Aerosol Jet Printing for Neuroprosthetic Device Development
Abstract
1. Introduction
2. Principles of Aerosol Jet Printing (AJP)
2.1. Overview of AJP Technology
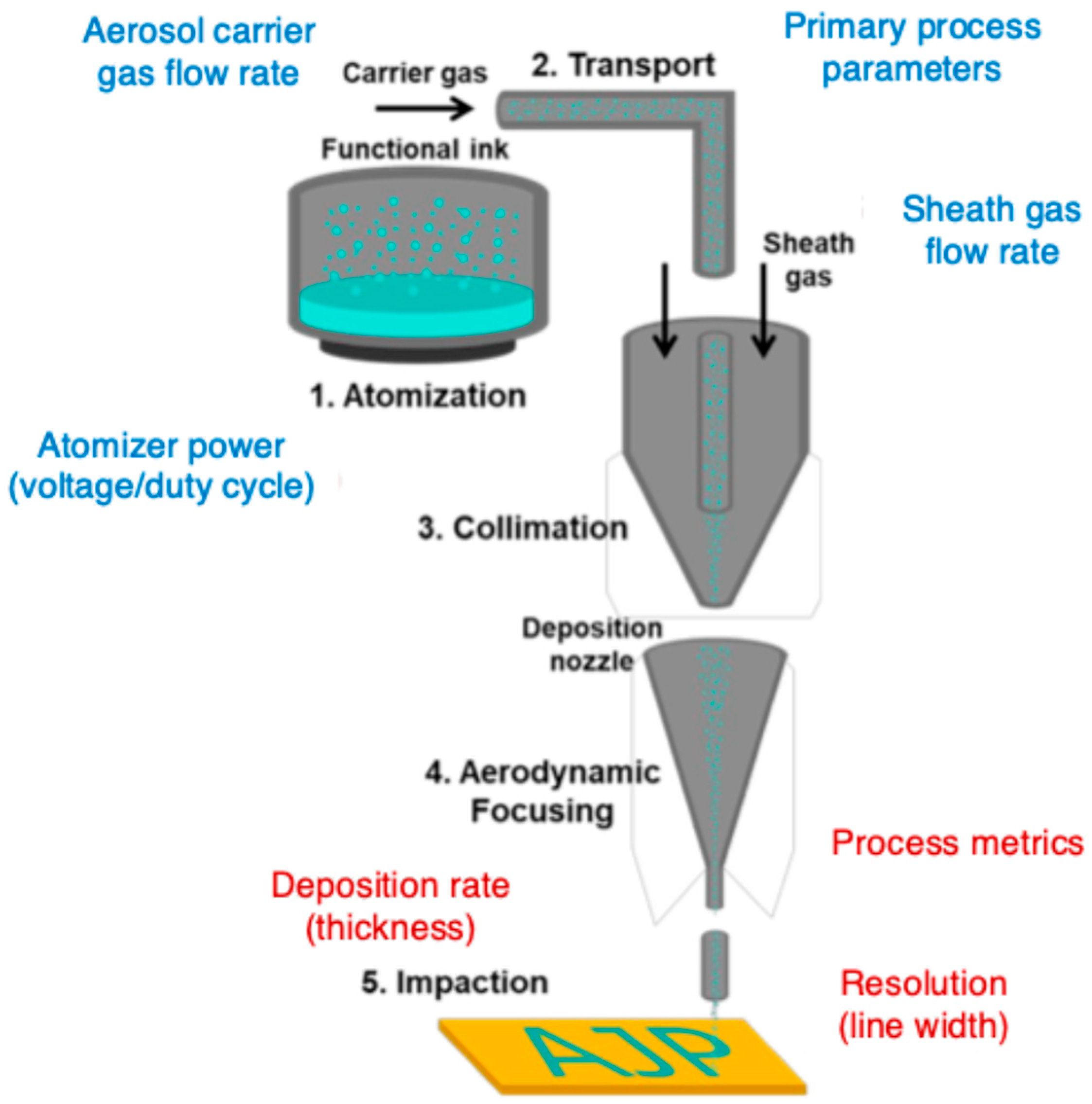
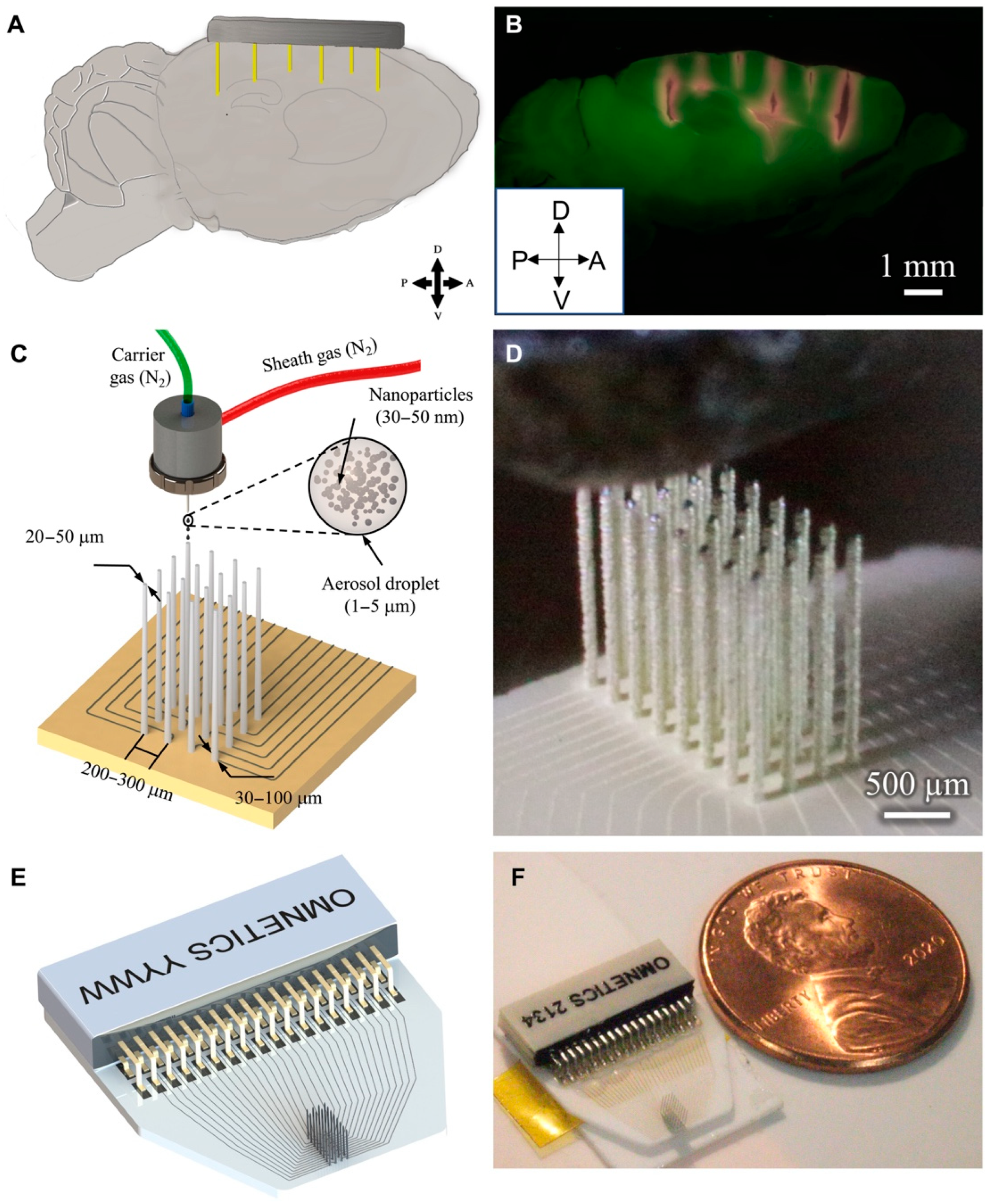
2.1.1. Mechanisms of Aerosol Generation and Deposition
2.1.2. Aerosol Generation: Atomizing Functional Materials into Micron-Sized Droplets
2.1.3. Aerosol Transport and Aerodynamic Focusing: High-Resolution Deposition
2.1.4. Material Deposition: Achieving Precision in Neuroprosthetic Applications
2.2. Materials Used in AJP for Neuroprosthetic Applications
2.2.1. Conductive Materials for Neural Interfaces and Bioelectronic Devices
2.2.2. Dielectric and Biocompatible Polymers for Insulation and Encapsulation
2.2.3. Functional Inks for Biointegrated Neuroprosthetic Systems
3. Applications of AJP in Neuroprosthetic Devices
3.1. Fabrication of Neural Interfaces
3.2. Development of Flexible and Stretchable Electronics
3.3. Creation of Microelectrode Arrays
3.4. Integration with Other Fabrication Technologies
4. Shortcomings and Challenges
4.1. Technical Challenges in AJP for Neuroprosthetic Devices
4.2. Material Compatibility and Biocompatibility
4.3. Reliability and Long-Term Stability of Printed Devices
5. Emerging Developments in AJP-Based Neuroprosthetic Devices
5.1. Technological Advances in AJP
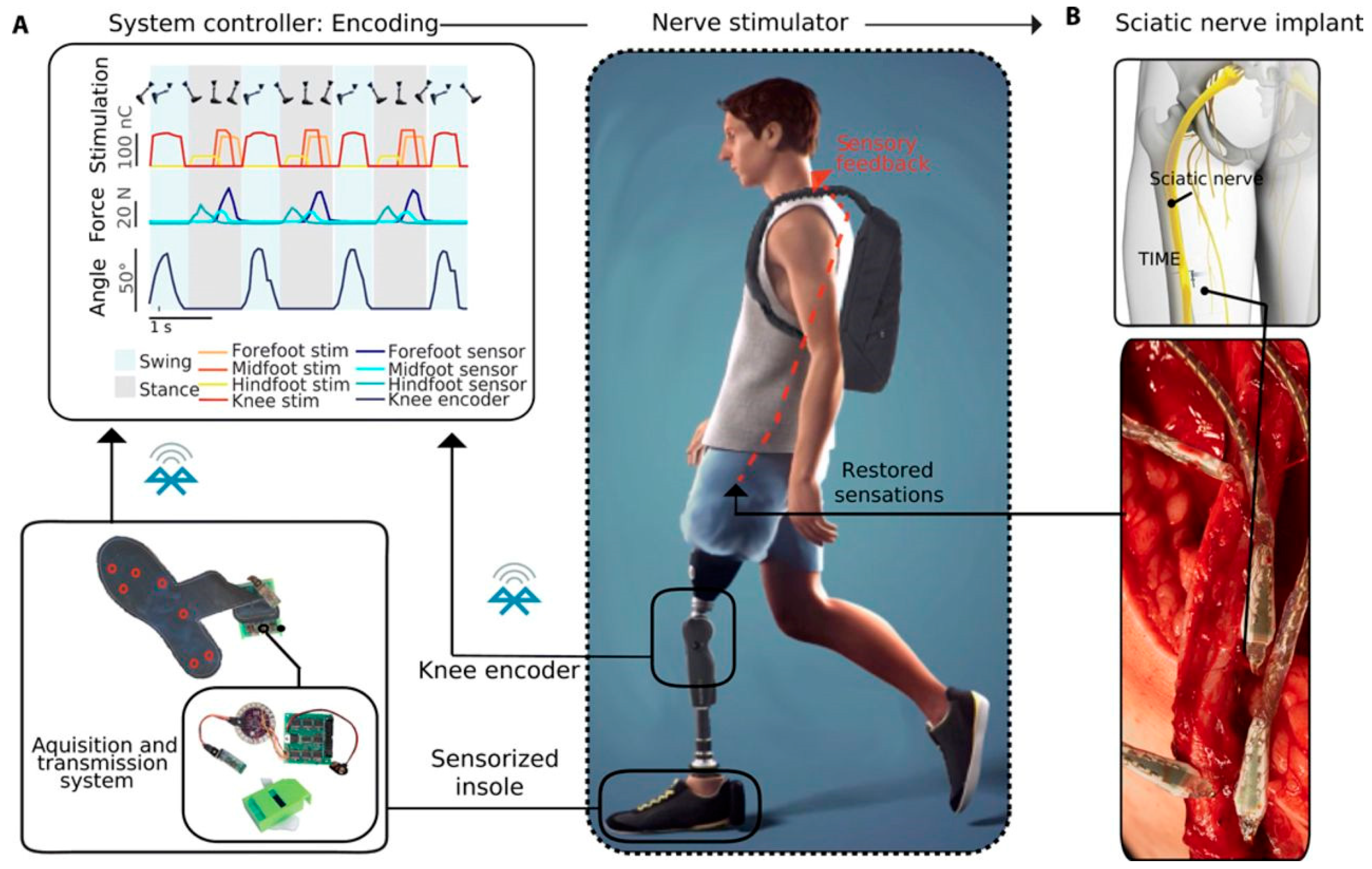
5.2. Realization of Feedback Restoration in Bionic Limbs
5.3. Integration of Biosensors for Monitoring Physiological Parameters
5.4. Control of Bionic Limbs
5.5. Prospects for Clinical Translation and Commercialization
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AJP | Aerosol Jet Printing |
| BCI | Brain–Computer Interface |
| CNT | Carbon Nanotube |
| EMA | European Medicines Agency |
| EMG | Electromyography |
| FDA | Food and Drug Administration |
| MEA | Microelectrode Array |
| MEMS | Microelectromechanical Systems |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate |
| PVDF | Polyvinylidene Fluoride |
| sEMG | Surface Electromyography |
References
- Choi, J.; Kim, S.-M.; Ryu, R.-H.; Kim, S.-P.; Sohn, J. Implantable Neural Probes for Brain-Machine Interfaces–Current Developments and Future Prospects. Exp. Neurobiol. 2018, 27, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Micera, S.; Caleo, M.; Chisari, C.; Hummel, F.C.; Pedrocchi, A. Advanced Neurotechnologies for the Restoration of Motor Function. Neuron 2020, 105, 604–620. [Google Scholar] [CrossRef]
- Gupta, A.; Vardalakis, N.; Wagner, F.B. Neuroprosthetics: From Sensorimotor to Cognitive Disorders. Commun. Biol. 2023, 6, 14. [Google Scholar] [CrossRef]
- Shokur, S.; Mazzoni, A.; Schiavone, G.; Weber, D.J.; Micera, S. A Modular Strategy for Next-Generation Upper-Limb Sensory-Motor Neuroprostheses. Med 2021, 2, 912–937. [Google Scholar] [CrossRef]
- Gadia, A.; Shah, K.; Nene, A. Emergence of Three-Dimensional Printing Technology and Its Utility in Spine Surgery. Asian Spine J. 2018, 12, 365–371. [Google Scholar] [CrossRef]
- Scholten, K.; Xu, H.; Lu, Z.; Jiang, W.; Ortigoza-Diaz, J.; Petrossians, A.; Orler, S.; Gallonio, R.; Liu, X.; Song, D.; et al. Polymer Implantable Electrode Foundry: A Shared Resource for Manufacturing Polymer-Based Microelectrodes for Neural Interfaces. bioRxiv 2023. bioRxiv:2023.11.05.565048. [Google Scholar] [CrossRef]
