Mechanotransduction: A Master Regulator of Alveolar Cell Fate Determination
Abstract
1. Introduction
2. Roadmap of Mechanotransduction
3. Mechanotransduction Forces Govern Alveolar Cell Development and Differentiation
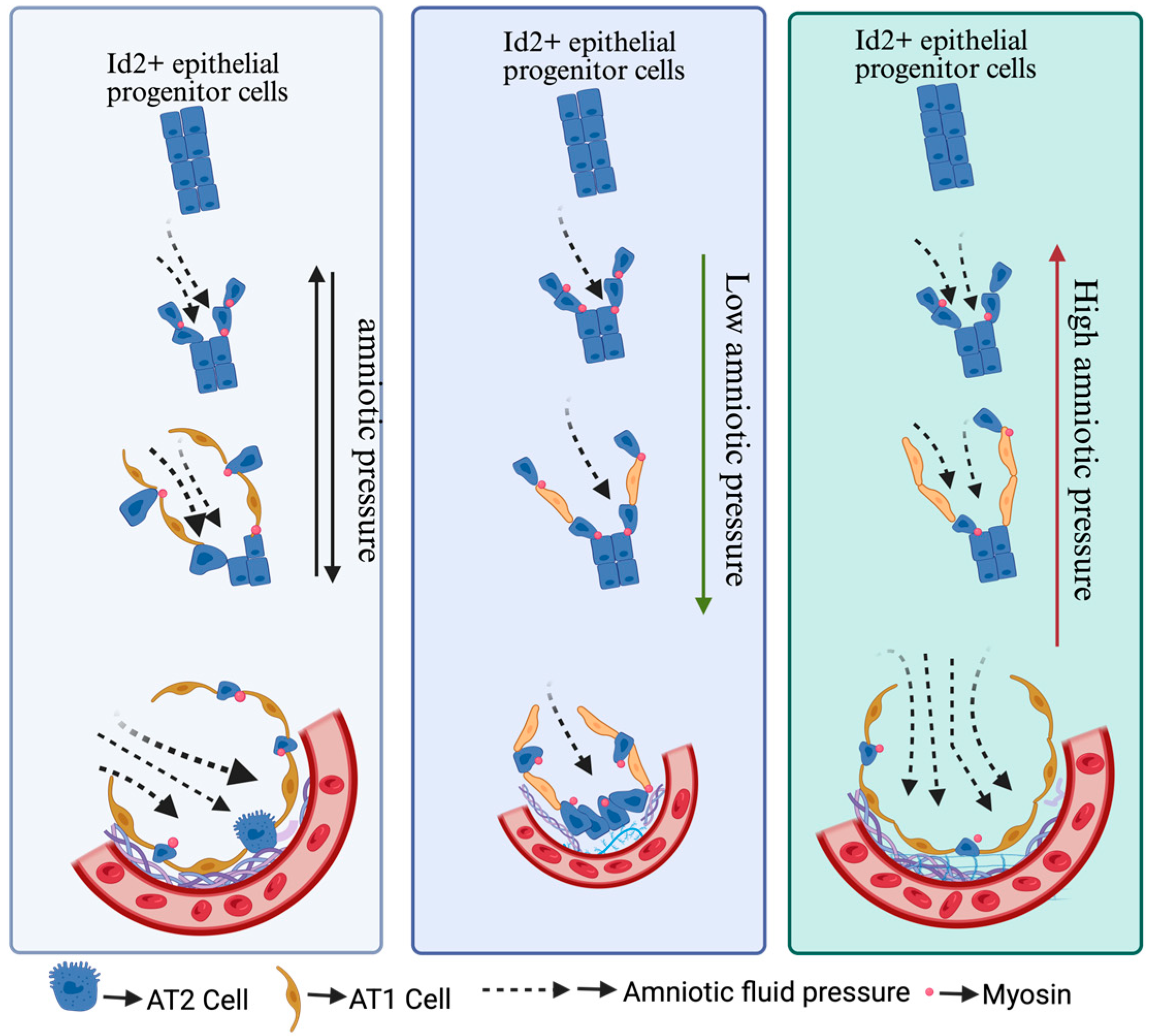
4. Mechanical Force Intensity Regulates the Differentiation of Alveolar Epithelial Cells
5. Chemical and Mechanical Cues Interplay in Alveolar Cell Fate Determination
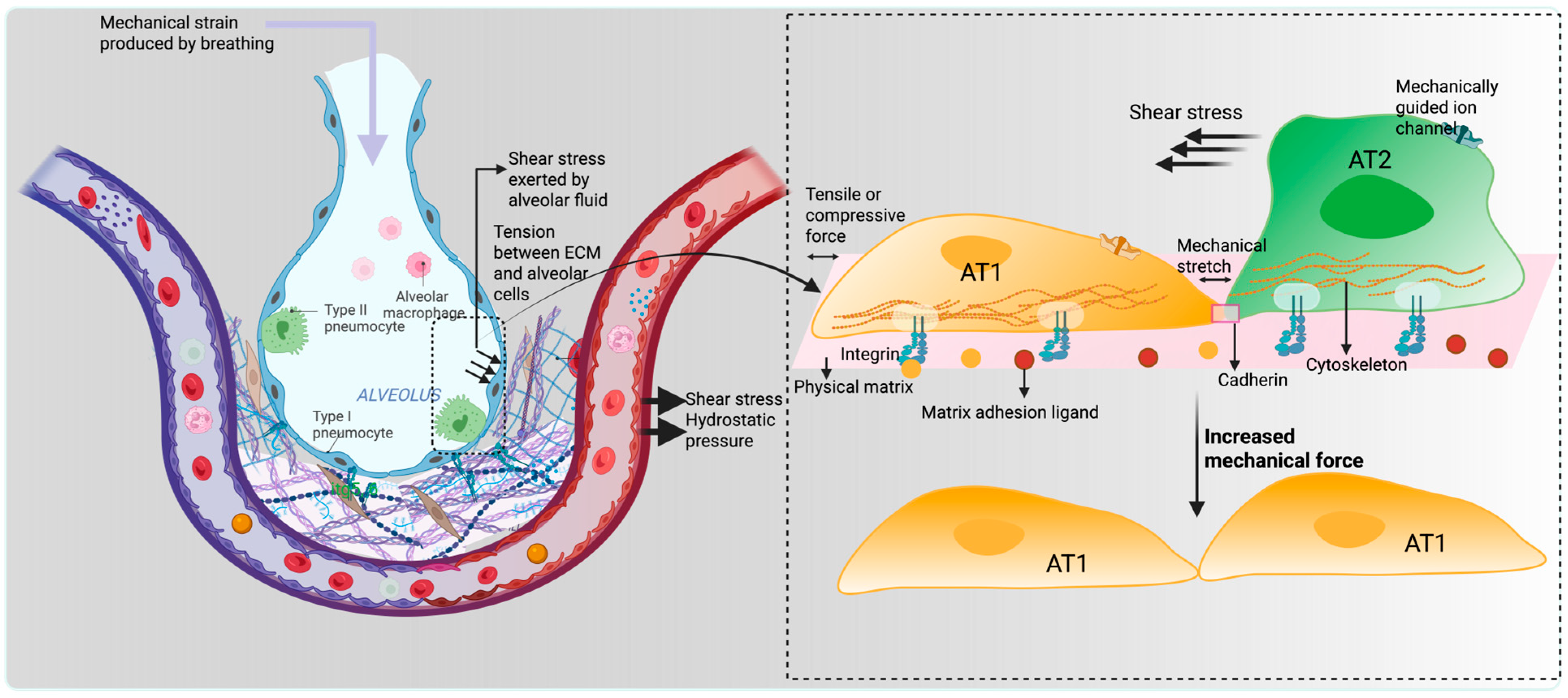
6. Types of Mechanical Cues Responsible for Alveolar Cell Development in the Lung
7. Extracellular Matrix (ECM) Is Critically Important in Alveolar Differentiation
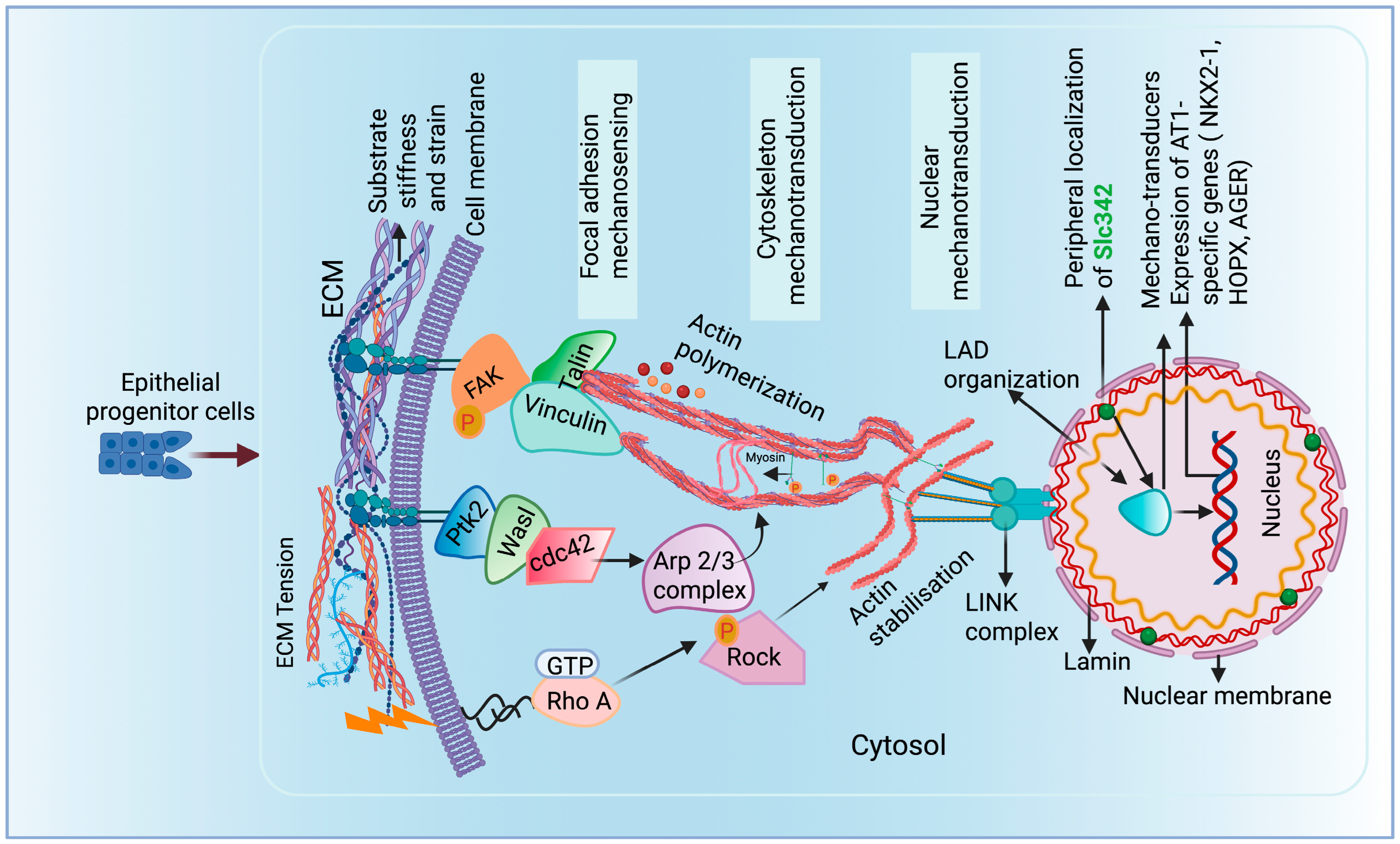
8. Involvement of Focal Cell Adhesion Molecules and Cytoskeleton Proteins in Alveolar Differentiation and Fate Maintenance
9. Impact of Mechanotransduction on Nuclear Lamina–Chromatin Interactions to Direct Alveolar Cell Fates
10. Involvement of Ion Channels in Mechanotransduction in Alveolar Cell Differentiation
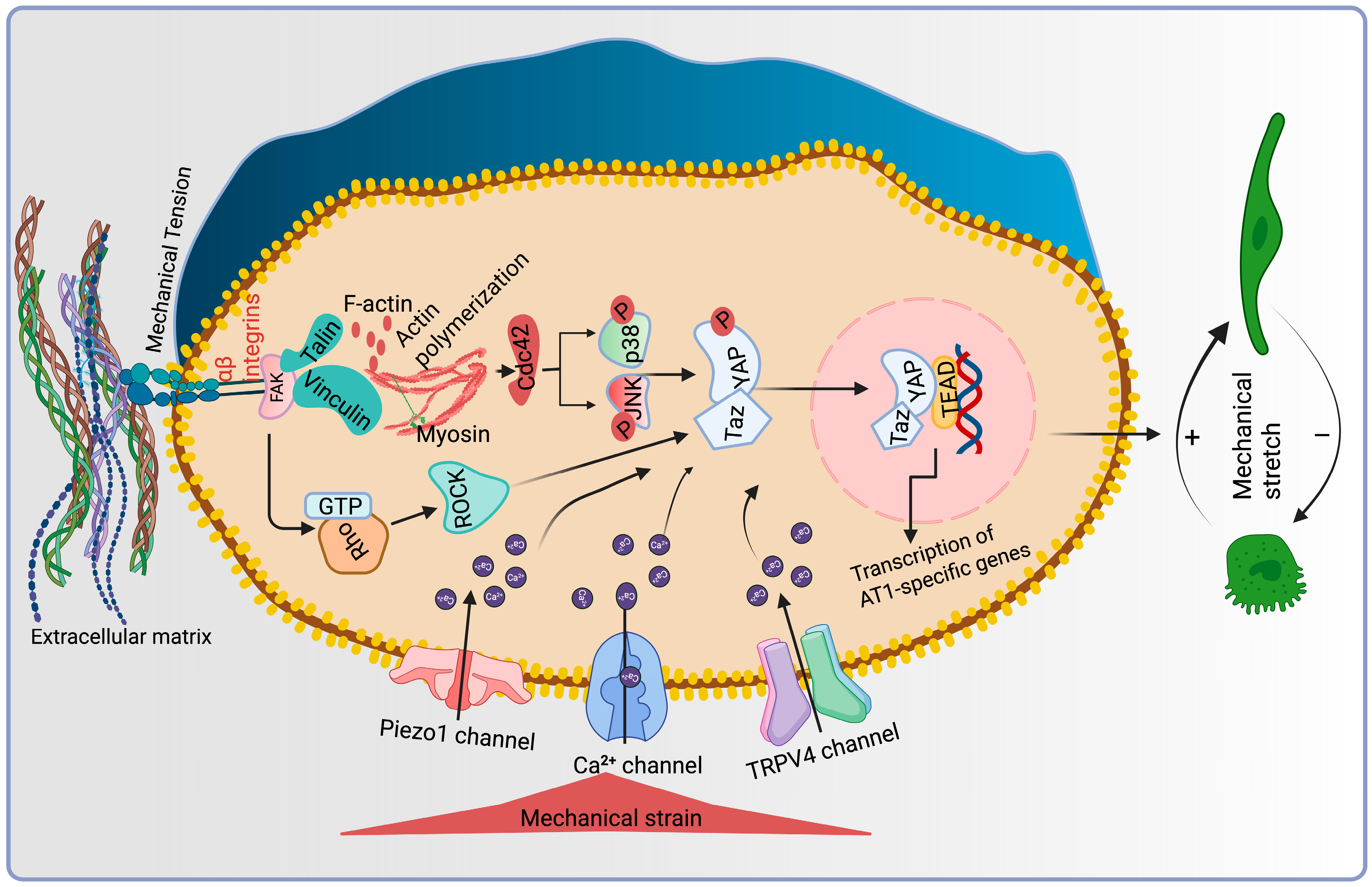
11. Mechanosensitive Signaling Pathways Regulating AT1-AT2 Cell Differentiation
11.1. RhoA/ROCK Pathway
11.2. YAP/TAZ Signaling
11.3. MAPK (ERK1/2, JNK and p38) Signaling
12. Nuclear Relocation Acts as a Crucial Regulator for AT1-AT2 Differentiation
13. Pathological Mechanical Stress Triggers the Pathological Condition
13.1. Ventilator-Induced Lung Injuries
13.2. Pulmonary Fibrosis
14. Conclusive Remark and Future Direction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AT1 | Alveolar type I |
| AT2 | Alveolar type II |
| CAR4 | Carbonic anhydrase 4 |
| CCN1 | Cellular communication network factor 1 |
| ECM | Extracellular matrix |
| FGF10/12 | Fibroblast growth factor 10/12 |
| FAK | Focal adhesion protein |
| Id2 | Inhibitor of DNA binding 2 |
| PDGFRB | Platelet-derived growth factor B |
| PTK2 | Protein Tyrosine Kinase 2 |
| TMEM63A | Transmembrane protein 63A |
| ROCK | Rho-associated coiled-coil-containing kinases |
| YAP/TAZ | Yes-associated protein (1) and TAZ (transcriptional coactivator with PDZ motif) |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-related kinase |
| JNK | c-Jun N-terminal kinases |
| LADs | Lamina-associated domains |
| LINC | Linker of the nucleoskeleton and cytoskeleton |
References
- Tschumperlin, D.J. Mechanotransduction. Compr. Physiol. 2011, 1, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanchez, M.-E.; Brunet, T.; Röper, J.-C.; Farge, E. Mechanotransduction’s impact on animal development, evolution, and tumorigenesis. Annu. Rev. Cell Dev. Biol. 2015, 31, 373–397. [Google Scholar] [CrossRef] [PubMed]
- Mascharak, S.; Talbott, H.E.; Januszyk, M.; Griffin, M.; Chen, K.; Davitt, M.F.; Demeter, J.; Henn, D.; Bonham, C.A.; Foster, D.S. Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell Stem Cell 2022, 29, 315–327.e316. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Integrin signaling in cancer: Mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Long, Y.; Niu, Y.; Liang, K.; Du, Y. Mechanical communication in fibrosis progression. Trends Cell Biol. 2022, 32, 70–90. [Google Scholar] [CrossRef]
- Marinval, N.; Chew, S.Y. Mechanotransduction assays for neural regeneration strategies: A focus on glial cells. APL Bioeng. 2021, 5, 021505. [Google Scholar] [CrossRef]
