1. Introduction
Pharmacological treatments are associated with a broad spectrum of clinical risks, including medication errors, adverse drug events, adverse drug reactions (ADRs), and drug–drug interactions (DDIs), which are collectively referred to as drug-related problems. The lack of universally accepted terminology and consistent definitions for these categories often hinders comparability between studies and impedes the calculation of incidence rates [
1].
DDIs constitute one of the most pressing and resource-intensive challenges within healthcare systems, being implicated in a significant proportion of ADRs, with estimates indicating that they account for approximately 6% to 30% of all ADRs. Furthermore, DDIs are responsible for nearly 2.8% of hospital admissions associated with ADRs [
2,
3]. The likelihood of potential drug–drug interactions (pDDIs) rises substantially in settings characterized by polypharmacy, a condition typically described as the simultaneous daily administration of five or more medications to an individual [
4].
The probability of pDDIs increases substantially with the number of medications administered concurrently, rising from an estimated 6% when two drugs are used to approximately 50% with five medications and nearly 100% when eight drugs are taken simultaneously [
2]. Across various geographical regions, the reported prevalence rates of pDDIs exhibit considerable variability. In the United States, these rates have been observed to fall between 7.7% and 30.2%, whereas in European cohorts, estimates span from 0.8% up to 54.3%. In contrast, data from Australia indicate that roughly 1.5% of the elderly population may be at risk. Such discrepancies are likely influenced by heterogeneity in the population characteristics, including disease prevalence and pharmacological load, as well as methodological differences such as sample composition and study design [
5].
Approximately six decades ago, the medical community, prompted by reports of life-threatening hypertensive episodes in individuals receiving monoamine oxidase inhibitors for depressive disorders following the ingestion of specific types of cheese, first began to acknowledge the clinical consequences of drug interactions. These adverse events were subsequently linked to the presence of high concentrations of tyramine, a pressor amine, whose intestinal degradation was markedly reduced due to monoamine oxidase inhibition. Around the same period, a separate pharmacokinetic interaction was described involving the concomitant use of the sulfonamide antibiotic sulphaphenazole and the antidiabetic agent tolbutamide, which resulted in hypoglycemic episodes. The underlying mechanism was attributed to a potential inhibition of tolbutamide metabolism by sulphaphenazole [
6].
Drug interactions are generally categorized based on their underlying mechanisms into pharmaceutical, pharmacokinetic, and pharmacodynamic types. Pharmaceutical interactions, often referred to as pharmaceutical incompatibilities, occur when the precipitating substance influences the object drug prior to its administration [
7]. Pharmacokinetic drug interactions commonly evaluate how one drug (i.e., the precipitant drug) alters the ADME processes of another by comparing these processes in the presence and absence of the interacting agent. In contrast, pharmacodynamic interactions are typically categorized as synergistic, additive, or antagonistic, depending on the observed modifications in the pharmacological effects of the drugs involved [
8].
Patients diagnosed with cardiovascular diseases, where therapeutic regimens often involve multiple pharmacological agents, represent the group most frequently affected by clinically significant pDDIs [
9]. Additionally, infectious diseases pose a high risk for drug interactions due to the susceptibility of antibiotics to such effects [
3], particularly because of the frequent use of proton pump inhibitors [
10,
11]. A notable distinction exists between pDDIs and DDIs that are clinically significant. Therefore, cautious clinical judgment is essential prior to modifying treatment regimens based solely on pDDIs flagged by various software systems [
12]. Recognizing the most clinically impactful DDIs within primary-care settings is critical for ensuring patient safety. Strategies to minimize the risk of DDIs include limiting the quantity of prescribed drugs, conducting frequent therapy evaluations, considering non-drug treatment options, closely observing signs of adverse effects or therapeutic outcomes, adjusting dosages when needed, and tailoring the timing of drug administration [
13].
A wide range of scientific information resources support the dissemination and retrieval of drug-related knowledge across the biomedical research community. Among these, PubMed [
14] and PubMed Central [
15], maintained by the U.S. National Library of Medicine, offer open and indexed access to millions of peer-reviewed articles, including many free full-text manuscripts relevant to DDIs. Scopus [
16], Web of Science [
17], and Embase [
18] further provide comprehensive indexing and citation data critical for bibliometric analyses and evidence synthesis. Full-text platforms such as ScienceDirect [
19], ClinicalKey [
20], and Wiley Online Library [
21] further enable advanced literature navigation across medical and pharmaceutical domains, including the domain of DDIs. In parallel, authoritative regulatory databases hosted by agencies like the U.S. Food and Drug Administration [
22] and the European Medicines Agency [
23] offer structured drug interaction guidelines, labeling information, and pharmacovigilance data. These curated resources complement computational and AI-based tools by contextualizing their pharmacological mechanisms and clinical relevance. Their integration into both academic research and clinical workflows fosters informed decision-making and facilitates translational approaches to complex medication regimens. Moreover, several available online platforms are specifically designed to check for drug interactions, including Drugs.com
®, Lexicomp
®, Medscape
® [
24], DDInter
® [
25], WebMD, and DrugBank [
3], with each providing advisory information intended to support clinical decision-making.
Certain drug interactions can be beneficial by improving their therapeutic effects through specific mechanisms. Synergistic pairings, such as ampicillin with gentamicin for enterococcal endocarditis, produce bactericidal effects unattainable by either drug alone, while tuberculosis treatment combining rifampin and isoniazid limits resistance development. Enhancement of drug levels occurs when ritonavir inhibits CYP3A4, thereby boosting the efficacy of darunavir [
26] or lopinavir [
7]. Beta-lactamase inhibitors like clavulanic acid aid amoxicillin by neutralizing bacterial resistance. Some combinations reduce toxicity; for instance, aluminum–magnesium hydroxide in antacids balances adverse reactions. Antidotal use of folic acid counters methotrexate toxicity. Combinations like carbidopa–levodopa provide dual benefits by increasing efficacy and lowering side effects, as carbidopa prevents peripheral dopamine conversion, minimizing systemic toxicity while preserving its central action [
7].
The rapid growth in studies of drug interactions has occurred despite the fact that research has focused almost exclusively on combinations of two drugs, whereas in real-world prescribing scenarios, patients are often treated with ten or more drugs concomitantly. This expanding body of literature highlights the need for systematic bibliometric analyses to track scientific trends, identify influential publications, and evaluate key bibliometric parameters such as publication volume, citation impact, and collaborative networks. Such analyses provide valuable insights into methodological evolution and thematic trends, thereby serving as a critical tool for advancing scientific knowledge and assessing the state of research across pre-research, basic, and applied stages. However, bibliometric data on DDIs remain scarce and fragmented, with limited attempts to systematically map the intellectual, thematic, and collaborative evolution of this critical research field. Given the escalating global burden of polypharmacy, the scarcity of comprehensive bibliometric assessments represents a research gap.
Our research aims to provide a high-resolution, multidimensional mapping of global DDI research through a comprehensive bibliometric and network analysis, with a focus on identifying structural shifts, knowledge clusters, and the most relevant bibliometric parameters. The contribution lies not only in the scale of the dataset and the limited bibliometric approaches in this field but also in the integration of artificial intelligence (AI)-driven terminology normalization, enabling high accuracy in thematic classification.
