Artificial Intelligence in the Diagnostic Use of Transcranial Doppler and Sonography: A Scoping Review of Current Applications and Future Directions
Abstract
1. Introduction
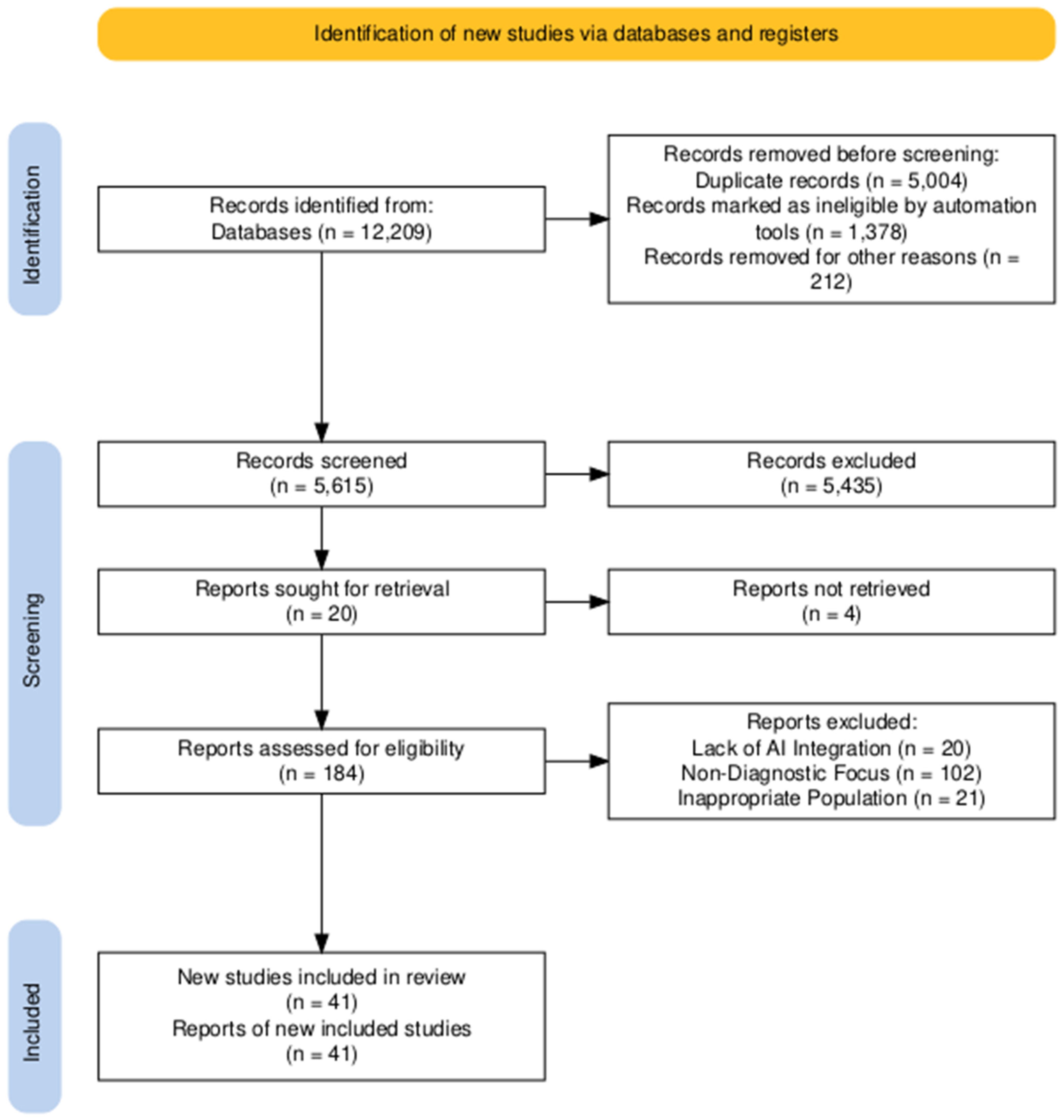
2. Research Strategy
3. Data Provenance and Analysis
Data Synthesis
4. Neurosonology: Indications and Utility
5. Transcranial Doppler and Transcranial Color-Coded Doppler: Advantages and Limitations
6. Generality of Artificial Intelligence
7. Intracranial Stenosis, Occlusions, and Cerebral Perfusion
7.1. Application of AI to TCD for ICAS Diagnosis
7.2. Transcranial Doppler for the Diagnosis of MCA Occlusion
7.3. Maintaining Cerebral Perfusion: Transcranial Doppler in Cerebral Autoregulation
8. Subarachnoid Hemorrhage
New Boundaries in Transcranial Doppler Applications: Artificial Intelligence in the Diagnosis and Monitoring of Vasospasms
9. Microemboli Detection and Right–Left Shunt
9.1. Patent Foramen Ovale
9.2. Cardiac Valve Embolism
9.3. Atrial Fibrillation
9.4. Carotid Embolism
9.5. Other Causes of ESUS
10. Monitoring in Acute Neurovascular Care and Non-Invasive Intracranial Pressure Measurement
10.1. TCD-Applied Artificial Intelligence Strategies to Improve Non-Invasive ICP Monitoring
10.2. Optic Nerve Sheath Diameters for Non-Invasive ICP Monitoring
11. Transcranial Brain Parenchyma Sonography
11.1. TCS in the Evaluation of Neurodegenerative Dementias
11.1.1. Parkinson’s Disease and Parkinsonian Syndromes
11.1.2. Alzheimer’s Disease and Vascular Dementia
11.2. TCS in the Detection of Space-Occupying Lesions
12. Application of AI for the Spectral Feature Extraction in Doppler Ultrasounds
13. Discussion
AI Use-Case in a Clinical Scenario and Its Limitations
14. Limitations
15. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Teng, X.; Li, S.; Yang, Y. Application of transcranial Doppler in cerebrovascular diseases. Front. Aging Neurosci. 2022, 14, 1035086. [Google Scholar] [CrossRef] [PubMed]