- Zhu, P.; Lu, H.; Wang, M.; Chen, K.; Chen, Z.; Yang, L. Targeted mechanical forces enhance the effects of tumor immunotherapy by regulating immune cells in the tumor microenvironment. Cancer Biol. Med. 2023, 20, 44. [Google Scholar] [CrossRef]
- Tschumperlin, D.J.; Ligresti, G.; Hilscher, M.B.; Shah, V.H. Mechanosensing and fibrosis. J. Clin. Investig. 2018, 128, 74–84. [Google Scholar] [CrossRef]
- Shiraishi, K.; Shah, P.P.; Morley, M.P.; Loebel, C.; Santini, G.T.; Katzen, J.; Basil, M.C.; Lin, S.M.; Planer, J.D.; Cantu, E. Biophysical forces mediated by respiration maintain lung alveolar epithelial cell fate. Cell 2023, 186, 1478–1492.e1415. [Google Scholar] [CrossRef]
- Goldmann, W.H. Mechanosensation: A basic cellular process. Prog. Mol. Biol. Transl. Sci. 2014, 126, 75–102. [Google Scholar] [CrossRef]
- Li, C.B.; Hu, L.L.; Wang, Z.D.; Zhong, S.Q.; Lei, L. Regulation of compaction initiation in mouse embryo. Yi Chuan 2009, 31, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Maître, J.L.; Niwayama, R.; Turlier, H.; Nédélec, F.; Hiiragi, T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat. Cell Biol. 2015, 17, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Stress-Strain, L.T.U. An Analytical Model for Estimating. Understanding Lung Acinar Micromechanics in Health and Disease: Linking Quantitative Imaging and Organ Scale Mechanics by Computational Modeling. Front. Physiol. 2021, 11, 12158. [Google Scholar]
- Roan, E.; Waters, C.M. What do we know about mechanical strain in lung alveoli? Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011, 301, L625–L635. [Google Scholar] [CrossRef]
- Desai, L.P.; Chapman, K.E.; Waters, C.M. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 295, L958–L965. [Google Scholar] [CrossRef]
- Garcia, C.; Prota, L.; Morales, M.; Romero, P.V.; Zin, W.; Rocco, P. Understanding the mechanisms of lung mechanical stress. Braz. J. Med. Biol. Res. 2006, 39, 697–706. [Google Scholar] [CrossRef][Green Version]
- Lin, C.; Zheng, X.; Lin, S.; Zhang, Y.; Wu, J.; Li, Y. Mechanotransduction regulates the interplays between alveolar epithelial and vascular endothelial cells in lung. Front. Physiol. 2022, 13, 818394. [Google Scholar] [CrossRef]
- Liao, H.; Qi, Y.; Ye, Y.; Yue, P.; Zhang, D.; Li, Y. Mechanotranduction pathways in the regulation of mitochondrial homeostasis in cardiomyocytes. Front. Cell Dev. Biol. 2021, 8, 625089. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Q.; Zheng, Z.L.; Gao, Y.T.; Zhu, G.Y.; Wei, Q.; Xu, J.Z.; Li, Z.M.; Zhao, C.S. Topographic cues guiding cell polarization via distinct cellular mechanosensing pathways. Small 2022, 18, 2104328. [Google Scholar] [CrossRef]
- de Aja, J.S.; Kim, C.F. May the (mechanical) force be with AT2. Cell 2020, 180, 20–22. [Google Scholar] [CrossRef]
- West, J.B. How well designed is the human lung? Am. J. Respir. Crit. Care Med. 2006, 173, 583–584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gillich, A.; Zhang, F.; Farmer, C.G.; Travaglini, K.J.; Tan, S.Y.; Gu, M.; Zhou, B.; Feinstein, J.A.; Krasnow, M.A.; Metzger, R.J. Capillary cell-type specialization in the alveolus. Nature 2020, 586, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hernandez, B.J.; Martinez Alanis, D.; Narvaez del Pilar, O.; Vila-Ellis, L.; Akiyama, H.; Evans, S.E.; Ostrin, E.J.; Chen, J. The development and plasticity of alveolar type 1 cells. Development 2016, 143, 54–65. [Google Scholar] [CrossRef]