2. Materials and Methods
The present bibliometric assessment utilized the Web of Science Core Collection as the primary data repository for comprehensive literature retrieval. The selection of this database was strategically motivated by several methodological considerations to ensure robust analytical outcomes. The Web of Science Core Collection provides extensive multidisciplinary coverage spanning the pharmaceutical sciences, clinical medicine, pharmacology, and toxicology domains essential for DDI research. The database maintains high-quality bibliographic records with comprehensive metadata, including complete author affiliations, citation networks, and keyword indexing systems that facilitate advanced bibliometric mapping techniques. Furthermore, the database’s rigorous peer-review standards and established indexing protocols ensure the inclusion of high-impact, methodologically sound research publications. The consistency of data formatting and standardized field structures within the Web of Science Core Collection minimizes technical complications during data processing and enhances the reliability of cross-reference analysis. By utilizing a single, comprehensive database, methodological complexities associated with multi-database integration were circumvented, thereby strengthening the reproducibility and validity of the analytical framework [
27].
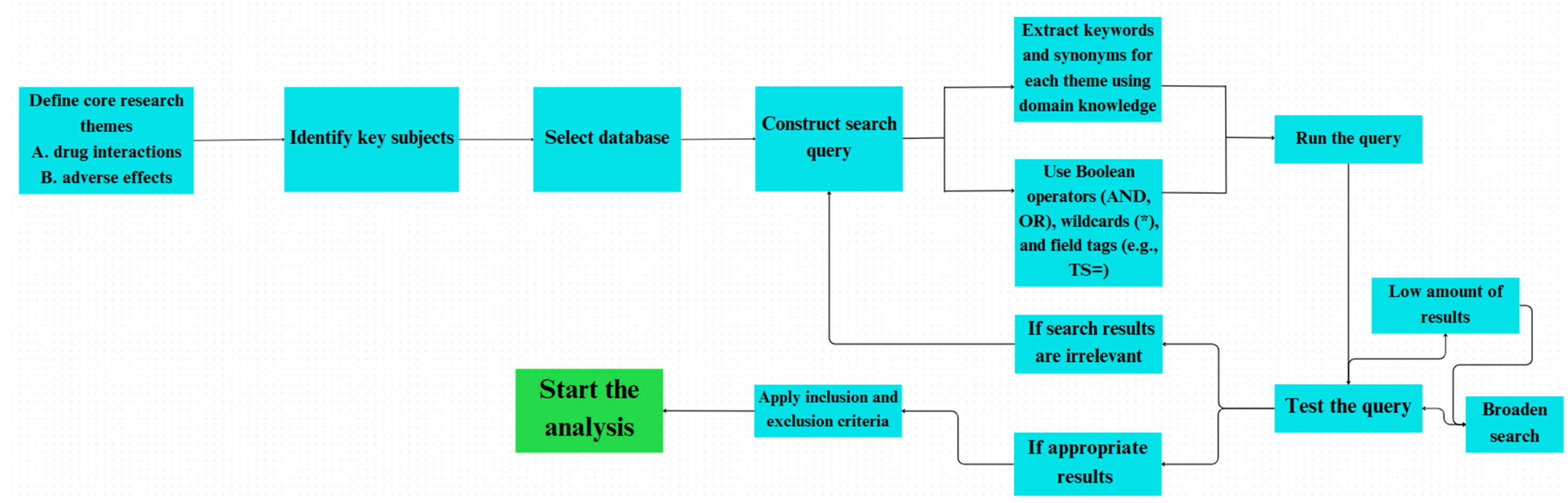
An extensive search strategy was developed to thoroughly explore the literature on DDIs while ensuring high precision in the results. This approach was based on two main concepts: terminology related to interactions and terms describing clinical outcomes. By combining these elements, the search effectively captured both the mechanistic aspects and the practical implications of DDI research. The primary search query is displayed descriptively in
Figure 1 [
28].
To enhance specificity and minimize false positives, the Boolean operator “AND” was used to ensure that retrieved documents addressed both drug interaction mechanisms and their clinical relevance. Within each conceptual group, the “OR” operator broadened the search by including synonyms and alternative expressions frequently used in various medical disciplines and research settings. Truncation symbols (e.g., *) were strategically used to account for different word forms, such as singular, plural, and related derivatives of core terms. This method increased the sensitivity of the search by retrieving a wider range of relevant records while still aligning with the central concepts of the research area.
The initial search execution yielded 20,710 documents spanning the complete temporal range available in the Web of Science database. To optimize the dataset quality and analytical consistency, a systematic filtering protocol was implemented according to predetermined inclusion criteria (
Figure 2).
The inclusion criteria encompassed exclusively articles and reviews published in the English language, ensuring linguistic consistency, essential for subsequent keyword analysis and thematic interpretation. Application of these criteria resulted in a refined dataset of 19,151 documents, representing a 7.5% reduction, which significantly enhanced the data quality while preserving comprehensive coverage of the research domain. This filtering approach ensured linguistic homogeneity, essential for accurate keyword co-occurrence analysis, and eliminated publication types with limited citation potential that could skew bibliometric indicators.
A comprehensive visualization assessment of collaborative networks and the thematic evolution was facilitated through the implementation of a bibliometric analysis that employed multiple integrated platforms utilizing diverse, mutually reinforcing software applications. Network visualizations were primarily generated using VOSviewer (version 1.6.20) [
29], which served as the principal instrument for constructing bibliometric maps that depicted keyword co-occurrence structures and research collaboration patterns at the country level. The R statistical environment’s Biblioshiny web interface [
30] provided access to the Bibliometrix package (version 5.0.0) [
31], which was utilized to conduct trend analysis in the field of DDIs. Data preprocessing, descriptive statistics generation, and the creation of supplementary visualizations were accomplished utilizing Microsoft Excel (Microsoft Office Professional Plus 2019), thereby improving the interpretability and clarity of intricate bibliometric results.
The significant discrepancy technically observed between China’s publication counts from VOSviewer) and Bibliometrix reflects different counting methodologies employed by these bibliometric tools. VOSviewer uses fractional counting, dividing credit for international collaborations among participating countries, while Bibliometrix applies full counting, crediting each country fully for collaborative publications. This difference is particularly significant for China, given its substantial increase in international research partnerships since 2015. Fractional counting provides a more conservative estimate of unique national contributions, while full counting captures the complete scope of research participation.
Annual publication trends across leading countries were visualized using Python 3.12.3 libraries, including Pandas version 2.3.1 [
32], Matplotlib version 3.10.3 [
33], Seaborn version 0.13.2 [
34], and NumPy version 2.3.1 [
35]. Cumulative publication data were converted to annual increments and organized in country-by-year matrices. Heat maps were created using a yellow–orange–red colormap to represent publication intensity, with grid lines for clarity and 5-year-interval labeling on the
x-axis. This approach facilitated identification of temporal research patterns and national productivity trends across the 51-year study period.
Total link strength (TLS) is a VOSviewer metric representing the cumulative strength of a node’s connections with all other nodes in the network. It was employed to quantify collaborative relationships and keyword associations.