- Liu, X.; Chen, P.; Ding, X.; Liu, A.; Li, P.; Sun, C.; Guan, H. A Narrative Review of Cortical Visual Prosthesis Systems: The Latest Progress and Significance of Nanotechnology for the Future. Ann. Transl. Med. 2022, 10, 716. [Google Scholar] [CrossRef]
- Matta, R.; Moreau, D.; O’Connor, R. Printable Devices for Neurotechnology. Front. Neurosci. 2024, 18, 1332827. [Google Scholar] [CrossRef] [PubMed]
- Capel, A.J.; Smith, M.A.A.; Taccola, S.; Pardo-Figuerez, M.; Rimington, R.P.; Lewis, M.P.; Christie, S.D.R.; Kay, R.W.; Harris, R.A. Digitally Driven Aerosol Jet Printing to Enable Customisable Neuronal Guidance. Front. Cell Dev. Biol. 2021, 9, 722294. [Google Scholar] [CrossRef]
- Seiti, M.; Ginestra, P.S.; Ferraro, R.M.; Giliani, S.; Vetrano, R.M.; Ceretti, E.; Ferraris, E. Aerosol Jet® Printing of Poly(3,4-Ethylenedioxythiophene): Poly(Styrenesulfonate) onto Micropatterned Substrates for Neural Cells In Vitro Stimulation. Int. J. Bioprinting 2022, 8, 504. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.; Sluyter, R.; Konstantinov, K.; Kim, J.H.; Kim, S.; Kim, Y.H. Wearable and Implantable Bioelectronics as Eco-Friendly and Patient-Friendly Integrated Nanoarchitectonics for next-Generation Smart Healthcare Technology. EcoMat 2023, 5, e12356. [Google Scholar] [CrossRef]
- Wilkinson, N.J.; Smith, M.A.A.; Kay, R.W.; Harris, R.A. A Review of Aerosol Jet Printing—A Non-Traditional Hybrid Process for Micro-Manufacturing. Int. J. Adv. Manuf. Technol. 2019, 105, 4599–4619. [Google Scholar] [CrossRef]
- Fisher, C.; Skolrood, L.N.; Li, K.; Joshi, P.C.; Aytug, T. Aerosol-Jet Printed Sensors for Environmental, Safety, and Health Monitoring: A Review. Adv. Mater. Technol. 2023, 8, 2300030. [Google Scholar] [CrossRef]
- Secor, E.B. Principles of Aerosol Jet Printing. Flex. Print. Electron. 2018, 3, 035002. [Google Scholar] [CrossRef]
- Skarżyński, K.; Krzemiński, J.; Jakubowska, M.; Słoma, M. Highly Conductive Electronics Circuits from Aerosol Jet Printed Silver Inks. Sci. Rep. 2021, 11, 18141. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, S.; Fattahi, R.; Safshekan, F.; Saremi, J.; Hasanzadeh, E. Advances in 4D Bioprinting: The Next Frontier in Regenerative Medicine and Tissue Engineering Applications. Adv. Healthc. Mater. 2025, 14, e2403065. [Google Scholar] [CrossRef] [PubMed]
- Seiti, M.; Degryse, O.; Ferraris, E. Aerosol Jet® Printing 3D Capabilities for Metal and Polymeric Inks. Mater. Today Proc. 2022, 70, 38–44. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, D.; Yan, X.; Liu, X.; Sun, Q.; Yang, H.; Liu, X.; Jia, H. Organic Electrochemical Transistors: From Lithography to Large-Scale Printing. Adv. Electron. Mater. 2025, 11, 2400474. [Google Scholar] [CrossRef]
- Kudryashova, O.; Shalunov, A.; Terentiev, S.; Khmelev, V. High-Performance Acousto-Hydraulic Method for Generating Fine Aerosols for Air and Surface Disinfection. Ultrason. Sonochem. 2024, 111, 107149. [Google Scholar] [CrossRef]
- Salary, R.; Lombardi, J.P.; Weerawarne, D.L.; Rao, P.; Poliks, M.D. A Computational Fluid Dynamics Investigation of Pneumatic Atomization, Aerosol Transport, and Deposition in Aerosol Jet Printing Process. J. Micro Nano-Manuf. 2021, 9, 010903. [Google Scholar] [CrossRef]