- Little, D.R.; Gerner-Mauro, K.N.; Flodby, P.; Crandall, E.D.; Borok, Z.; Akiyama, H.; Kimura, S.; Ostrin, E.J.; Chen, J. Transcriptional control of lung alveolar type 1 cell development and maintenance by NK homeobox 2-1. Proc. Natl. Acad. Sci. USA 2019, 116, 20545–20555. [Google Scholar] [CrossRef]
- Crapo, J.D.; Barry, B.E.; Gehr, P.; Bachofen, M.; Weibel, E.R. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 1982, 126, 332–337. [Google Scholar]
- Mizgerd, J.P. Acute lower respiratory tract infection. N. Engl. J. Med. 2008, 358, 716–727. [Google Scholar] [CrossRef]
- Han, S.; Mallampalli, R.K. The role of surfactant in lung disease and host defense against pulmonary infections. Ann. Am. Thorac. Soc. 2015, 12, 765–774. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Hagood, J.; Zhu, M.-S.; Sun, X. Myofibroblast contraction is essential for generating and regenerating the gas-exchange surface. J. Clin. Investig. 2021, 130, 2859–2871. [Google Scholar] [CrossRef]
- He, H.; Ma, C.; Wei, W.; Wang, H.; Lai, Y.; Liu, M.; Sun, S.; Ma, Q.; Lai, J.; Liu, H. Heparan sulfate regulates myofibroblast heterogeneity and function to mediate niche homeostasis during alveolar morphogenesis. Nat. Commun. 2025, 16, 1834. [Google Scholar] [CrossRef]
- Khan, I.S.; Molina, C.; Ren, X.; Auyeung, V.C.; Cohen, M.; Tsukui, T.; Atakilit, A.; Sheppard, D. Impaired myofibroblast proliferation is a central feature of pathologic post-natal alveolar simplification. eLife 2024, 13, RP94425. [Google Scholar] [CrossRef]
- Travaglini, K.J.; Nabhan, A.N.; Penland, L.; Sinha, R.; Gillich, A.; Sit, R.V.; Chang, S.; Conley, S.D.; Mori, Y.; Seita, J. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020, 587, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ying, L.; Lang, J.K.; Hinz, B.; Zhao, R. Modeling mechanical activation of macrophages during pulmonary fibrogenesis for targeted anti-fibrosis therapy. Sci. Adv. 2024, 10, eadj9559. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Du, H.; Winer, D.A.; Clemente-Casares, X.; Tsai, S. Mechanosensing in macrophages and dendritic cells in steady-state and disease. Front. Cell Dev. Biol. 2022, 10, 1044729. [Google Scholar] [CrossRef] [PubMed]
- Spieth, P.; Bluth, T.; Gama De Abreu, M.; Bacelis, A.; Goetz, A.; Kiefmann, R. Mechanotransduction in the lungs. Minerva Anestesiol. 2013, 80, 933–941. [Google Scholar]
- Arold, S.P.; Bartolák-Suki, E.; Suki, B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L574–L581. [Google Scholar] [CrossRef]
- López-Martínez, C.; Huidobro, C.; Albaiceta, G.M.; López-Alonso, I. Mechanical stretch modulates cell migration in the lungs. Ann. Transl. Med. 2018, 6, 28. [Google Scholar] [CrossRef]
- Noskovičová, N.; Petřek, M.; Eickelberg, O.; Heinzelmann, K. Platelet-derived growth factor signaling in the lung. From lung development and disease to clinical studies. Am. J. Respir. Cell Mol. Biol. 2015, 52, 263–284. [Google Scholar] [CrossRef]