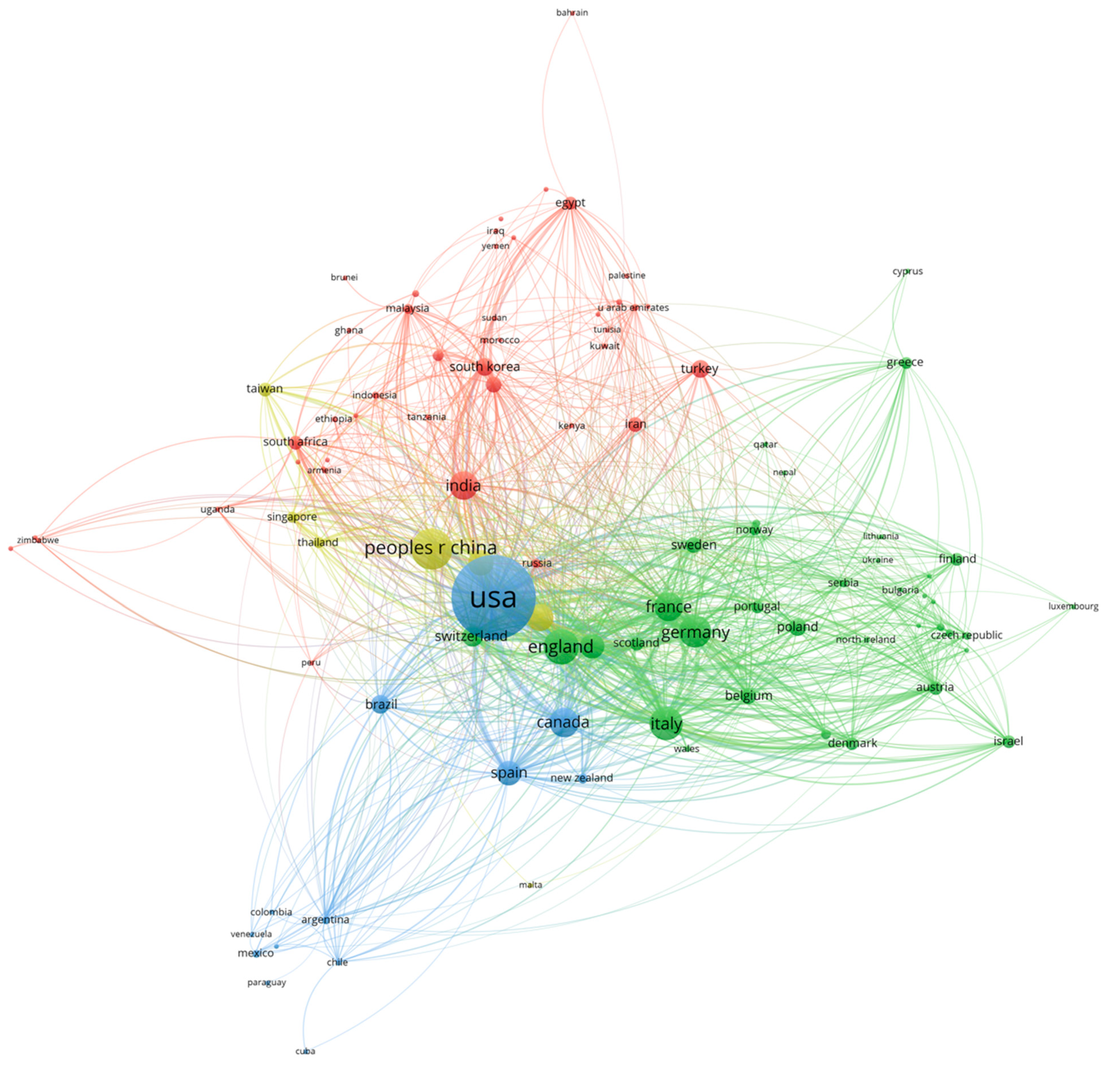
The analysis of country collaboration networks incorporated nations contributing a minimum of 5 publications to establish meaningful collaboration patterns while excluding countries with an insufficient publication volume for reliable network positioning. Node dimensions in the network visualization reflected total publication counts, with larger nodes representing nations demonstrating greater research productivity. The strength of collaborative relationships between countries was indicated through the line thickness, which served as a visual representation of both the frequency and intensity of international research partnerships. Data normalization was achieved through manually identifying different spellings of the same country (i.e., Turkiye and Turkey).
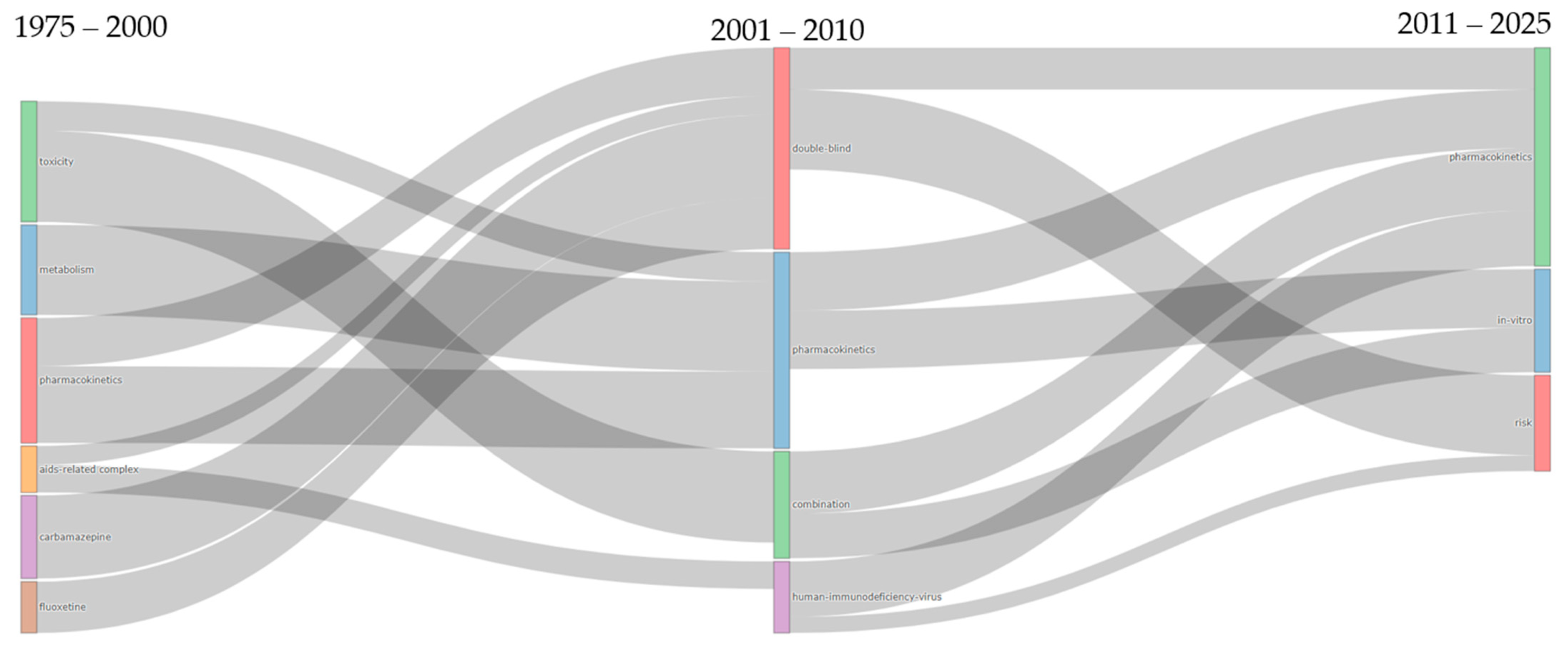
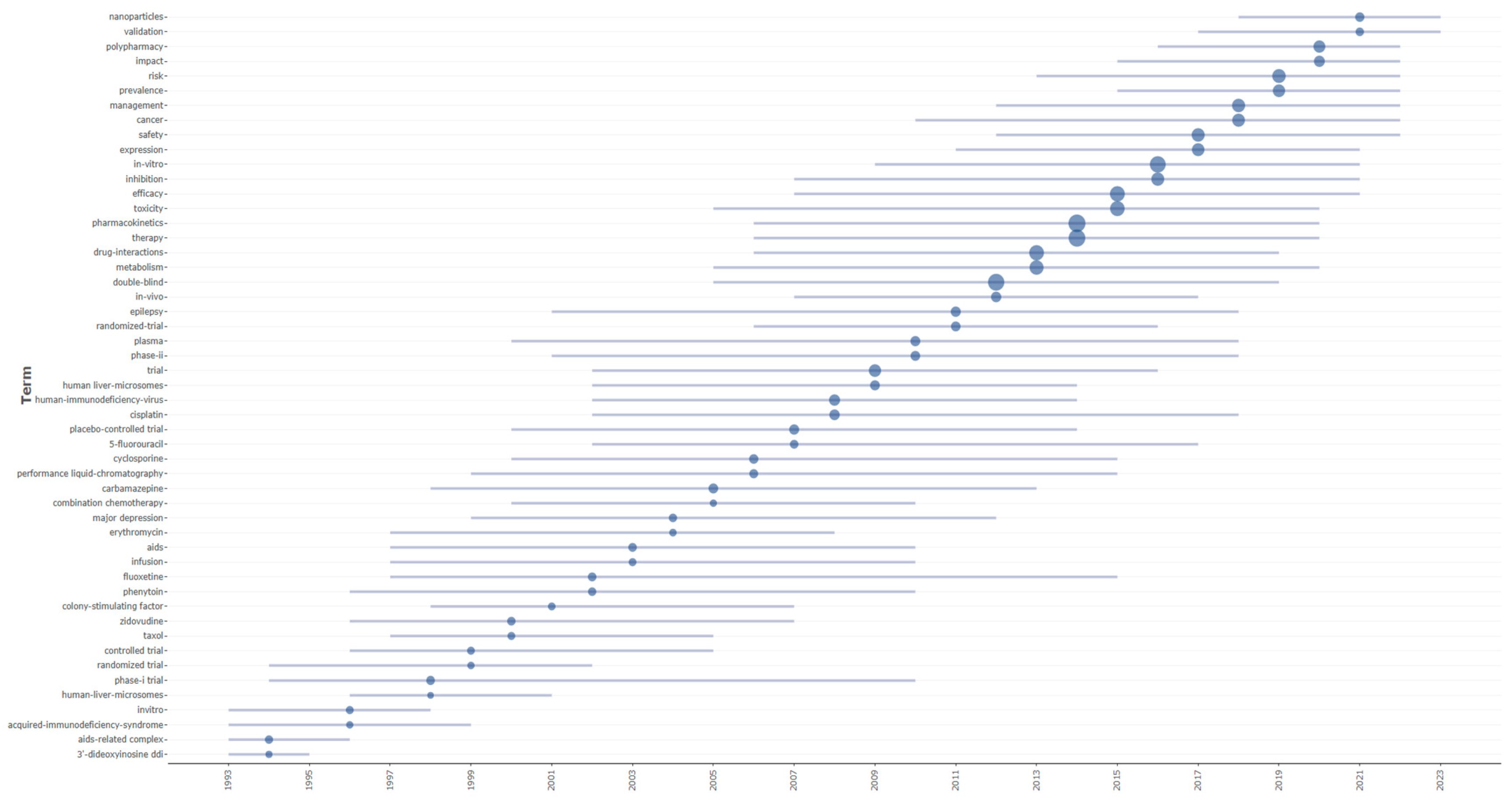
The thematic evolution and temporal distribution analyses were conducted using the Bibliometrix package (version 5.0.0) through its specialized functions for longitudinal bibliometric assessment. Thematic evolution was examined using the thematic evolution function of the Biblioshiny web interface, which generated Sankey diagrams to visualize the transformation and flow of research themes across three predefined temporal segments (1975–2000, 2001–2010, and 2011–2025), with inclusion thresholds set to capture themes appearing in at least 50 documents per period to ensure statistical relevance. The temporal distribution of the research terminology was analyzed using the trend topics, enabling the identification of temporal patterns in keyword emergence and their persistence throughout the dataset.
Network visualizations for keyword co-occurrence analysis included terms appearing in at least 100 publications, ensuring statistical significance while capturing the comprehensive breadth of research terminology within the field. Node proximity in these visualizations was determined by the co-occurrence frequency, with closely positioned terms indicating regular simultaneous usage throughout the literature, while VOSviewer’s association strength calculations enabled the identification of thematically related keyword clusters through systematic color-coding.
A multi-method AI-enhanced system for automated synonym detection and terminology standardization in pharmaceutical literature has been developed (437 lines of Python code) that is specifically designed for DDI research vocabularies. The system employs a three-tiered approach combining exact character variation detection using regular expressions to identify formatting inconsistencies, semantic similarity clustering using pre-trained transformer models (BioBERT and SentenceTransformers) with DBSCAN clustering (cosine similarity threshold ≥0.85), and medical context-aware fuzzy string matching (FuzzyWuzzy library) that considers pharmaceutical components such as dosage forms, concentrations, and active ingredients [