- Chen, S.; Surendran, A.; Wu, X.; Lee, S.Y.; Stephen, M.; Leong, W.L. Recent Technological Advances in Fabrication and Application of Organic Electrochemical Transistors. Adv. Mater. Technol. 2020, 5, 2000523. [Google Scholar] [CrossRef]
- Lu, S.; Zheng, J.; Cardenas, J.A.; Williams, N.X.; Lin, Y.-C.; Franklin, A.D. Uniform and Stable Aerosol Jet Printing of Carbon Nanotube Thin-Film Transistors by Ink Temperature Control. ACS Appl. Mater. Interfaces 2020, 12, 43083–43089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ahmadi, M.; Fargas, G.; Perinka, N.; Reguera, J.; Lanceros-Méndez, S.; Llanes, L.; Jiménez-Piqué, E. Silver Nanoparticles for Conductive Inks: From Synthesis and Ink Formulation to Their Use in Printing Technologies. Metals 2022, 12, 234. [Google Scholar] [CrossRef]
- Qian, X.; Liao, C. Engineering Liquid Metal-Based Implantable Electrodes toward Brain-Machine Interfaces. Health Sci. Rev. 2023, 9, 100118. [Google Scholar] [CrossRef]
- Ramesh, S.; Xu, Z.; Rivero, I.V.; Cormier, D.R. Computational Fluid Dynamics and Experimental Validation of Aerosol Jet Printing with Multi-Stage Flow Focusing Lenses. J. Manuf. Process. 2023, 95, 312–329. [Google Scholar] [CrossRef]
- Ramesh, S.; Mahajan, C.; Gerdes, S.; Gaikwad, A.; Rao, P.; Cormier, D.R.; Rivero, I.V. Numerical and Experimental Investigation of Aerosol Jet Printing. Addit. Manuf. 2022, 59, 103090. [Google Scholar] [CrossRef]
- Tafoya, R.R.; Secor, E.B. Understanding Effects of Printhead Geometry in Aerosol Jet Printing. Flex. Print. Electron. 2020, 5, 035004. [Google Scholar] [CrossRef]
- Mosa, M.A.; Jo, J.Y.; Kwon, K.-S. Fast On-off Jet Control of Aerosol Jet Printing (AJP) Using Internal Rotary Valve. Addit. Manuf. 2023, 67, 103466. [Google Scholar] [CrossRef]
- Areias, C.; Akyurtlu, A. An Examination of Aerosol Jet-Printed Surface Roughness and Its Impact on the Performance of High-Frequency Electronics. Adv. Eng. Mater. 2025, 2402715. [Google Scholar] [CrossRef]
- Zhong, K.; Rozsa, J.; Patel, D.K.; Yao, L.; Fedder, G.K.; Islam, M.F. Aerosol Jet Printing of Superhydrophobic Surfaces. Adv. Mater. Technol. 2025, 10, 2401878. [Google Scholar] [CrossRef]
- Gori, M.; Vadalà, G.; Giannitelli, S.M.; Denaro, V.; Di Pino, G. Biomedical and Tissue Engineering Strategies to Control Foreign Body Reaction to Invasive Neural Electrodes. Front. Bioeng. Biotechnol. 2021, 9, 659033. [Google Scholar] [CrossRef] [PubMed]
- Cutrone, A.; Micera, S. Implantable Neural Interfaces and Wearable Tactile Systems for Bidirectional Neuroprosthetics Systems. Adv. Healthc. Mater. 2019, 8, 1801345. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Q.; Yang, L.; Cui, Y. Materials and Biomedical Applications of Implantable Electronic Devices. Adv. Mater. Technol. 2023, 8, 2200853. [Google Scholar] [CrossRef]
- Kim, T.; Cho, M.; Yu, K.J. Flexible and Stretchable Bio-Integrated Electronics Based on Carbon Nanotube and Graphene. Materials 2018, 11, 1163. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Farzad, R.; Tamayol, A.; Manoharan, V.; Mostafalu, P.; Zhang, Y.S.; Akbari, M.; Jung, S.M.; Kim, D.; Commotto, M.; et al. A Bioactive Carbon Nanotube-Based Ink for Printing 2D and 3D Flexible Electronics. Adv. Mater. 2016, 28, 3280–3289. [Google Scholar] [CrossRef]