- Chen, G.-L.; Li, J.-Y.; Chen, X.; Liu, J.-W.; Zhang, Q.; Liu, J.-Y.; Wen, J.; Wang, N.; Lei, M.; Wei, J.-P. Mechanosensitive channels TMEM63A and TMEM63B mediate lung inflation–induced surfactant secretion. J. Clin. Investig. 2024, 134, e174508. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Chu, Q.; Jiang, K.; Li, J.; Tang, N. The strength of mechanical forces determines the differentiation of alveolar epithelial cells. Dev. Cell 2018, 44, 297–312.e295. [Google Scholar] [CrossRef]
- Kwinta, P.; Pietrzyk, J.J. Preterm birth and respiratory disease in later life. Expert Rev. Respir. Med. 2010, 4, 593–604. [Google Scholar] [CrossRef]
- Desai, T.J.; Brownfield, D.G.; Krasnow, M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014, 507, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Morrisey, E.E.; Hogan, B.L. Preparing for the first breath: Genetic and cellular mechanisms in lung development. Dev. Cell 2010, 18, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Brownfield, D.G.; de Arce, A.D.; Ghelfi, E.; Gillich, A.; Desai, T.J.; Krasnow, M.A. Alveolar cell fate selection and lifelong maintenance of AT2 cells by FGF signaling. Nat. Commun. 2022, 13, 7137. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; De Coppi, P.; Michielin, F. Leveraging mechanobiology and biophysical cues in lung organoids for studying lung development and disease. Front. Chem. Eng. 2023, 5, 1255783. [Google Scholar] [CrossRef]
- Zepp, J.A.; Morley, M.P.; Loebel, C.; Kremp, M.M.; Chaudhry, F.N.; Basil, M.C.; Leach, J.P.; Liberti, D.C.; Niethamer, T.K.; Ying, Y. Genomic, epigenomic, and biophysical cues controlling the emergence of the lung alveolus. Science 2021, 371, eabc3172. [Google Scholar] [CrossRef]
- Nelson, C.M.; Gleghorn, J.P.; Pang, M.F.; Jaslove, J.M.; Goodwin, K.; Varner, V.D.; Miller, E.; Radisky, D.C.; Stone, H.A. Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development 2017, 144, 4328–4335. [Google Scholar] [CrossRef]
- Nguyen, T.M.; van der Merwe, J.; Elowsson Rendin, L.; Larsson-Callerfelt, A.-K.; Deprest, J.; Westergren-Thorsson, G.; Toelen, J. Stretch increases alveolar type 1 cell number in fetal lungs through ROCK-Yap/Taz pathway. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 321, L814–L826. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.; Zhang, C.; Shang, Y.; Lin, J. YAP-mediated crosstalk between the Wnt and Hippo signaling pathways (Review). Mol. Med. Rep. 2020, 22, 4101–4106. [Google Scholar] [CrossRef]
- Kechagia, Z.; Roca-Cusachs, P. Cytoskeletal safeguards: Protecting the nucleus from mechanical perturbations. Curr. Opin. Biomed. Eng. 2023, 28, 100494. [Google Scholar] [CrossRef]
- Swift, J.; Discher, D.E. The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J. Cell Sci. 2014, 127, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Vashisth, M.; Abbas, A.; Majkut, S.; Vogel, K.; Xia, Y.; Ivanovska, I.L.; Irianto, J.; Tewari, M.; Zhu, K.; et al. Mechanosensing by the Lamina Protects Against Nuclear Rupture, DNA Damage, and Cell-Cycle Arrest. Dev. Cell 2019, 49, 920–935.e925. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.E.; Collins, J.M.; Dawahare, J.H.; Nguyen, T.D.; Lin, Y.; Voytik-Harbin, S.L.; Zorlutuna, P.; Yoder, M.C.; Boerckel, J.D. YAP and TAZ limit cytoskeletal and focal adhesion maturation to enable persistent cell motility. J. Cell Biol. 2019, 218, 1369–1389. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, N.; Chen, H.; Fernandez, G.E.; Warburton, D.; Moats, R.; Mecham, R.P.; Krenitsky, D.; Pryhuber, G.S.; Shi, W. Spatial and temporal changes in extracellular elastin and laminin distribution during lung alveolar development. Sci. Rep. 2018, 8, 8334. [Google Scholar] [CrossRef]
