, S. Metagenomic insights into bacterial dynamics and niche partitioning in response to varying oxygen gradients in the Arabian Sea oxygen minimum zone (OMZ). Reg. Stud. Mar. Sci. 2024, 78, 103768. [Google Scholar] [CrossRef]
- Li, Z.; Feng, Q.; Zhang, F.; Zhao, F.; Lu, M.; Qin, F.; Guo, R. Simultaneous denitrification enhancement and sludge reduction based on novel suspended carrier modified using activated carbon and magnetite at low carbon/nitrogen ratio. Bioresour. Technol. 2024, 395, 130360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, G. Leveraging anaerobic biodegradation of tetracycline in anaerobic digestion systems with different operational modes. Environ. Technol. Innov. 2023, 32, 103373. [Google Scholar] [CrossRef]
- Song, T.; Li, S.; Ding, W.; Li, H.; Bao, M.; Li, Y. Biodegradation of hydrolyzed polyacrylamide by the combined expanded granular sludge bed reactor-aerobic biofilm reactor biosystem and key microorganisms involved in this bioprocess. Bioresour. Technol. 2018, 263, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Thontowi, A.; Yetti, E.; Yopi, Y. Medium Chain and Long Chain Alkanes Hydroxylase Producing Whole Cell Biocatalyst From Marine Bacteria. Ann. Bogor. 2018, 22, 12–19. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Liu, M.; Sun, H.; Bao, M. Study on the biodegradation of crude oil by free and immobilized bacterial consortium in marine environment. PLoS ONE 2017, 12, e0174445. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, Y.Y.; Wang, C.; Ma, Z.; Liu, X.; Li, S. Biodegradation of n-alkanes in crude oil by three identified bacterial strains. Fuel 2020, 275, 117897. [Google Scholar] [CrossRef]
- Dai, H.; Sun, Y.; Wan, D.; Abbasi, H.N.; Guo, Z.; Geng, H.; Wang, X.; Chen, Y. Simultaneous denitrification and phosphorus removal: A review on the functional strains and activated sludge processes. Sci. Total Environ. 2022, 835, 155409. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Hu, H.-Y.; Shi, Z.-J.; Yang, C.-C.; Li, P.; Huang, M.; Ni, W.-M.; Shi, M.-L.; Jin, R.-C. Towards simultaneously removing nitrogen and sulfur by a novel process: Anammox and autotrophic desulfurization–denitrification (AADD). Chem. Eng. J. 2016, 297, 207–216. [Google Scholar] [CrossRef]
- Ding, B.; Li, Z.; Qin, Y. Nitrogen loss from anaerobic ammonium oxidation coupled to Iron(III) reduction in a riparian zone. Environ. Pollut. 2017, 231, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Y.; Zhang, M.; Xiong, P.; Liu, L.; Bao, Y.; Zhao, Z. Link between characteristics of Fe(III) oxides and critical role in enhancing anaerobic methanogenic degradation of complex organic compounds. Environ. Res. 2021, 194, 110498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, C.; Wang, L.; Wang, M.; Zhang, D.; Tang, G. Exploring the promoting effect of nitrilotriacetic acid on hydroxyl radical and humification during magnetite-amended composting of sewage sludge. Bioresour. Technol. 2024, 403, 130863. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, R.; Cui, Y.; Zhang, Z.; Jing, H.; Chen, Z.; Tian, S.; Yang, Z.; Liu, Y. Effects of Fe3O4 on production of even-carbon volatile fatty acids by anaerobic fermentation of kitchen waste under ultrasonic-alkali pretreatment: Performance, metabolic functions, and metabolic pathways. J. Environ. Chem. Eng. 2025, 13, 116615. [Google Scholar] [CrossRef]