- Kostarelos, K.; Vincent, M.; Hebert, C.; Garrido, J.A. Graphene in the Design and Engineering of Next-Generation Neural Interfaces. Adv. Mater. 2017, 29, 1700909. [Google Scholar] [CrossRef]
- Wang, M.; Mi, G.; Shi, D.; Bassous, N.; Hickey, D.; Webster, T.J. Nanotechnology and Nanomaterials for Improving Neural Interfaces. Adv. Funct. Mater. 2018, 28, 1700905. [Google Scholar] [CrossRef]
- Ferlauto, L.; D’Angelo, A.N.; Vagni, P.; Airaghi Leccardi, M.J.I.; Mor, F.M.; Cuttaz, E.A.; Heuschkel, M.O.; Stoppini, L.; Ghezzi, D. Development and Characterization of PEDOT:PSS/Alginate Soft Microelectrodes for Application in Neuroprosthetics. Front. Neurosci. 2018, 12, 648. [Google Scholar] [CrossRef]
- Go, G.-T.; Lee, Y.; Seo, D.-G.; Lee, T.-W. Organic Neuroelectronics: From Neural Interfaces to Neuroprosthetics. Adv. Mater. 2022, 34, 2201864. [Google Scholar] [CrossRef]
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and Technologies for Soft Implantable Neuroprostheses. Nat. Rev. Mater. 2016, 1, 16063. [Google Scholar] [CrossRef]
- Mariello, M.; Kim, K.; Wu, K.; Lacour, S.P.; Leterrier, Y. Recent Advances in Encapsulation of Flexible Bioelectronic Implants: Materials, Technologies, and Characterization Methods. Adv. Mater. 2022, 34, 2201129. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Jo, J.-W.; Kim, J.; Park, S.K. Stretchable Electronics Based on Inorganic Semiconducting Materials. J. Mater. Chem. C 2024, 12, 19323–19351. [Google Scholar] [CrossRef]
- Novikov, A.; Goding, J.; Chapman, C.; Cuttaz, E.; Green, R.A. Stretchable Bioelectronics: Mitigating the Challenges of the Percolation Threshold in Conductive Elastomers. APL Mater. 2020, 8, 101105. [Google Scholar] [CrossRef]
- Caldwell, R.; Mandal, H.; Sharma, R.; Solzbacher, F.; Tathireddy, P.; Rieth, L. Analysis of Al2O3—Parylene C Bilayer Coatings and Impact of Microelectrode Topography on Long Term Stability of Implantable Neural Arrays. J. Neural Eng. 2017, 14, 046011. [Google Scholar] [CrossRef]
- Gogoi, D.; Kumar, M.; Singh, J. A Comprehensive Review on Hydrogel-Based Bio-Ink Development for Tissue Engineering Scaffolds Using 3D Printing. Ann. 3D Print. Med. 2024, 15, 100159. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and Novel Polymeric Biomaterials for Neural Tissue Engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef]
- Liao, W.; Shi, Y.; Li, Z.; Yin, X. Advances in 3D Printing Combined with Tissue Engineering for Nerve Regeneration and Repair. J. Nanobiotechnol. 2025, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Goos, R.; Weerdt, J.D.; Pelgrims, P.; Ferraris, E. Design, Fabrication, and Testing of a Fully 3D-Printed Pressure Sensor Using a Hybrid Printing Approach. Sensors 2022, 22, 7531. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sekine, T.; Miura, R.; Abe, M.; Shouji, Y.; Ito, K.; Wang, Y.-F.; Hong, J.; Takeda, Y.; Kumaki, D.; et al. Optimization of a Soft Pressure Sensor in Terms of the Molecular Weight of the Ferroelectric-Polymer Sensing Layer. Adv. Funct. Mater. 2022, 32, 2107434. [Google Scholar] [CrossRef]