- Rannels, D.E.; Rannels, S.R. Influence of the extracellular matrix on type 2 cell differentiation. Chest 1989, 96, 165–173. [Google Scholar] [CrossRef]
- Sucre, J.M.; Bock, F.; Negretti, N.M.; Benjamin, J.T.; Gulleman, P.M.; Dong, X.; Ferguson, K.T.; Jetter, C.S.; Han, W.; Liu, Y. Alveolar repair following LPS-induced injury requires cell-ECM interactions. JCI Insight 2023, 8, e167211. [Google Scholar] [CrossRef]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef]
- Le, H.Q.; Ghatak, S.; Yeung, C.-Y.C.; Tellkamp, F.; Günschmann, C.; Dieterich, C.; Yeroslaviz, A.; Habermann, B.; Pombo, A.; Niessen, C.M. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 2016, 18, 864–875. [Google Scholar] [CrossRef]
- Tajik, A.; Zhang, Y.; Wei, F.; Sun, J.; Jia, Q.; Zhou, W.; Singh, R.; Khanna, N.; Belmont, A.S.; Wang, N. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 2016, 15, 1287–1296. [Google Scholar] [CrossRef]
- Szczesny, S.E.; Mauck, R.L. The nuclear option: Evidence implicating the cell nucleus in mechanotransduction. J. Biomech. Eng. 2017, 139, 021006. [Google Scholar] [CrossRef]
- Kirby, T.J.; Lammerding, J. Emerging views of the nucleus as a cellular mechanosensor. Nat. Cell Biol. 2018, 20, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Takenawa, T.; Suetsugu, S. The WASP–WAVE protein network: Connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2007, 8, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Bourke, S. Investigation of Ion Channel Activity in Alveolar Epithelial Cells. The Development of Lung Slice In-Vitro Model. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2010. [Google Scholar]
- Mustafa, S.B.; Isaac, J.; Seidner, S.R.; Dixon, P.S.; Henson, B.M.; DiGeronimo, R.J. Mechanical stretch induces lung α-epithelial Na+ channel expression. Exp. Lung Res. 2014, 40, 380–391. [Google Scholar] [CrossRef]
- Coutino, B.C.; Mayor, R. Mechanosensitive ion channels in cell migration. Cells Dev. 2021, 166, 203683. [Google Scholar] [CrossRef]
- Zechini, L.; Camilleri-Brennan, J.; Walsh, J.; Beaven, R.; Moran, O.; Hartley, P.S.; Diaz, M.; Denholm, B. Piezo buffers mechanical stress via modulation of intracellular Ca2+ handling in the Drosophila heart. Front. Physiol. 2022, 13, 1003999. [Google Scholar] [CrossRef]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H. Chemical activation of the mechanotransduction channel Piezo1. eLife 2015, 4, e07369. [Google Scholar] [CrossRef]
- Ratnasingham, M.; Bradding, P.; Roach, K. The role of TRP channels in lung fibrosis: Mechanisms and therapeutic potential. Int. J. Biochem. Cell Biol. 2025, 180, 106728. [Google Scholar] [CrossRef]
