Heterologous Production of the Structurally Complex Diterpenoid Forskolin in Synechocystis sp. PCC. 6803
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Starter Cultures
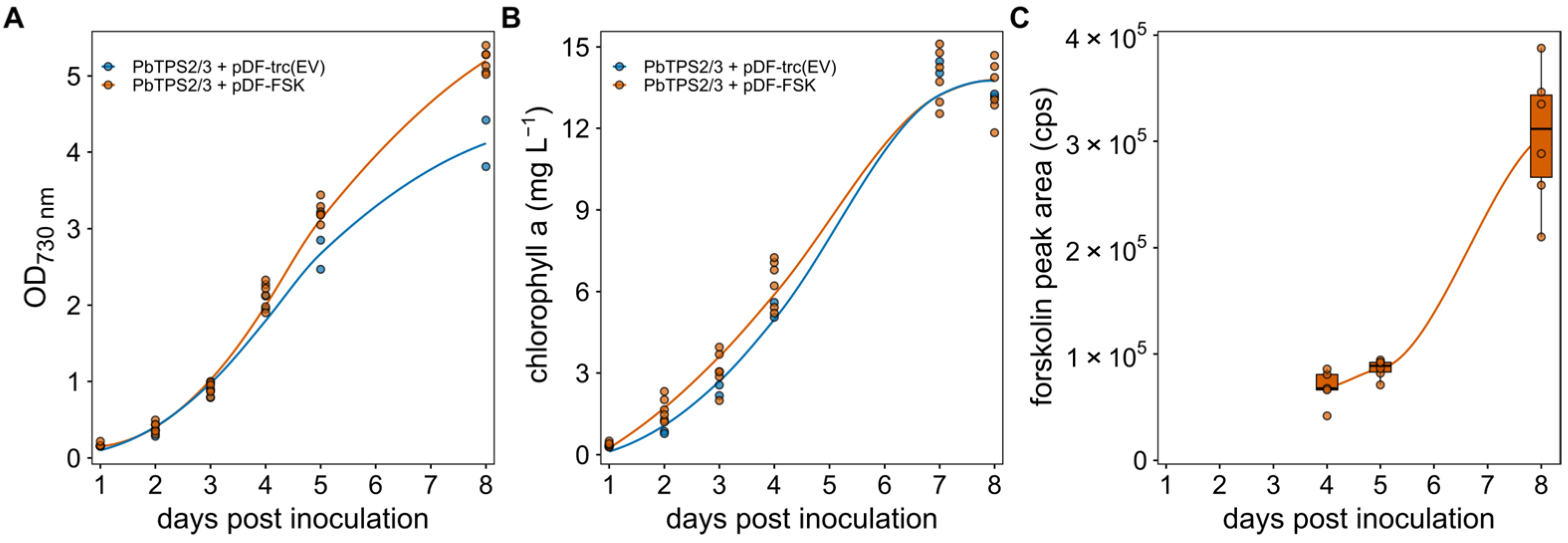
2.3. Photobioreactor Cultures
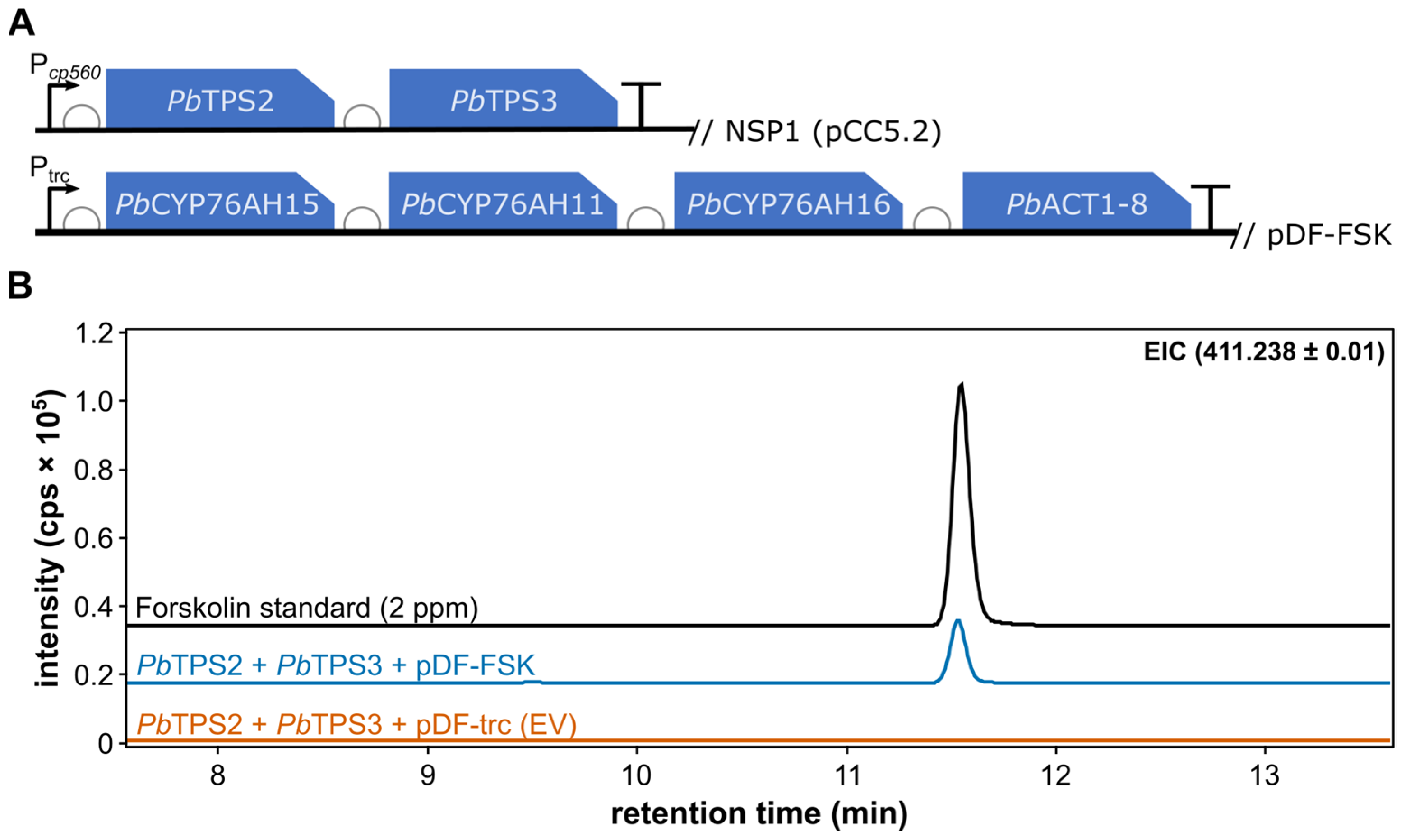
2.4. Cloning and Transformation
2.5. Quantification of Forskolin
2.6. Chlorophyll Extraction and Quantification
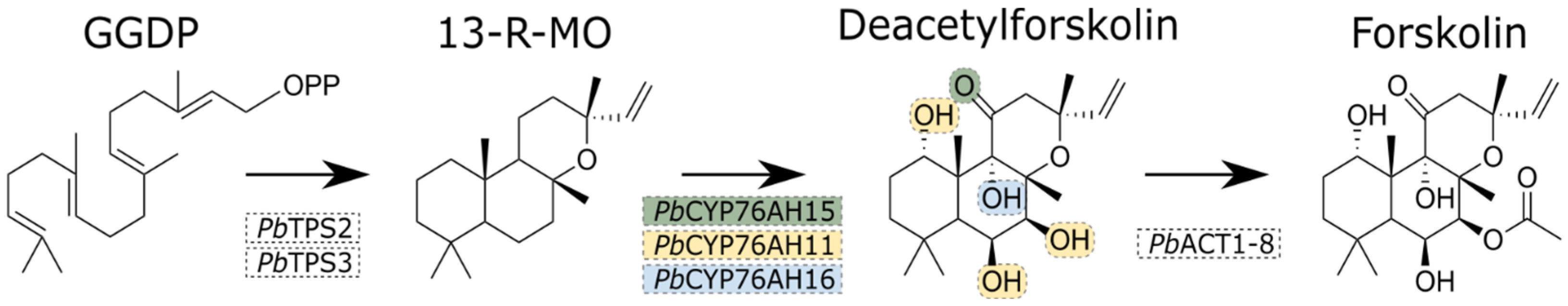
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klaus, O.; Hilgers, F.; Nakielski AHasenklever, D.; Jaeger, K.-E.; Axmann, I.M.; Drepper, T. Engineering phototrophic bacteria for the production of terpenoids. Curr. Opin. Biotechnol. 2022, 77, 102764. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Wang, F.; Chen, G.Q.; Zhang, H.B.; Xian, M. Biosynthesis of natural rubber: Current state and perspectives. Int. J. Mol. Sci. 2019, 20, 50. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Abdel-Ghany, S.E.; Ali, G.S. Genome editing approaches: Manipulating of lovastatin and taxol synthesis of filamentous fungi by CRISPR/Cas9 system. Appl. Microbiol. Biotechnol. 2017, 101, 3953–3976. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.; Andersen-Ranberg JHankamer, B.; Møller, B.L. Circular biomanufacture through harvesting solar energy and CO2. Trends Plant Sci. 2022, 27, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Pateraki, I.; Andersen-Ranberg, J.; Hamberger Britta Heskes, A.M.; Martens, H.J.; Zerbe, P.; Bach, S.S.; Møller, B.L.; Bohlmann, J.; Hamberger, B. Manoyl oxide (13R), the biosynthetic precursor of forskolin, is synthesized in specialized root cork cells in Coleus forskohlii. Plant Physiol. 2014, 164, 1222–1236. [Google Scholar] [CrossRef]
- Pateraki, I.; Andersen-Ranberg, J.; Jensen, N.B.; Wubshet, S.G.; Heskes, A.M.; Forman, V.; Hallström, B.; Hamberger Britta Motawia, M.S.; Olsen, C.E.; Staerk, D.; et al. Total biosynthesis of the cyclic AMP booster forskolin from Coleus forskohlii. eLife 2017, 6, e23001. [Google Scholar] [CrossRef]
- Forman, V.; Luo, D.; Lemcke, R.; Nelson, D.R.; Staerk, D.; Kampranis, S.; Møller, B.L.; Pateraki, I. A gene cluster in Ginkgo biloba encoding for unique multifunctional cytochrome P450s orchestrates key steps in ginkgolide biosynthesis. Nat. Commun. 2022, 13, 5143. [Google Scholar] [CrossRef]