- Hartman Wyatt, H.; Bueno de Mesquita Clifton, P.; Theroux Susanna, M.; Morgan-Lang, C.; Baldocchi Dennis, D.; Tringe Susannah, G. Multiple microbial guilds mediate soil methane cycling along a wetland salinity gradient. mSystems 2024, 9, e00936-23. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, Q.; Feng, J.; Yang, Z.; Yu, C.; Zhang, J.; Ling, J.; Dong, J. Introduction of exotic species Sonneratia apetala alters diazotrophic community and stimulates nitrogen fixation in mangrove sediments. Ecol. Indic. 2022, 142, 109179. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Chen, H. Evaluating optimized volatile fatty acids production from carbon-rich wastewater during hydrolysis acidification process by Fe(Ⅱ) and Fe(Ⅲ) addition. J. Environ. Chem. Eng. 2023, 11, 110724. [Google Scholar] [CrossRef]
- Zhang, E.; Wu, S.; Liu, J.; Li, H.; Liu, X.; Lu, Y.; Ge, C.; Zhou, D. Activated carbon as a strong DOM adsorbent mitigates antimony and arsenic release in flooded mining-impacted soils. J. Hazard. Mater. 2024, 473, 134663. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Sun, H.; Gao, B.; Dang, J.; Zhang, M.; Li, M.; Dong, J.; Wu, H.; Zhang, J.; Guo, Z. Enhanced reduction of Cr(VI) in iron-carbon micro-electrolysis constructed wetlands: Mechanisms of iron cycle and microbial interactions. Chem. Eng. J. 2022, 439, 135742. [Google Scholar] [CrossRef]
- Luan, Y.-N.; Yin, Y.; Guo, Z.; Wang, Q.; Xu, Y.; Zhang, F.; Xiao, Y.; Liu, C. Partial nitrification-denitrification and enrichment of paracoccus induced by iron-chitosan beads addition in an intermittently-aerated activated sludge system. J. Environ. Manag. 2024, 353, 120189. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Gao, X.; Su, L.; Lei, Y.; Li, T.; Dong, X.; Li, X.; Yan, Z. Unveiling the impact of flooding and salinity on iron oxides-mediated binding of organic carbon in the rhizosphere of Scirpus mariqueter. Sci. Total Environ. 2024, 908, 168447. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Garcia, I.N.; Belgini, D.R.B.; Torres-Ballesteros, A.; Paez-Espino, D.; Capilla, R.; Santos Neto, E.V.; Gray, N.; de Oliveira, V.M. In depth metagenomic analysis in contrasting oil wells reveals syntrophic bacterial and archaeal associations for oil biodegradation in petroleum reservoirs. Sci. Total Environ. 2020, 715, 136646. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lin, W.-H.; Yu, Y.-L.; Dong, C.-D.; Zhang, H.; Hu, Z.; Kao, C.-M. Transitioning weathered oil fields towards new energy: A review on utilizing hydrogenotrophic methanogens for petroleum hydrocarbons remediation. J. Hazard. Mater. 2024, 477, 135279. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Altamirano, M.J.; Maza-Márquez, P.; Montemurro, N.; Rodelas, B.; Osorio, F.; Pozo, C. Linking microbial diversity and population dynamics to the removal efficiency of pharmaceutically active compounds (PhACs) in an anaerobic/anoxic/aerobic (A2O) system. Chemosphere 2019, 233, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Han, Y.; Zeng, Y.; Wang, T.; Wang, Z.; Wu, Y.; Li, N.; Lobo, F.L.; Wang, X. Understanding the microbial processes on carbon brushes that accelerate methanogenesis of long-chain fatty acids in anaerobic digestion. Water Res. 2025, 273, 123084. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lu, X. Start-up performance and granular sludge features of an improved external circulating anaerobic reactor for algae-laden water treatment. Saudi J. Biol. Sci. 2017, 24, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sar, P.; Sarkar, J.; Dutta, A.; Sarkar, P.; Gupta, A.; Mohapatra, B.; Pal, S.; Kazy, S.K. Petroleum hydrocarbon rich oil refinery sludge of North-East India harbours anaerobic, fermentative, sulfate-reducing, syntrophic and methanogenic microbial populations. BMC Microbiol. 2018, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Cabral, L.; Giovanella, P.; Pellizzer, E.P.; Teramoto, E.H.; Kiang, C.H.; Sette, L.D. Microbial communities in petroleum-contaminated sites: Structure and metabolisms. Chemosphere 2022, 286, 131752. [Google Scholar] [CrossRef] [PubMed]