- Liang, G.P.; Xu, J.; Cao, L.L.; Zeng, Y.H.; Chen, B.X.; Yang, J.; Zhang, Z.W.; Kang, Y. Piezo1 induced apoptosis of type II pneumocytes during ARDS. Respir. Res. 2019, 20, 118. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Li, L.F.; Fu, J.Y.; Kao, K.C.; Huang, C.C.; Chien, Y.; Liao, Y.W.; Chiou, S.H.; Chang, Y.L. Induced pluripotent stem cell therapy ameliorates hyperoxia-augmented ventilator-induced lung injury through suppressing the Src pathway. PLoS ONE 2014, 9, e109953. [Google Scholar] [CrossRef]
- Ingber, D.E. Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 2003, 116, 1397–1408. [Google Scholar]
- Moore, K.A.; Polte, T.; Huang, S.; Shi, B.; Alsberg, E.; Sunday, M.E.; Ingber, D.E. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 232, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Olivera, D.S.; Boggs, S.E.; Beenhouwer, C.; Aden, J.; Knall, C. Cellular mechanisms of mainstream cigarette smoke-induced lung epithelial tight junction permeability changes in vitro. Inhal. Toxicol. 2007, 19, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Silbert, O.; Wang, Y.; Maciejewski, B.S.; Lee, H.S.; Shaw, S.K.; Sanchez–Esteban, J. Roles of RhoA and Rac1 on actin remodeling and cell alignment and differentiation in fetal type II epithelial cells exposed to cyclic mechanical stretch. Exp. Lung Res. 2008, 34, 663–680. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Esteban, J.; Wang, Y.; Gruppuso, P.A.; Rubin, L.P. Mechanical stretch induces fetal type II cell differentiation via an epidermal growth factor receptor–extracellular-regulated protein kinase signaling pathway. Am. J. Respir. Cell Mol. Biol. 2004, 30, 76–83. [Google Scholar] [CrossRef]
- Chen, S.P.; Zhou, B.; Willis, B.C.; Sandoval, A.J.; Liebler, J.M.; Kim, K.-J.; Ann, D.K.; Crandall, E.D.; Borok, Z. Effects of transdifferentiation and EGF on claudin isoform expression in alveolar epithelial cells. J. Appl. Physiol. 2005, 98, 322–328. [Google Scholar] [CrossRef]
- Foster, C.D.; Varghese, L.S.; Gonzales, L.W.; Margulies, S.S.; Guttentag, S.H. The Rho pathway mediates transition to an alveolar type I cell phenotype during static stretch of alveolar type II cells. Pediatr. Res. 2010, 67, 585–590. [Google Scholar] [CrossRef]
- Wilhelm, K.R.; Roan, E.; Ghosh, M.C.; Parthasarathi, K.; Waters, C.M. Hyperoxia increases the elastic modulus of alveolar epithelial cells through Rho kinase. FEBS J. 2014, 281, 957–969. [Google Scholar] [CrossRef]
- Zanconato, F.; Forcato, M.; Battilana, G.; Azzolin, L.; Quaranta, E.; Bodega, B.; Rosato, A.; Bicciato, S.; Cordenonsi, M.; Piccolo, S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 2015, 17, 1218–1227. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef]
- Yu, F.-X.; Zhao, B.; Guan, K.-L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.C.; Halder, G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Proc. Semin. Cell Dev. Biol. 2012, 23, 803–811. [Google Scholar] [CrossRef]
- Gonzalez, R.F.; Dobbs, L.G. Isolation and culture of alveolar epithelial Type I and Type II cells from rat lungs. In Epithelial Cell Culture Protocols, 2nd ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 145–159. [Google Scholar]
- Halder, G.; Dupont, S.; Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012, 13, 591–600. [Google Scholar] [CrossRef]