- Hansen, N.L.; Kjærulff LHeck, Q.; Forman, V.; Stærk, D.; Møller, B.L.; Andersen-Ranberg, J. Tripterygium wilfordii cytochrome P450s catalyze the key methyl shift and epoxidations in biosynthesis of triptonide. Nat. Commun. 2022, 13, 5011. [Google Scholar] [CrossRef]
- Pattanaik, B.; Lindberg, P. Terpenoids and their biosynthesis in cyanobacteria. Life 2015, 5, 269–293. [Google Scholar] [CrossRef]
- Mendoza-Poudereux, I.; Kutzner, E.; Huber, C.; Segura, J.; Eisenreich, W.; Arrillaga, I. Metabolic cross-talk between pathways of terpenoid backbone biosynthesis in spike lavender. Plant Physiol. Biochem. 2015, 95, 113–120. [Google Scholar] [CrossRef]
- Rohmer, M. Mevalonate-independent methylerythritol phosphate pathway for isoprenoid biosynthesis. Elucidation and distribution. Pure Appl. Chem. 2003, 75, 375–387. [Google Scholar] [CrossRef]
- Bathe, U.; Tissier, A. Cytochrome P450 enzymes: A driving force of plant diterpene diversity. Phytochemistry 2019, 161, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Jensen, P.E.; Møller, B.L. Light-driven chemical synthesis. Trends Plant Sci. 2012, 17, 1360–1385. [Google Scholar] [CrossRef]
- Jensen, K.; Møller, B.L. Plant NADPH-cytochrome P450 0xidoreductases. Phytochemistry 2010, 71, 132–141. [Google Scholar] [CrossRef]
- Jensen, K.; Jensen, P.E.; Møller, B.L. Light-driven cytochrome P450 hydroxylations. ACS Chem. Biol. 2011, 6, 533–539. [Google Scholar] [CrossRef]
- Jensen, S.B.; Thodberg, S.; Parween, S.; Moses, M.E.; Hansen, C.C.; Thomsen, J.; Sletfjerding, M.B.; Knudsen, C.; Giudice, R.D.; Lund, P.M.; et al. Biased cytochrome P450-mediated metabolism via small-molecule ligands binding P450 oxidoreductase. Nat. Commun. 2021, 12, 2260. [Google Scholar] [CrossRef]
- Liu, D.; Liberton, M.; Hendry, J.I.; Aminian-Dehkordi, J.; Maranas, C.D.; Pakrasi, H.B. Engineering biology approaches for food and nutrient production by cyanobacteria. Curr. Opin. Biotechnol. 2020, 67, 1–6. [Google Scholar] [CrossRef]
- Melis, A.; Martinez, D.A.H.; Betterle, N. Perspectives of cyanobacterial cell factories. Photosynth. Res. 2023, 162, 459–471. [Google Scholar] [CrossRef]
- Satta AEsquirol, L.; Ebert, B.E. Current Metabolic Engineering Strategies for Photosynthetic Bioproduction in Cyanobacteria. Microorganisms 2023, 11, 455. [Google Scholar] [CrossRef]
- Dietsch, M.; Behle, A.; Westhoff, P.; Axmann, I.M. Metabolic engineering of Synechocystis sp. PCC 6803 for the photoproduction of the sesquiterpene valencene. Metab. Eng. Commun. 2021, 13, e00178. [Google Scholar] [CrossRef]