- Singer, A.; Dutta, S.; Lewis, E.; Chen, Z.; Chen, J.C.; Verma, N.; Avants, B.; Feldman, A.K.; O’Malley, J.; Beierlein, M.; et al. Magnetoelectric Materials for Miniature, Wireless Neural Stimulation at Therapeutic Frequencies. Neuron 2020, 107, 631–643.e5. [Google Scholar] [CrossRef]
- Yu, W.; Cai, P.J.; Liu, R.; Shen, F.P.; Zhang, T. A Flexible Ultrasensitive IgG-Modified rGO-Based FET Biosensor Fabricated by Aerosol Jet Printing. Appl. Mech. Mater. 2015, 748, 157–161. [Google Scholar] [CrossRef]
- Ma, T.; Li, Y.; Cheng, H.; Niu, Y.; Xiong, Z.; Li, A.; Jiang, X.; Park, D.; Zhang, K.; Yi, C. Enhanced Aerosol-Jet Printing Using Annular Acoustic Field for High Resolution and Minimal Overspray. Nat. Commun. 2024, 15, 6317. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.S.; Ritchie, S.M.; Nicholas, M.A.; Gordon, H.L.; Hu, C.; Jahan, S.; Yuan, B.; Bezbaruah, R.; Reddy, J.W.; Ahmed, Z.; et al. CMU Array: A 3D Nanoprinted, Fully Customizable High-Density Microelectrode Array Platform. Sci. Adv. 2022, 8, eabj4853. [Google Scholar] [CrossRef]
- Dijk, G.; Pas, J.; Markovic, K.; Scancar, J.; O’Connor, R.P. PEDOT:PSS-Coated Platinum Electrodes for Neural Stimulation. APL Bioeng. 2023, 7, 046117. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Lee, Y.J.; Kim, K.R.; Kim, J.-H.; Yeo, W.-H. Advances in Materials, Sensors, and Integrated Systems for Monitoring Eye Movements. Biosensors 2022, 12, 1039. [Google Scholar] [CrossRef]
- Ganesh, S.; Sarreal, R.R.S.; Blake, D.; Tridandapani, S.; Bhatti, P.T. An Aerosol Jet Printed Microcoil for Cochlear Micromagnetic Stimulation. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 15–19 July 2024; pp. 1–5. [Google Scholar]
- Sarreal, R.R.; Bhatti, P. Characterization and Miniaturization of Silver-Nanoparticle Microcoil via Aerosol Jet Printing Techniques for Micromagnetic Cochlear Stimulation. Sensors 2020, 20, 6087. [Google Scholar] [CrossRef]
- Yu, C.-S.; Chen, S.-Y.; Lin, J.-S.; Chen, Y.-Y.; Huang, W.-C. Highly Hydroresponsive Nacre-like Oligo Proanthocyanidin-Intercalated Ca–Al-Layered Double Hydroxides/Graphene Oxide/Polyvinyl Alcohol as a Potential Neural Implant Material. J. Mater. Res. Technol. 2021, 15, 595–605. [Google Scholar] [CrossRef]
- Lee, Y.; Tian, X.; Park, J.; Nam, D.H.; Wu, Z.; Choi, H.; Kim, J.; Park, D.-W.; Zhou, K.; Lee, S.W.; et al. Rapidly Self-Healing Electronic Skin for Machine Learning–Assisted Physiological and Movement Evaluation. Sci. Adv. 2025, 11, eads1301. [Google Scholar] [CrossRef]
- Williams, N.X.; Watson, N.; Joh, D.Y.; Chilkoti, A.; Franklin, A.D. Aerosol Jet Printing of Biological Inks by Ultrasonic Delivery. Biofabrication 2020, 12, 025004. [Google Scholar] [CrossRef]
- Perilli, S.; Di Pietro, M.; Mantini, E.; Regazzetti, M.; Kiper, P.; Galliani, F.; Panella, M.; Mantini, D. Development of a Wearable Electromyographic Sensor with Aerosol Jet Printing Technology. Bioengineering 2024, 11, 1283. [Google Scholar] [CrossRef]
- Park, J.; Seong, D.; Choi, H.; Lee, J.; Song, J.; Shin, M.; Son, D. A Nerve-Adhesive Stretchable Electrode for Stable Neural Signal Recording and Stimulation. MRS Bull. 2025, 50, 9–19. [Google Scholar] [CrossRef]