- Sun, T.; Huang, Z.; Zhang, H.; Posner, C.; Jia, G.; Ramalingam, T.R.; Xu, M.; Brightbill, H.; Egen, J.G.; Dey, A. TAZ is required for lung alveolar epithelial cell differentiation after injury. JCI Insight 2019, 4, e128674. [Google Scholar] [CrossRef]
- Mitani, A.; Nagase, T.; Fukuchi, K.; Aburatani, H.; Makita, R.; Kurihara, H. Transcriptional coactivator with PDZ-binding motif is essential for normal alveolarization in mice. Am. J. Respir. Crit. Care Med. 2009, 180, 326–338. [Google Scholar] [CrossRef]
- Penkala, I.J.; Liberti, D.C.; Pankin, J.; Sivakumar, A.; Kremp, M.M.; Jayachandran, S.; Katzen, J.; Leach, J.P.; Windmueller, R.; Stolz, K. Age-dependent alveolar epithelial plasticity orchestrates lung homeostasis and regeneration. Cell Stem Cell 2021, 28, 1775–1789.e1775. [Google Scholar] [CrossRef]
- LaCanna, R.; Liccardo, D.; Zhang, P.; Tragesser, L.; Wang, Y.; Cao, T.; Chapman, H.A.; Morrisey, E.E.; Shen, H.; Koch, W.J. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J. Clin. Investig. 2019, 129, 2107–2122. [Google Scholar] [CrossRef]
- Wu, A.; Song, H. Regulation of alveolar type 2 stem/progenitor cells in lung injury and regeneration. Acta Biochim. Et Biophys. Sin. 2020, 52, 716–722. [Google Scholar] [CrossRef]
- Liu, K.; Meng, X.; Liu, Z.; Tang, M.; Lv, Z.; Huang, X.; Jin, H.; Han, X.; Liu, X.; Pu, W. Tracing the origin of alveolar stem cells in lung repair and regeneration. Cell 2024, 187, 2428–2445.e2420. [Google Scholar] [CrossRef] [PubMed]
- Olajuyin, A.M.; Zhang, X.; Ji, H.-L. Alveolar type 2 progenitor cells for lung injury repair. Cell Death Discov. 2019, 5, 63. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H.; Jiang, K.; Wang, Y.; Zhang, W.; Chu, Q.; Li, J.; Huang, H.; Cai, T.; Ji, H. MAPK-mediated YAP activation controls mechanical-tension-induced pulmonary alveolar regeneration. Cell Rep. 2016, 16, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Diem, K.; Fauler, M.; Fois, G.; Hellmann, A.; Winokurow, N.; Schumacher, S.; Kranz, C.; Frick, M. Mechanical stretch activates piezo1 in caveolae of alveolar type I cells to trigger ATP release and paracrine stimulation of surfactant secretion from alveolar type II cells. Faseb J 2020, 34, 12785–12804. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, A.; Desjardins, J.; Shabbir, W.; Roy, A.; Filion, D.; Sauvé, R.; Berthiaume, Y. Loss of barrier integrity in alveolar epithelial cells downregulates ENaC expression and activity via Ca2+ and TRPV4 activation. Pflügers Arch.-Eur. J. Physiol. 2018, 470, 1615–1631. [Google Scholar] [CrossRef]
- Ning, Q.M.; Sun, X.N.; Zhao, X.K. Role of mechanical stretching and lipopolysaccharide in early apoptosis and IL-8 of alveolar epithelial type II cells A549. Asian Pac. J. Trop. Med. 2012, 5, 638–644. [Google Scholar] [CrossRef]
- Valentine, M.S.; Link, P.A.; Herbert, J.A.; Kamga Gninzeko, F.J.; Schneck, M.B.; Shankar, K.; Nkwocha, J.; Reynolds, A.M.; Heise, R.L. Inflammation and Monocyte Recruitment due to Aging and Mechanical Stretch in Alveolar Epithelium are Inhibited by the Molecular Chaperone 4-phenylbutyrate. Cell Mol. Bioeng. 2018, 11, 495–508. [Google Scholar] [CrossRef]
- Davidovich, N.; DiPaolo, B.C.; Lawrence, G.G.; Chhour, P.; Yehya, N.; Margulies, S.S. Cyclic stretch-induced oxidative stress increases pulmonary alveolar epithelial permeability. Am. J. Respir. Cell Mol. Biol. 2013, 49, 156–164. [Google Scholar] [CrossRef]