- Bolay, P.; Dodge, N.; Janssen, K.; Jensen, P.E.; Lindberg, P. Tailoring regulatory components for metabolic engineering in cyanobacteria. Physiol. Plantarum. 2024, 176, e14316. [Google Scholar] [CrossRef] [PubMed]
- Kukil, K.; Lindberg, P. Metabolic engineering of Synechocystis sp. PCC 6803 for the improved production of phenylpropanoids. Microb. Cell Factories 2024, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring, and Management; Chorus, I., Bertram, J., Eds.; World Health Organization: Geneva, Switzerland, 1999; ISBN 0419239308. [Google Scholar]
- Mellor, S.B.; Vinde, M.H.; Nielsen, A.Z.; Hanke, G.T.; Abdiaziz, K.; Roessler, M.M.; Burow, M.; Motawia, M.S.; Møller, B.L.; Jensen, P.E. Defining optimal electron transfer partners for light-driven cytochrome P450 reactions. Metab. Eng. 2019, 55, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.Z.; Ziersen, B.; Jensen, K.; Lassen, L.M.; Olsen, C.E.; Møller, B.L.; Jensen, P.E. Redirecting Photosynthetic Reducing Power toward Bioactive Natural Product Synthesis. ACS Synth. Biol. 2013, 2, 308–315. [Google Scholar] [CrossRef]
- Wlodarczyk, A.; Gnanasekaran, T.; Nielsen, A.Z.; Zulu, N.N.; Mellor, S.B.; Luckner, M.; Thøfner, J.F.B.; Olsen, C.E.; Mottawie, M.S.; Burow, M.; et al. Metabolic engineering of light-driven cytochrome P450 dependent pathways into Synechocystis sp. PCC 6803. Metab. Eng. 2016, 33, 1–11. [Google Scholar] [CrossRef]
- Berepiki, A.; Hitchcock, A.; Moore, C.M.; Bibby, T.S. Tapping the unused potential of photosynthesis with a heterologous electron sink. ACS Synth. Biol. 2016, 5, 1369–1375. [Google Scholar] [CrossRef]
- Berepiki, A.; Gittins, J.R.; Moore, C.M.; Bibby, T.S. Rational engineering of photosynthetic electron flux enhances light-powered cytochrome P450 activity. Synth. Biol. 2018, 3, ysy009. [Google Scholar] [CrossRef]
- Santos-Merino, M.; Torrado, A.; Davis, G.A.; Röttig, A.; Bibby, T.S.; Kramer, D.M.; Ducat, D.C. Improved photosynthetic capacity and photoprotection via heterologous metabolism engineering in cyanobacteria. Proc. Natl. Acad. Sci. USA 2020, 118, e20215231. [Google Scholar] [CrossRef]
- Teicher, H.B.; Møller, B.L.; Scheller, H.V. Photoinhibition of Photosystem I in field-grown barley (Hordeum vulgare L.): Induction, recovery and acclimation. Photosynth. Res. 2000, 64, 53–61. [Google Scholar] [CrossRef]