- Aubé, J.; Senin, P.; Bonin, P.; Pringault, O.; Jeziorski, C.; Bouchez, O.; Klopp, C.; Guyoneaud, R.; Goñi-Urriza, M. Meta-omics Provides Insights into the Impact of Hydrocarbon Contamination on Microbial Mat Functioning. Microb. Ecol. 2020, 80, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Xu, T.; Wang, Z.; Wen, X.; Jiao, Z.; Liu, J. Metagenomic analysis of petroleum biodegradation coupled to specific N-cycling process in oil-contaminated soil. Appl. Soil Ecol. 2024, 193, 105144. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Liu, Z.; Ou, Y.; Duan, X.; Wu, M. Sensitive indicator microorganisms and C,N-cycle processes in soil with different petroleum hydrocarbon pollution levels. Biochem. Eng. J. 2025, 220, 109752. [Google Scholar] [CrossRef]
- Ou, Y.; Wu, M.; Yu, Y.; Liu, Z.; Kang, H.; Hu, M.; Zhang, C.; Chen, X. Influence mechanisms underlying the degradation of petroleum hydrocarbons in response to various nitrogen dosages supplementation through metatranscriptomics analysis. J. Hazard. Mater. 2025, 487, 137074. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Hao, D.; Xuji, Z.; Tom, G.; Zuotao, Z.; Nan, Z.; Wang, H. Dissimilatory iron-reducing microorganisms: The phylogeny, physiology, applications and outlook. Crit. Rev. Environ. Sci. Technol. 2025, 55, 73–98. [Google Scholar] [CrossRef]
- Scherr, K.E.; Lundaa, T.; Klose, V.; Bochmann, G.; Loibner, A.P. Changes in bacterial communities from anaerobic digesters during petroleum hydrocarbon degradation. J. Biotechnol. 2012, 157, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Toth, C.R.A.; Gieg, L.M. Time Course-Dependent Methanogenic Crude Oil Biodegradation: Dynamics of Fumarate Addition Metabolites, Biodegradative Genes, and Microbial Community Composition. Front. Microbiol. 2018, 8, 2610. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Value |
|---|---|
| COD (mg/L) | 492.69 ± 192.03 |
| Oil content (mg/L) | 129.63 ± 92.57 |
| SS content (mg/L) | 144.62 ± 44.29 |
| Polymer concentration (mg/L) | 42.51 ± 13.24 |
| NH4+-N (mg/L) | 15.17 ± 3.30 |
| Viscosity (mPa∙s) | 0.70 ± 0.03 |
| pH | 7.56 ± 0.05 |
| Sample | Ace | Chao | Coverage | Shannon | Simpson | Sobs |
|---|---|---|---|---|---|---|
| AN1 | 1416 | 1412 | 0.9939 | 3.51 | 0.17 | 1156 |
| AN2 | 1516 | 1506 | 0.9941 | 4.16 | 0.12 | 1259 |
| ANO | 1607 | 1615 | 0.9930 | 3.59 | 0.21 | 1283 |
| O1 | 1555 | 1557 | 0.9933 | 4.38 | 0.05 | 1254 |
| O2 | 1091 | 1076 | 0.9958 | 4.24 | 0.04 | 913 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Chen, C.; Chen, Z.; Shan, H.; Liang, J. Enhanced Oilfield-Produced-Water Treatment Using Fe3+-Augmented Composite Bioreactor: Performance and Microbial Community Dynamics. Bioengineering 2025, 12, 784. https://doi.org/10.3390/bioengineering12070784
Zhao Q, Chen C, Chen Z, Shan H, Liang J. Enhanced Oilfield-Produced-Water Treatment Using Fe3+-Augmented Composite Bioreactor: Performance and Microbial Community Dynamics. Bioengineering. 2025; 12(7):784. https://doi.org/10.3390/bioengineering12070784
Chicago/Turabian StyleZhao, Qiushi, Chunmao Chen, Zhongxi Chen, Hongman Shan, and Jiahao Liang. 2025. "Enhanced Oilfield-Produced-Water Treatment Using Fe3+-Augmented Composite Bioreactor: Performance and Microbial Community Dynamics" Bioengineering 12, no. 7: 784. https://doi.org/10.3390/bioengineering12070784
APA StyleZhao, Q., Chen, C., Chen, Z., Shan, H., & Liang, J. (2025). Enhanced Oilfield-Produced-Water Treatment Using Fe3+-Augmented Composite Bioreactor: Performance and Microbial Community Dynamics. Bioengineering, 12(7), 784. https://doi.org/10.3390/bioengineering12070784