- Song, M.J.; Davidovich, N.; Lawrence, G.G.; Margulies, S.S. Superoxide mediates tight junction complex dissociation in cyclically stretched lung slices. J. Biomech. 2016, 49, 1330–1335. [Google Scholar] [CrossRef]
- Kroon, A.A.; Delriccio, V.; Tseu, I.; Kavanagh, B.P.; Post, M. Mechanical ventilation-induced apoptosis in newborn rat lung is mediated via FasL/Fas pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L795–L804. [Google Scholar] [CrossRef]
- Morrell, E.D.; Grazioli, S.; Hung, C.; Kajikawa, O.; Kosamo, S.; Stapleton, R.D.; Gharib, S.A.; Amado-Rodríguez, L.; Albaiceta, G.; Wurfel, M.M.; et al. Alveolar CCN1 is associated with mechanical stretch and acute respiratory distress syndrome severity. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L825–L832. [Google Scholar] [CrossRef] [PubMed]
- Freeberg, M.A.T.; Perelas, A.; Rebman, J.K.; Phipps, R.P.; Thatcher, T.H.; Sime, P.J. Mechanical Feed-Forward Loops Contribute to Idiopathic Pulmonary Fibrosis. Am. J. Pathol. 2021, 191, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Pardo, A. The leading role of epithelial cells in the pathogenesis of idiopathic pulmonary fibrosis. Cell. Signal. 2020, 66, 109482. [Google Scholar] [CrossRef]
- Kuhn, H.; Zobel, C.; Vollert, G.; Gurcke, M.; Jenszöwski, C.; Barina, C.; Frille, A.; Wirtz, H. High amplitude stretching of ATII cells and fibroblasts results in profibrotic effects. Exp. Lung Res. 2019, 45, 167–174. [Google Scholar] [CrossRef]
- Froese, A.R.; Shimbori, C.; Bellaye, P.S.; Inman, M.; Obex, S.; Fatima, S.; Jenkins, G.; Gauldie, J.; Ask, K.; Kolb, M. Stretch-induced Activation of Transforming Growth Factor-β1 in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2016, 194, 84–96. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Y.; Huang, H.; Hu, Y.; Fu, S.; Wang, Z.; Shi, M.; Zhao, X.; Yuan, J.; Li, J.; et al. Progressive Pulmonary Fibrosis is Caused by Elevated Mechanical Tension on Alveolar Stem Cells. Cell 2020, 180, 107–121.e117. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 373, 96. [Google Scholar] [CrossRef]
- Ryter, S.W.; Rosas, I.O.; Owen, C.A.; Martinez, F.J.; Choi, M.E.; Lee, C.G.; Elias, J.A.; Choi, A.M.K. Mitochondrial Dysfunction as a Pathogenic Mediator of Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2018, 15, S266–S272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi, K.; Parekh, K.R. Mechanotransduction: A Master Regulator of Alveolar Cell Fate Determination. Bioengineering 2025, 12, 760. https://doi.org/10.3390/bioengineering12070760
Devi K, Parekh KR. Mechanotransduction: A Master Regulator of Alveolar Cell Fate Determination. Bioengineering. 2025; 12(7):760. https://doi.org/10.3390/bioengineering12070760
Chicago/Turabian StyleDevi, Kusum, and Kalpaj R. Parekh. 2025. "Mechanotransduction: A Master Regulator of Alveolar Cell Fate Determination" Bioengineering 12, no. 7: 760. https://doi.org/10.3390/bioengineering12070760
APA StyleDevi, K., & Parekh, K. R. (2025). Mechanotransduction: A Master Regulator of Alveolar Cell Fate Determination. Bioengineering, 12(7), 760. https://doi.org/10.3390/bioengineering12070760