36]. The architecture of the system (
Figure 3) follows a structured pipeline: after the initial term cleaning and validation, the three modules—exact matching, semantic embedding-based clustering, and fuzzy rule-based matching—operate in parallel. Their outputs are subsequently merged using a graph-based resolution mechanism, where overlapping synonym groups are unified, and canonical terms are selected through component-wise analysis. The final output is a normalized term dictionary that is optimized for downstream text mining and information retrieval in biomedical domains.
The algorithm incorporates domain-specific validation patterns for medical terminology, handles missing dependencies through graceful fallbacks, and uses graph-based merging (NetworkX) to resolve overlapping synonym groups while maintaining precision through minimum group-size constraints. Input terms undergo preprocessing to normalize punctuation and to extract core pharmaceutical components, with the final output formatted as comma-separated value files that are compatible with VOSviewer visualization software, enabling standardized thesaurus integration for bibliometric and systematic review applications. The system’s precision was validated using a stratified random sample of 300 term pairs from the generated thesaurus. Three domain experts in pharmaceutical sciences independently evaluated each synonym grouping as correct or incorrect based on semantic equivalence criteria. The system’s performance was deemed acceptable only when all three evaluators unanimously agreed that ≥90% of the groupings were correct.
To clarify the role of AI in our methodology, we distinguish between two phases of our analysis. In the first phase, we use AI/machine learning techniques for terminology standardization. We utilize transformer-based language models (BioBERT) and unsupervised clustering algorithms (DBSCAN with a cosine similarity threshold of ≥0.85) to intelligently identify semantic relationships between different terms. This AI-driven pre-processing step is crucial for overcoming the significant challenge of keyword variability in bibliometric studies, where authors may use dozens of variations to describe the same concept. The second phase applies traditional bibliometric and data-mining techniques, which include publication counting, citation analysis, and network visualization, to the standardized dataset. While these established methods do not constitute AI, the quality and accuracy of their outputs are substantially enhanced by the initial AI-driven terminology normalization. This enables a more reliable identification of research trends and thematic clusters than would be possible with conventional keyword matching alone.