- Cantù, E.; Fapanni, T.; Giorgi, G.; Narduzzi, C.; Sardini, E.; Serpelloni, M.; Tonello, S. Printed Multi-EMG Electrodes on the 3D Surface of an Orthosis for Rehabilitation: A Feasibility Study. IEEE Sens. J. 2021, 21, 14407–14417. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J.H.; Kim, S.; Han, S.; Moon, H.; Song, J.-Y.; Park, A.-Y. Optimization of Process Parameters in Micro-Scale Pneumatic Aerosol Jet Printing for High-Yield Precise Electrodes. Sci. Rep. 2023, 13, 21297. [Google Scholar] [CrossRef]
- Ding, J.; Zeng, M.; Tian, Y.; Chen, Z.; Qiao, Z.; Xiao, Z.; Wu, C.; Wei, D.; Sun, J.; Fan, H. Flexible Silk-Fibroin-Based Microelectrode Arrays for High-Resolution Neural Recording. Mater. Horiz. 2024, 11, 4338–4347. [Google Scholar] [CrossRef]
- Zeng, Q.; Yu, S.; Fan, Z.; Huang, Y.; Song, B.; Zhou, T. Nanocone-Array-Based Platinum-Iridium Oxide Neural Microelectrodes: Structure, Electrochemistry, Durability and Biocompatibility Study. Nanomaterials 2022, 12, 3445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, T.; Jiao, J.; Song, S.; Wang, Z.; Wang, Z. Experimental and Numerical Investigation on the Aerosol Micro-Jet 3D Printing of Flexible Electronic Devices. Materials 2023, 16, 7099. [Google Scholar] [CrossRef]
- Armando, I.; Borghetti, M.; Sardini, E.; Serpelloni, M. A Feasibility Study of Customized and Fully Aerosol-Jet-Printed Micro-Electrode Arrays for In Vitro Application. IEEE Sens. J. 2023, 23, 24205–24213. [Google Scholar] [CrossRef]
- Ali, M.A.; Hu, C.; Yttri, E.A.; Panat, R. Recent Advances in 3D Printing of Biomedical Sensing Devices. Adv. Funct. Mater. 2022, 32, 2107671. [Google Scholar] [CrossRef]
- Rao, C.H.; Avinash, K.; Varaprasad, B.K.S.V.L.; Goel, S. A Review on Printed Electronics with Digital 3D Printing: Fabrication Techniques, Materials, Challenges and Future Opportunities. J. Electron. Mater. 2022, 51, 2747–2765. [Google Scholar] [CrossRef]
- Smith, M.; Fry, N.; Kay, R.; Harris, R. Digitally-Driven Micro Surface Patterning by Hybrid Manufacturing. In Proceedings of the 29th Annual International Solid Freeform Fabrication Symposium 2018: An Additive Manufacturing Conference, Austin, TX, USA, 13–15 August 2018. [Google Scholar]
- Green, R.; Abidian, M.R. Conducting Polymers for Neural Prosthetic and Neural Interface Applications. Adv. Mater. 2015, 27, 7620–7637. [Google Scholar] [CrossRef]
- Goh, G.D.; Wong, K.K.; Tan, N.; Seet, H.L.; Nai, M.L.S. Large-Format Additive Manufacturing of Polymers: A Review of Fabrication Processes, Materials, and Design. Virtual Phys. Prototyp. 2024, 19, e2336160. [Google Scholar] [CrossRef]
- Venturi, F.; Taylor, R. Additive Manufacturing in the Context of Repeatability and Reliability. J. Mater. Eng. Perform. 2023, 32, 6589–6609. [Google Scholar] [CrossRef]
- Vinchurkar, K.; Bukke, S.P.N.; Jain, P.; Bhadoria, J.; Likhariya, M.; Mane, S.; Suryawanshi, M.; Veerabhadrappa, K.V.; Eftekhari, Z.; Onohuean, H. Advances in Sustainable Biomaterials: Characterizations, and Applications in Medicine. Discov. Polym. 2025, 2, 2. [Google Scholar] [CrossRef]
- Agarwala, S.; Goh, G.L.; Yeong, W.Y. Optimizing Aerosol Jet Printing Process of Silver Ink for Printed Electronics. IOP Conf. Ser. Mater. Sci. Eng. 2017, 191, 012027. [Google Scholar] [CrossRef]
- Airaghi Leccardi, M.J.I.; Vagni, P.; Ghezzi, D. Multilayer 3D Electrodes for Neural Implants. J. Neural. Eng. 2019, 16, 026013. [Google Scholar] [CrossRef]