- Alasbahi, R.H.; Melzig, M.F. Forskolin and derivatives as tools for studying the role of cAMP. Pharmazie 2012, 67, 5–13. [Google Scholar] [CrossRef]
- D’Orazio, J.A.; Nobuhisa, T.; Cui, R.; Arya, M.; Spry, M.; Wakamatsu, K.; Igras, V.; Kunisada, T.; Granter, S.R.; Nishimura, E.K.; et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature 2006, 443, 340–344. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Vaidyanathan, P.; Karri, S.K.; Jose, J.A. Efficacy and safety of 1% forskolin eye drops in open angle glaucoma—An open label study. Saudi J. Ophthalmol. 2015, 29, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Mulieri, L.A.; Leavitt, B.J.; Martin, B.J.; Haeberle, J.R.; Alpert, N.R. Myocardial force-frequency defect in mitral regurgitation heart failure is reversed by forskolin. Circulation 1993, 88, 2700–2704. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, F.; Carbonell, V.; Cossu, M.; Correddu, D.; Jones, P.R. Ethylene Synthesis and Regulated Expression of Recombinant Protein in Synechocystis sp. PCC 6803. PLoS ONE 2012, 7, e50470. [Google Scholar] [CrossRef]
- Ng, A.H.; Berla, B.M.; Pakrasi, H.B. 2015. Fine-tuning of photoautotrophic protein production by combining promoters and neutral sites in the cyanobacterium Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 2015, 81, 6857–6863. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Forman, V.; Bjerg-Jensen, N.; Dyekjær, J.D.; Møller, B.L.; Pateraki, I. Engineering of CYP76AH15 can improve activity and specificity towards forskolin biosynthesis in yeast. Microb. Cell Factories 2018, 17, 181. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Meng, H.; Zhu, Y.; Bao, G.; Zhang, Y.; Li, Y.; Ma, Y. Discovery of a super-strong promoter enables efficient production of heterologous proteins in cyanobacteria. Sci. Rep. 2014, 4, 4500. [Google Scholar] [CrossRef]
- Sutradja, L.C.; Dodge, N.; Walby, S.L.; Butler, N.J.; Gnanasekaran, T.; Møller, B.L.; Jensen, P.E. Modulation of the MEP Pathway for Overproduction of 13-R-manoyl Oxide in Cyanobacteria. Synth. Biol. Eng. 2024, 2, 10005. [Google Scholar] [CrossRef]
- Casella, S.; Huang, F.; Mason, D.; Zhao, G.-Y.; Johnson, G.N.; Mullineaux, C.W.; Liu, L.-N. Dissecting the Native Architecture and Dynamics of Cyanobacterial Photosynthetic Machinery. Mol. Plant 2017, 10, 1434–1448. [Google Scholar] [CrossRef]