4. Discussion
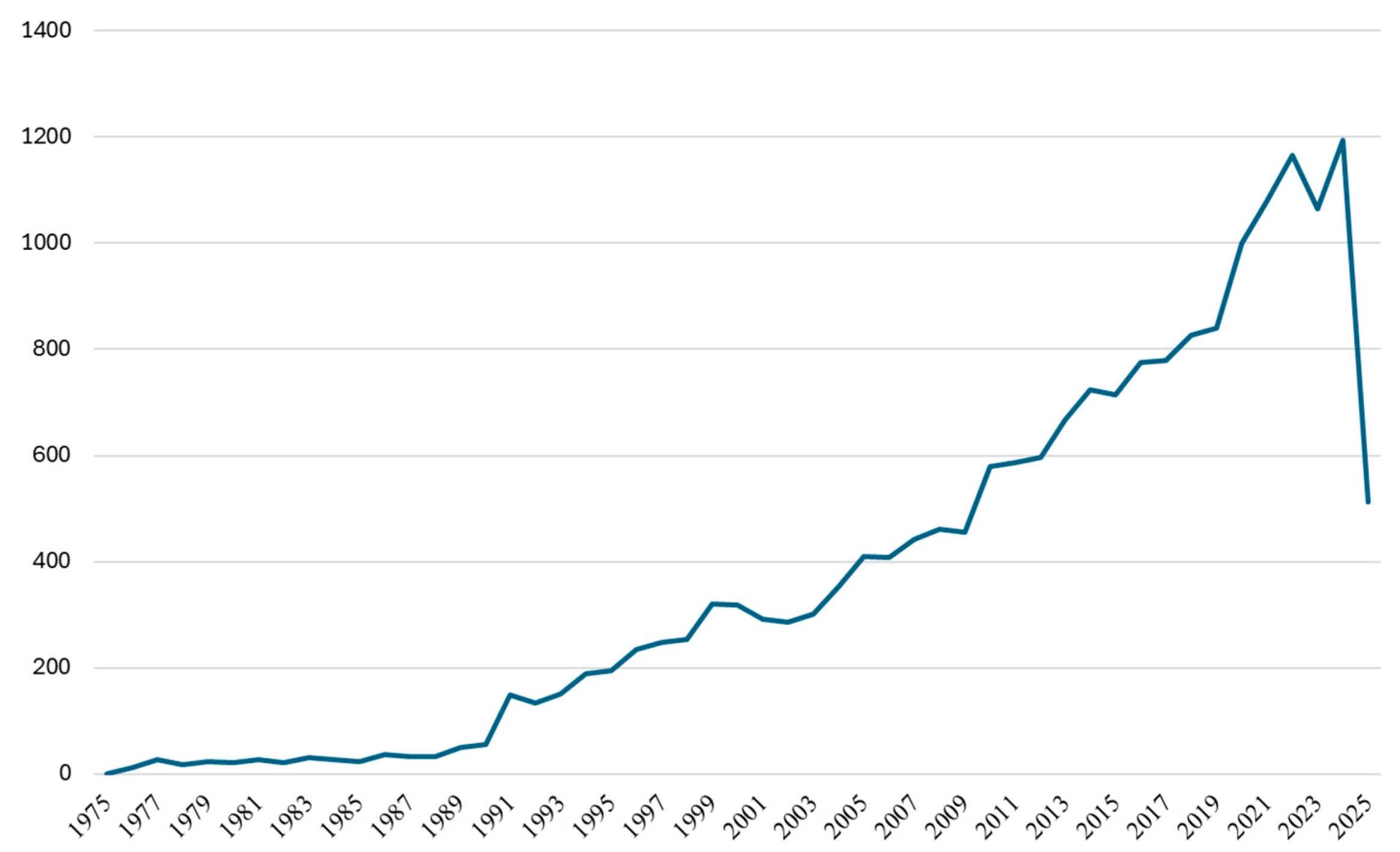
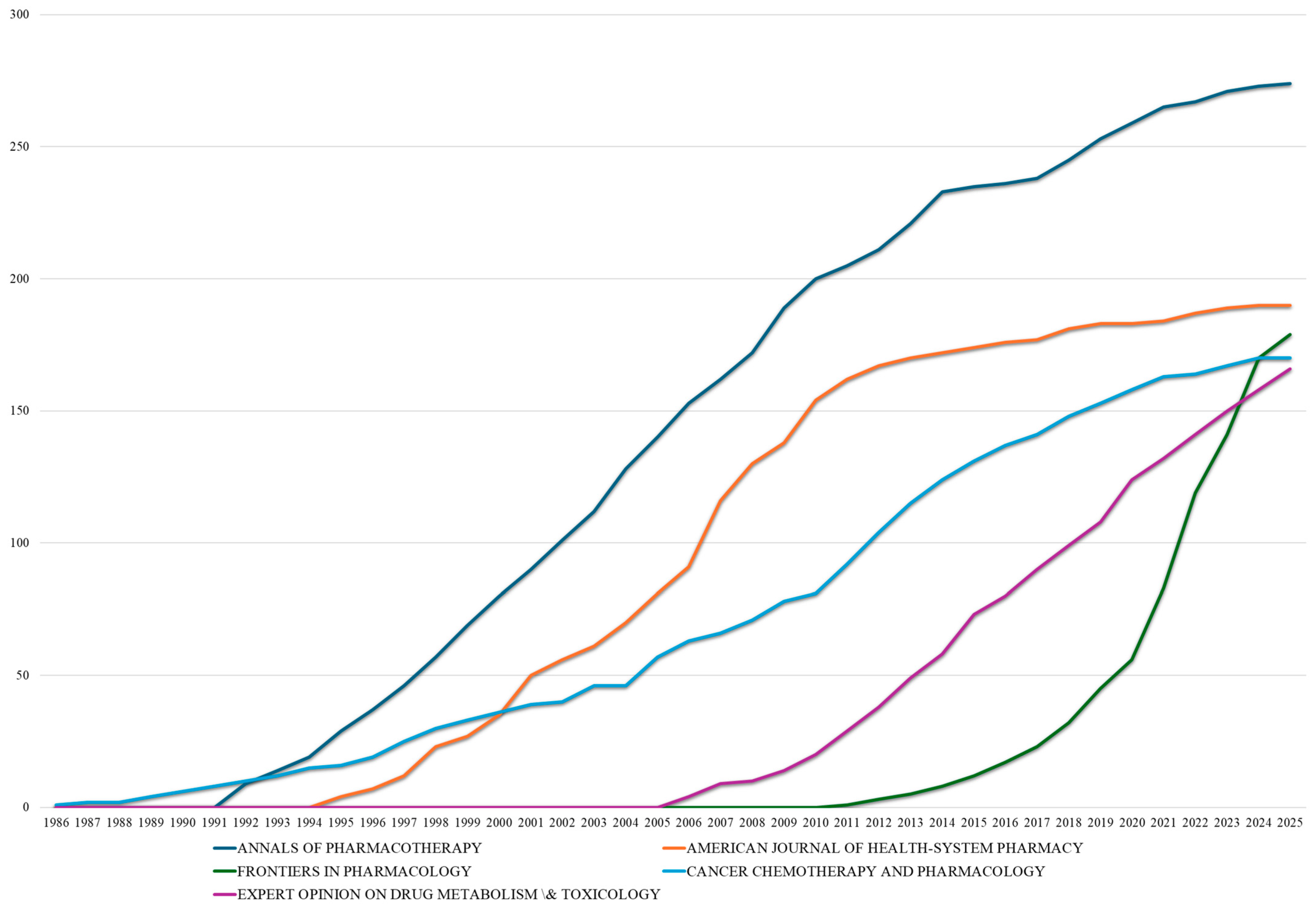
This comprehensive bibliometric analysis spanning five decades (1975–2025) reveals the transformation of drug–drug interaction research from a peripheral pharmacological concern to a central pillar of pharmaceutical safety. The exponential growth trajectory, particularly the increase during 2010–2024, reflects not merely quantitative expansion but fundamental shifts in research paradigms, methodological sophistication, and clinical integration. The five-decade evolution reveals exponential growth patterns consistent with emerging scientific disciplines achieving mainstream recognition. The total accumulation of 19,151 publications represents substantial intellectual investment in understanding and predicting drug–drug interactions, transitioning from isolated clinical observations to a comprehensive mechanistic understanding supported by sophisticated analytical frameworks. This sustained momentum indicates that drug–drug interaction research has achieved mature scientific status while continuing to evolve in response to new therapeutic challenges and technological capabilities, with 2024 representing the most productive year in the field’s history.