- Obata, K.; Schonewille, A.; Slobin, S.; Hohnholz, A.; Unger, C.; Koch, J.; Suttmann, O.; Overmeyer, L. Hybrid 2D Patterning Using UV Laser Direct Writing and Aerosol Jet Printing of UV Curable Polydimethylsiloxane. Appl. Phys. Lett. 2017, 111, 121903. [Google Scholar] [CrossRef]
- Hohnholz, A.; Obata, K.; Nakajima, Y.; Koch, J.; Terakawa, M.; Suttmann, O.; Overmeyer, L. Hybrid UV Laser Direct Writing of UV-Curable PDMS Thin Film Using Aerosol Jet Printing. Appl. Phys. A 2019, 125, 120. [Google Scholar] [CrossRef]
- Feng, J.; Klett, J.D.; Renn, M.J. Mist Generation Behavior in Ultrasonic Atomizer for Aerosol Jet® Printing. Aerosol Sci. Eng. 2024, 8, 77–86. [Google Scholar] [CrossRef]
- Rurup, J.D.; Secor, E.B. A Real-Time Process Diagnostic to Support Reliability, Control, and Fundamental Understanding in Aerosol Jet Printing. Adv. Eng. Mater. 2024, 26, 2301348. [Google Scholar] [CrossRef]
- Rurup, J.D.; Secor, E.B. Predicting Deposition Rate and Closing the Loop on Aerosol Jet Printing with In-Line Light Scattering Measurements. Adv. Eng. Mater. 2023, 25, 2201919. [Google Scholar] [CrossRef]
- Page, D.M.; George, J.A.; Kluger, D.T.; Duncan, C.; Wendelken, S.; Davis, T.; Hutchinson, D.T.; Clark, G.A. Motor Control and Sensory Feedback Enhance Prosthesis Embodiment and Reduce Phantom Pain After Long-Term Hand Amputation. Front. Hum. Neurosci. 2018, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Zangrandi, A.; D’Alonzo, M.; Cipriani, C.; Di Pino, G. Neurophysiology of Slip Sensation and Grip Reaction: Insights for Hand Prosthesis Control of Slippage. J. Neurophysiol. 2021, 126, 477–492. [Google Scholar] [CrossRef]
- Pandarinath, C.; Bensmaia, S.J. The Science and Engineering behind Sensitized Brain-Controlled Bionic Hands. Physiol. Rev. 2022, 102, 551–604. [Google Scholar] [CrossRef] [PubMed]
- Petrini, F.M.; Valle, G.; Bumbasirevic, M.; Barberi, F.; Bortolotti, D.; Cvancara, P.; Hiairrassary, A.; Mijovic, P.; Sverrisson, A.Ö.; Pedrocchi, A.; et al. Enhancing Functional Abilities and Cognitive Integration of the Lower Limb Prosthesis. Sci. Transl. Med. 2019, 11, eaav8939. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, E.; Naseri, Z.; Deigner, H.-P.; Rahimi, H.; Altintas, Z. Approaches of Wearable and Implantable Biosensor towards of Developing in Precision Medicine. Front. Med. 2024, 11, 1390634. [Google Scholar] [CrossRef]
- Herbert, R.; Mishra, S.; Lim, H.-R.; Yoo, H.; Yeo, W.-H. Fully Printed, Wireless, Stretchable Implantable Biosystem toward Batteryless, Real-Time Monitoring of Cerebral Aneurysm Hemodynamics. Adv. Sci. 2019, 6, 1901034. [Google Scholar] [CrossRef]
- Canepa, M.; Marucchi, L.; Boccardo, N.; Marinelli, A.; Domenico, D.D.; Gandolla, M.; Laffranchi, M.; Castellini, C. Towards a Real-Time, Interactive, Incremental Learning Algorithm for Prosthetic Myocontrol. In Proceedings of the 2025 International Conference on Rehabilitation Robotics (ICORR), Chicago, IL, USA, 12–16 May 2025. [Google Scholar]
| Technique | Resolution | Material Compatibility | Flexibility | Biocompatibility | Manufacturers |
|---|---|---|---|---|---|