- Lassen, L.M.M.; Nielsen, A.Z.; Olsen, C.E.; Bialek, W.; Jensen, K.; Møller, B.L.; Jensen, P.E. Anchoring a plant cytochrome P450 via PsaM to the thylakoids in Synechococcus sp. PCC 7002: Evidence for light-driven biosynthesis. PLoS ONE 2014, 9, e102184. [Google Scholar] [CrossRef] [PubMed]
- Brey, L.F.; Włodarczyk, A.J.; Bang Thøfner, J.F.; Burow, M.; Crocoll, C.; Nielsen, I.; Zygadlo Nielsen, A.J.; Jensen, P.E. Metabolic engineering of Synechocystis sp. PCC 6803 for the production of aromatic amino acids and derived phenylpropanoids. Metab. Eng. 2020, 57, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, B.; Englund ENolte, N.; Lindberg, P. Introduction of a green algal squalene synthase enhances squalene accumulation in a strain of Synechocystis sp. PCC 6803. Metab. Eng. Commun. 2020, 10, e00125. [Google Scholar] [CrossRef] [PubMed]
- Dienst, D.; Wichmann, J.; Mantovani, O.; Rodrigues, J.S.; Lindberg, P. High density cultivation for efficient sesquiterpenoid biosynthesis in Synechocystis sp. PCC 6803. Sci. Rep. 2020, 10, 5932. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodge, N.; Sutardja, L.C.; Mellor, S.; Gnanasekaran, T.; Lassen, L.M.M.; Nielsen, A.Z.; Møller, B.L.; Jensen, P.E. Heterologous Production of the Structurally Complex Diterpenoid Forskolin in Synechocystis sp. PCC. 6803. Bioengineering 2025, 12, 683. https://doi.org/10.3390/bioengineering12070683
Dodge N, Sutardja LC, Mellor S, Gnanasekaran T, Lassen LMM, Nielsen AZ, Møller BL, Jensen PE. Heterologous Production of the Structurally Complex Diterpenoid Forskolin in Synechocystis sp. PCC. 6803. Bioengineering. 2025; 12(7):683. https://doi.org/10.3390/bioengineering12070683
Chicago/Turabian StyleDodge, Nadia, Lawrence Chuk Sutardja, Silas Mellor, Thiyagarajan Gnanasekaran, Lærke Marie Münter Lassen, Agnieszka Zygadlo Nielsen, Birger Lindberg Møller, and Poul Erik Jensen. 2025. "Heterologous Production of the Structurally Complex Diterpenoid Forskolin in Synechocystis sp. PCC. 6803" Bioengineering 12, no. 7: 683. https://doi.org/10.3390/bioengineering12070683
APA StyleDodge, N., Sutardja, L. C., Mellor, S., Gnanasekaran, T., Lassen, L. M. M., Nielsen, A. Z., Møller, B. L., & Jensen, P. E. (2025). Heterologous Production of the Structurally Complex Diterpenoid Forskolin in Synechocystis sp. PCC. 6803. Bioengineering, 12(7), 683. https://doi.org/10.3390/bioengineering12070683