While early combination therapy successes like Einhorn and Donohue’s (1977) groundbreaking regimen combining cisplatin, vinblastine, and bleomycin for testicular cancer [
37] demonstrated the power of multi-drug approaches, they also underscored the critical need to understand DDIs. This recognition led to systematic investigations of drug metabolism, particularly through cytochrome P450 pathways, which became the foundation of modern drug interaction science.
Throughout the 1990s and early 2000s, the research focus shifted toward a mechanistic understanding of specific drug–drug interactions, particularly cytochrome P450-mediated interactions. Key studies documented critical CYP3A4 interactions, such as the profound effects of ketoconazole, itraconazole, and macrolide antibiotics (clarithromycin, erythromycin) on drugs like terfenadine, cisapride, and statins [
38].
The emergence of HIV-protease-inhibitor therapy revealed complex interaction networks, with ritonavir identified as both a potent CYP3A4 inhibitor and inducer, dramatically affecting concentrations of co-administered drugs including saquinavir, indinavir, and immunosuppressants like cyclosporin [
39].
Antidepressant polypharmacy research highlighted significant interactions between selective serotonin reuptake inhibitors, particularly fluoxetine and paroxetine as CYP2D6 inhibitors affecting the metabolism of beta-blockers and tricyclic antidepressants [
40,
41].
Studies on statin interactions demonstrated that simvastatin and lovastatin, as CYP3A4 substrates, resulted in an increased risk of myopathy when combined with inhibitors like cyclosporine A and mibefradil [
42,
43]. This period established fundamental pharmacokinetic principles, including the recognition of drug-transporter involvement (particularly of P-glycoprotein) alongside CYP450 metabolism, that continue to guide drug interaction assessment today.
A paradigm shift occurred in the 2010s that was marked by the transition from studying isolated drug pairs to addressing complex polypharmacy scenarios. Gnjidic et al. established in 2012 that five or more medications significantly increased the risks of frailty, disability, and falls in elderly patients, with every additional medication increasing the odds of adverse outcome [
44].
Supporting this computational evolution, DrugBank 4.0 expanded to include over 1200 drug metabolites and 1300 drug metabolism reactions, providing comprehensive ADMET data and tools for analyzing drug-target–enzyme-transporter associations to predict drug–drug interactions [
45]. Real-world drug interaction data revealed critical safety signals through computational approaches, with Tatonetti et al. developing data-driven methods that identified novel interactions, including the QT prolongation risk with combined selective serotonin reuptake inhibitors and thiazides [
46].
Clinical complexity was exemplified in HIV care, where Smit et al. projected that by 2030, 40% of HIV patients could face complications with first-line regimens due to drug–drug interactions from multiple comorbidities [
47]. The management of complex therapies illustrated these challenges, as shown in the chronic pulmonary aspergillosis guidelines by Denning et al., which emphasized the careful monitoring of azole serum concentrations and drug interactions [
48]. Deprescribing emerged as essential, with Scott et al. providing the following five-step protocol to systematically reduce inappropriate polypharmacy: identifying all current medications and their indications, evaluating the patient-specific risk of drug-related harm to guide intervention intensity, balancing each drug’s benefits against potential harms, prioritizing the cessation of drugs with minimal benefit and low withdrawal risk, and executing a tapering plan with close monitoring for clinical changes or adverse events [
49]. This evolution culminated in Zitnik et al.’s (2018) graph convolutional networks (i.e., Decagon) for modeling polypharmacy side effects, which could predict the exact side effects of drug combinations and achieved up to 69% performance improvement over baselines, representing the field’s maturation from documenting interactions to developing comprehensive predictive frameworks [
50].
The network topology reveals therapeutically coherent clustering patterns where disease-specific medication groups naturally aggregate, together with their associated safety concerns. Within the red cluster, three distinct therapeutic subclusters emerge, each with characteristic co-occurrence patterns. The epilepsy-focused subcluster demonstrates strong associations between seizures, antiepileptic drugs, and specific agents such as lamotrigine and carbamazepine, reflecting the well-documented interaction potential of these narrow-therapeutic-index medications. The psychiatric medication subcluster, also positioned within the red cluster, centers around schizophrenia, with the closely linked antipsychotics risperidone and clozapine, indicating concentrated research attention on psychotropic drug interactions. The demographic risk subcluster completes the red cluster’s composition, revealing polypharmacy as strongly associated with elderly and older adults, highlighting this population’s heightened vulnerability to interaction-related adverse events due to age-related pharmacokinetic changes and medication complexity.
Within the blue cluster, three distinct therapeutic subclusters emerge, each with characteristic co-occurrence patterns. The oncology-focused subcluster dominates, demonstrating dense interconnections between cancer types (i.e., breast, lung, and ovarian), chemotherapeutic agents (i.e., cisplatin, paclitaxel, doxorubicin, carboplatin, gemcitabine, and 5-fluorouracil), and treatment modalities (i.e., chemotherapy and combination therapy). This reflects the critical importance of understanding drug interactions in multi-agent cancer treatment protocols, where therapeutic windows are narrow and managing toxicity is paramount. The drug-resistance mechanisms subcluster, also positioned within the blue cluster, centers around multi-drug resistance, drug resistance, and cellular mechanisms, including apoptosis, cytotoxicity, and synergism, coupled with delivery technologies such as nanoparticles, indicating concentrated research attention on overcoming therapeutic resistance through combination strategies and novel drug delivery approaches. The clinical-trial methodology subcluster completes the blue cluster, revealing strong associations between Phase I, Phase II, and Phase III trials, randomized trials, and survival outcomes. This highlights the systematic approach to evaluating the safety and efficacy of drug interactions in oncology, where combination regimens require rigorous clinical validation due to their complexity and the potential for both synergistic therapeutic effects and additive toxicities.
The green cluster reveals a mechanistic research domain organized around three interconnected areas. The cluster is founded on drug metabolism processes, featuring extensive networks that connect cytochrome P450 enzymes, hepatic microsomes, and metabolic pathways, as well as specific enzyme induction and inhibition mechanisms. This emphasizes an understanding of molecular-level interactions. Another major component is transport-protein research, where studies of P-glycoprotein, blood–brain barrier permeability, and drug disposition intersect with natural interaction modulators such as grapefruit juice and St John’s wort. This demonstrates how efflux transporters and herbal products influence drug bioavailability and interaction outcomes. Hepatic safety assessment, which investigates links between hepatotoxicity, liver function monitoring, gene expression profiling and predictive modelling approaches, completes this cluster. This establishes the liver’s central role in evaluating interaction risk and developing computational prediction tools.
The yellow cluster represents HIV therapeutics as a specialized research enclave, where antiretroviral therapy, protease inhibitors, and AIDS-related conditions converge with infected patient populations and specific antiretroviral agents. This clustering pattern underscores the unique pharmacological challenges of HIV treatment, where complex drug interaction profiles between antiretroviral combinations directly influence therapeutic success, viral-resistance patterns, and patient outcomes, requiring dedicated investigation into optimizing multi-drug regimens while navigating the intricate interaction landscape characteristic of HIV pharmacotherapy.