| Aerosol Jet Printing | ~10 µm | Conductive inks, polymers, carbon-based materials, hydrogels | High (prints on flexible/stretchable substrates) | High (supports biocompatible materials) | Optomec, Neotech AMT |
| Inkjet Printing | ~20–50 µm | Limited to low-viscosity inks, fewer biocompatible materials | Moderate (mostly for flexible electronics) | Moderate (some conductive inks need post-treatment) | Dimatix (Fujifilm), MicroFab |
| Photolithography | <1 µm | Metals, semiconductors, dielectrics | Low (requires rigid substrates) | High (if using biocompatible coatings) | SUSS MicroTec, EV Group |
| MEMS-based methods | <1 µm | Silicon-based materials, metals, polymers | Low (mainly rigid structures) | High (used in implantable neuroprosthetic devices) | Bosch, IMEC (custom foundries) |
| Laser-based printing | <1 µm | Optically responsive inks, limited bioinks | Low to moderate (mostly flat surfaces) | Variable (risk of thermal damage) | Luxexcel, InnoLaser, LPKF |
| Material Category | Examples | Function in Neuroprosthetic Devices |
|---|---|---|
| Conductive materials | Silver, gold, platinum, copper | High conductivity, corrosion resistance, used in neuroelectrodes and interconnects |
| Carbon nanotubes (CNTs), graphene | High flexibility, low impedance, improved signal transduction | |
| PEDOT:PSS (Conductive polymers) | Biocompatible, flexible, used for neural recording and stimulation | |
| Dielectric & biocompatible polymers | Polyimides | Flexible substrates for neural interfaces |
| Silicones | Encapsulation layers, long-term biostability | |
| Parylene | Moisture-resistant coating, chemical stability for implantable devices | |
| Functional inks | Bioactive/biomimetic Inks | Promote neural adhesion, growth, and differentiation |
| Piezoelectric polymers (PVDF) | Convert mechanical input into electrical signals or vice versa for sensing applications | |
| Magnetostrictive materials | Enable wireless neural stimulation using magnetic fields | |
| Biosensing materials | Monitor neurochemical activity, glucose levels, and inflammation for real-time feedback |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Waele, L.; Di Pietro, M.; Perilli, S.; Mantini, E.; Trevisan, G.; Simoncini, M.; Panella, M.; Betti, V.; Laffranchi, M.; Mantini, D. Aerosol Jet Printing for Neuroprosthetic Device Development. Bioengineering 2025, 12, 707. https://doi.org/10.3390/bioengineering12070707
De Waele L, Di Pietro M, Perilli S, Mantini E, Trevisan G, Simoncini M, Panella M, Betti V, Laffranchi M, Mantini D. Aerosol Jet Printing for Neuroprosthetic Device Development. Bioengineering. 2025; 12(7):707. https://doi.org/10.3390/bioengineering12070707
Chicago/Turabian StyleDe Waele, Lander, Massimo Di Pietro, Stefano Perilli, Emanuele Mantini, Giulio Trevisan, Michela Simoncini, Massimo Panella, Viviana Betti, Matteo Laffranchi, and Dante Mantini. 2025. "Aerosol Jet Printing for Neuroprosthetic Device Development" Bioengineering 12, no. 7: 707. https://doi.org/10.3390/bioengineering12070707
APA StyleDe Waele, L., Di Pietro, M., Perilli, S., Mantini, E., Trevisan, G., Simoncini, M., Panella, M., Betti, V., Laffranchi, M., & Mantini, D. (2025). Aerosol Jet Printing for Neuroprosthetic Device Development. Bioengineering, 12(7), 707. https://doi.org/10.3390/bioengineering12070707