The mechanistic understanding of pharmacokinetic drug–drug interactions evolved substantially throughout our study period. Hirota et al. demonstrated how CYP3A4 inhibitors dramatically increase plasma concentrations of simvastatin, lovastatin, and atorvastatin, while pravastatin and rosuvastatin remain unaffected due to minimal CYP metabolism [
51]. This complexity extends to phenoconversion, where drug-induced metabolic changes create mismatches between genetic and observable phenotypes [
52]. Clinical implications are evident in the Dutch Pharmacogenetics Working Group guidelines, which document CYP2C19 as poor metabolizers requiring 50% escitalopram dose reductions [
53]. Additionally, Tang et al. (2021) showed that fluconazole’s pronounced CYP3A4 inhibition significantly altered fedratinib pharmacokinetics, illustrating the clinical relevance of enzyme-mediated interactions [
54].
Our analysis reveals increasing attention to pharmacodynamic interactions affecting therapeutic outcomes through receptor-level mechanisms. Roberti et al. 2021 exemplified this complexity with cenobamate, which, acting through dual mechanisms, inhibited voltage-gated sodium channel persistent currents while modulating GABA(A) receptors at non-benzodiazepine sites [
55]. The clinical significance extends to oncology, where Bruin et al. documented exposure–efficacy relationships for talazoparib and exposure–toxicity correlations across poly (ADP-ribose) polymerase inhibitors, predominantly manifesting as hematological adverse events [
56]. These findings align with our bibliometric evolution, which showed a progression from documenting isolated receptor interactions to the development of an understanding of integrated pharmacodynamic networks crucial for managing polypharmacy in therapeutic areas with narrow therapeutic windows.
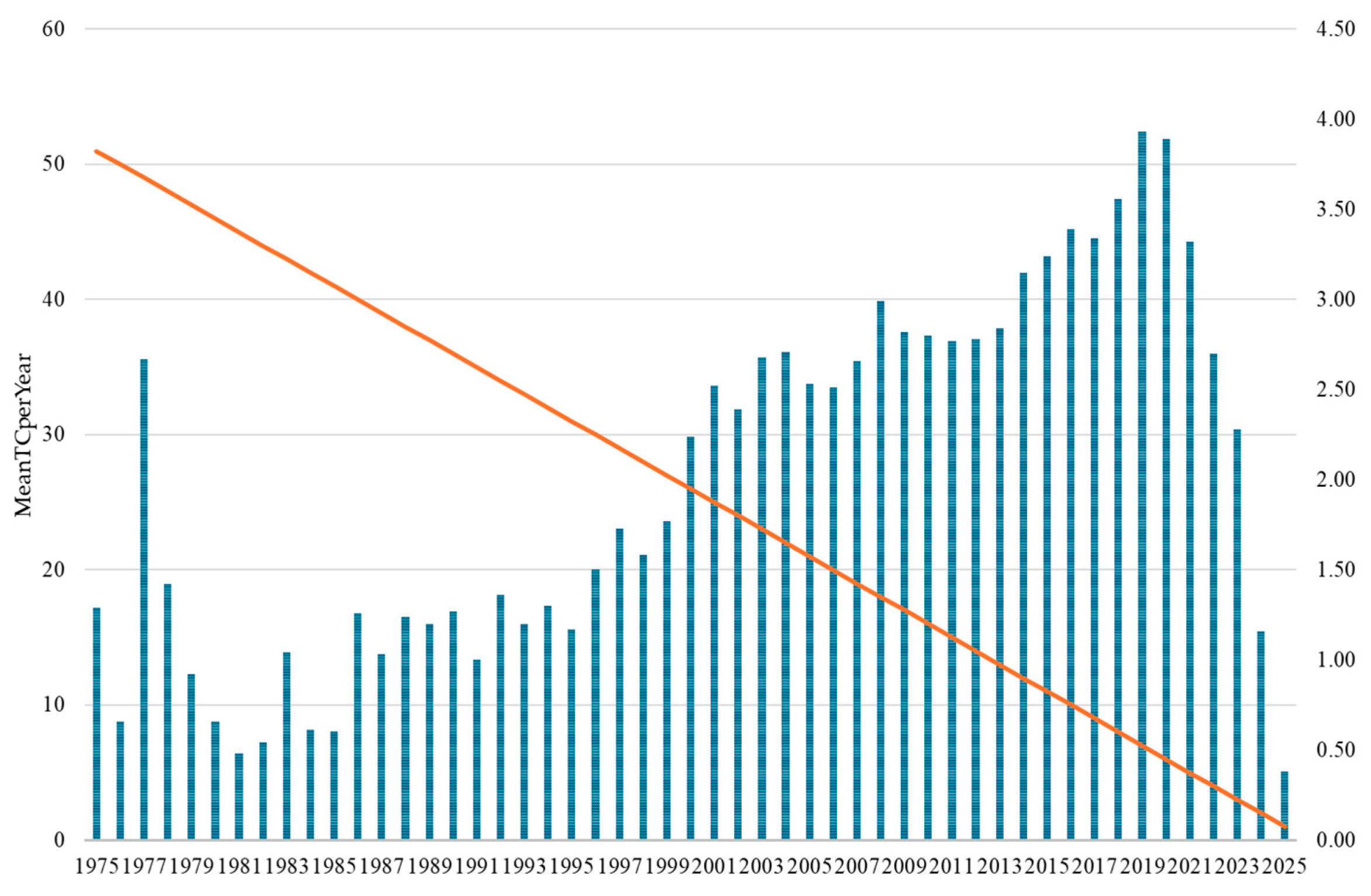
The temporal analysis of citation patterns provides crucial insights into the evolving scientific influence and maturation of drug–drug interaction research across five decades. The Mean Total Citations per Year (MeanTCperYear) metric reveals distinct phases of research impact that closely correspond to, yet uniquely complement, the publication volume trends previously identified. The overall citation trajectory reveals drug–drug interaction research’s transformation from a specialized pharmacological concern to a central pillar of modern pharmaceutical science. The progression from minimal early impact (<1.0) to peak contemporary influence (>3.5) demonstrates the successful integration of DDI considerations into mainstream medical practice, regulatory frameworks, and pharmaceutical development processes. This citation evolution indicates that the field has not only grown in volume but has achieved substantial scientific maturity and practical relevance, with recent research demonstrating an unprecedented influence on clinical practice and pharmaceutical innovation.
The international collaboration patterns in drug–drug interaction research reveal a multipolar research landscape that reflects both traditional pharmaceutical research hierarchies and emerging global partnerships. The United States’ central position, evidenced by its extensive collaboration network and highest TLS, aligns with its historical dominance in pharmaceutical research and regulatory science. However, the emergence of China as a major research contributor, coupled with the formation of distinct regional clusters, suggests a shift toward a more distributed global research architecture. The exceptionally high citation impacts observed in smaller European nations such as Switzerland and Denmark indicate that research quality and international influence are not solely dependent on publication volume but, rather, on specialized expertise and strategic collaborations within established pharmaceutical ecosystems. The four-cluster structure demonstrates how geographic proximity, linguistic similarities, and shared regulatory frameworks continue to shape international research partnerships while also revealing the emergence of transcontinental collaborations that bridge traditional research boundaries. Countries such as Australia and the Netherlands serve as crucial connectors between different regional research communities, facilitating knowledge transfer and methodological standardization across diverse pharmaceutical research traditions. This evolving collaboration landscape reflects the increasingly global nature of drug safety challenges and the recognition that a comprehensive understanding of drug–drug interactions requires diverse perspectives from different healthcare systems, patient populations, and regulatory environments.
The convergence of multiple drug-specific themes (i.e., carbamazepine, fluoxetine) and disease-focused research (i.e., AIDS-related complex) into the methodologically oriented double-blind cluster during 2001–2010 indicates a significant shift toward standardized clinical trial approaches. The remarkable persistence of pharmacokinetics across all three periods, visible as consistent flowing streams in the diagram, underscores its role as the foundational scientific framework of DDI research. Particularly revealing is the transformation of the dominant toxicity cluster into combination therapy research and, subsequently, into in vitro investigations, demonstrating a progression from reactive safety assessments to proactive mechanistic understanding of multi-drug interactions. The emergence of the risk assessment theme in the final period, drawing from multiple previous streams, represents a critical shift toward translational applications, suggesting that the field has evolved from purely mechanistic studies to encompass practical clinical risk-management strategies essential for addressing the complexities of modern polypharmacy.
The temporal evolution of drug–drug interaction research reveals distinct phases characterized by specific therapeutic compounds and a mechanistic understanding. Early research (1990s–early 2000s) concentrated heavily on antiretroviral therapy, with zidovudine and human-immunodeficiency-virus-related interactions dominating the landscape, reflecting the urgent clinical need to understand combination therapy safety during the AIDS epidemic. Concurrently, significant attention was directed toward anticonvulsants such as phenytoin and carbamazepine, known for their potent enzymatic induction properties affecting cytochrome P450 pathways. Cyclosporine, an immunosuppressant with narrow therapeutic windows, and fluoxetine, representing the selective serotonin reuptake inhibitor class, also emerged as critical compounds requiring interaction monitoring. Cancer therapeutics, including cisplatin and 5-fluorouracil, gained prominence in the mid-2000s, highlighting the complexity of managing combination chemotherapy regimens.
The research trajectory demonstrates evolving methodological approaches and expanding clinical considerations based on specific keyword patterns. The coexistence of both “in vivo” (2007, 2012, 2017) and “in vitro” (2009, 2016, 2021) methodologies throughout the timeline, with “in vitro” showing a substantially higher frequency (1237 vs. 245 occurrences), indicates that laboratory-based prediction methods gained prominence alongside clinical studies rather than replacing them. The emphasis on mechanistic understanding is evident through the topics of “human liver-microsomes” (2002, 2009, 2014) and “metabolism” (2005, 2013, 2020) appearing consistently throughout the research period. The emergence of “polypharmacy” (2016, 2020, 2022) as a distinct research focus in recent years directly reflects the growing clinical awareness of multiple-drug-regimen complexities, while “nanoparticles” (2018, 2021, 2023) represents the newest addition to interaction considerations, appearing exclusively in the most recent period. The persistence of “inhibition” (2007, 2016, 2021) across multiple time periods, combined with the consistent presence of “safety” (2012, 2017, 2022) and “risk” (2013, 2019, 2022) in recent years, demonstrates that enzymatic inhibition remains a central mechanistic concern, while risk assessment has become increasingly prioritized in contemporary drug–drug interaction research, as evidenced by the clustering of “management” (2012, 2018, 2022) in the latter portion of the timeline.
DDIs continue to present substantial clinical challenges, necessitating advanced predictive methodologies to improve patient safety and therapeutic outcomes. Recent innovations in computational modeling, such as DDINet, leverage deep sequential-learning architectures combined with attention mechanisms to accurately classify DDIs by their mechanistic pathways, including absorption, metabolism, and excretion processes, thereby potentially reducing the reliance on costly experimental assays [
57]. Complementing this, the TSEDDI framework integrates convolutional neural networks with multi-head attention and residual connections to effectively identify toxic side effects arising from drug combinations, underscoring the importance of computational approaches in the early detection of adverse reactions [
58].
However, rare, yet severe, DDIs remain difficult to detect due to limited clinical data; addressing this, meta-learning models like RareDDIE utilize biological semantic transferring and dual-granular variational representations to enhance the prediction accuracy in data-scarce scenarios, enabling zero-shot identification and improving drug-synergy evaluations [
59]. Furthermore, the timing of drug administration critically influences adverse drug events. The STEM model exemplifies advances in mining real-world datasets to detect DDIs with timing-dependent risks, offering superior false-positive control and increased detection power compared with traditional statistical methods [
60].
Beyond conventional pharmacology, investigations into traditional Chinese medicine illustrate the utility of quantitative pharmacology techniques such as the Chou–Talalay method for deciphering complex interactions within multi-compound formulations, providing a framework to optimize efficacy and safety through an understanding of synergistic and antagonistic drug combinations [
61]. Future research should focus on integrating these diverse computational and experimental approaches to refine predictive models, expanding databases to include rare DDIs and timing factors, and further exploring substructural drug properties that are linked to toxicity. Such multidisciplinary efforts are vital to advancing personalized medicine and minimizing the clinical risks associated with polypharmacy.
Despite the comprehensive analysis of 19,151 publications spanning five decades, several methodological constraints warrant consideration. The exclusive use of the Web of Science Core Collection, while ensuring data consistency and quality, potentially excludes relevant research published in regional databases or gray literature sources. Additionally, the English language restriction may underrepresent contributions from non-anglophone countries; this could partially explain the lower citation impacts observed for Asian nations compared with Western countries, which may reflect linguistic barriers rather than research quality differences.
The temporal aspects of bibliometric analysis introduce inherent biases, particularly when considering recent publications. Documents from 2020–2025 show artificially low citation rates (0.38–3.89 MeanTCperYear) due to the insufficient time for citation accumulation, potentially underestimating the influence of emerging research areas such as AI or machine learning applications and COVID-19-related drug interactions. Furthermore, although our Python-based thesaurus generator achieved a validation accuracy of 94.3%, automated keyword standardization cannot fully capture the semantic nuances present in the multidisciplinary landscape of DDI research. Although the standardization process is systematic, it may not adequately represent evolving terminology or context-specific meanings in the fields of pharmacology, clinical practice, and regulation.
The period of the COVID-19 pandemic introduces a unique temporal distortion to our dataset. Expedited publication processes and shifted research priorities may affect the thematic distribution and quality assessment of recent publications. This surge may overrepresent certain DDI topics, creating an unsustainable publication trajectory that does not reflect normal research patterns. Additionally, the fractional counting methodology used to calculate country contributions may undervalue nations with extensive international collaborations, which particularly affects smaller countries that predominantly participate in multinational research consortia. While these limitations do not undermine the validity of the study, they do suggest that absolute rankings, the impact of very recent research, and the completeness of global DDI research representation should be interpreted carefully.
Our analysis reveals that the emergence of computational and AI-driven approaches in DDI research offers a chance to democratize access to this important area of study. Traditional DDI research often requires expensive laboratory infrastructure, access to patient populations, and costly clinical trials. However, the recent shift toward computational methods, as evidenced by the emergence of “molecular docking” (2023), “machine learning”, and “in silico” approaches, enables researchers to conduct sophisticated DDI predictions using freely available databases and open-source software. This technological transformation is particularly beneficial for scientists in settings with limited resources, as they can leverage computational tools to conduct meaningful research without the need for extensive physical infrastructure.
Furthermore, the increasing availability of open-access DDI databases (i.e., DrugBank, DDInter), cloud-based computing resources (like Amazon Web Services, Google Cloud Platform, IMB Cloud, and Microsoft Azure), and collaborative platforms reduces traditional barriers to entry. Our collaboration network analysis showing successful partnerships across 100 countries suggests that international collaborations can bridge resource gaps, with computational methods serving as the common platform. This shift toward democratized, computation-based DDI research not only expands the global research community but also ensures that DDI safety profiles are studied across diverse populations and healthcare contexts, ultimately improving pharmaceutical safety worldwide.