- Krejza, J.; Baumgartner, R.W. Clinical applications of transcranial color-coded duplex sonography. J. Neuroimaging 2004, 14, 215–225. [Google Scholar] [CrossRef]
- Kirsch, J.D.; Mathur, M.; Johnson, M.H.; Gowthaman, G.; Scoutt, L.M. Advances in transcranial Doppler US: Imaging ahead. Radiographics 2013, 33, E1–E14. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Topcuoglu, M.A. Transcranial Doppler Ultrasound in Neurovascular Diseases: Diagnostic and Therapeutic Aspects. J. Neurochem. 2012, 123 (Suppl. S2), 39–51. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Goffi, A.; Geeraerts, T.; Cardim, D.; Via, G.; Czosnyka, M.; Park, S.; Sarwal, A.; Padayachy, L.; Rasulo, F.; et al. Brain Ultrasonography: Methodology, Basic and Advanced Principles and Clinical Applications. A narrative review. Intensive Care Med. 2019, 45, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Garami, Z.; Alexandrov, A.V. Neurosonology. Neurol. Clin. 2009, 27, 89–108. [Google Scholar] [CrossRef]
- Favaretto, S.; Walter, U.; Baracchini, C.; Cagnin, A. Transcranial Sonography in Neurodegenerative Diseases with Cognitive Decline. J. Alzheimer’s Dis. 2017, 61, 29–40. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Katsanos, A.H.; Papageorgiou, S.G.; Dardiotis, E.; Voumvourakis, K.; Giannopoulos, S. The Role of Neurosonology in the Diagnosis of Vascular Dementia. J. Alzheimer’s Dis. 2014, 42 (Suppl. S3), S251–S257. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Thomas, T.; Ekkehard, H.; Katharina, S. Transcranial Ultrasound of the Basal Ganglia in Sporadic Creutzfeldt-Jakob Disease. J. Neuroimaging 2008, 18, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Hu, H.; Luo, W.F.; Sheng, Y.J.; Chen, X.F.; Mao, C.J.; Xiong, K.P.; Yu, L.F.; Zhang, Y.; Liu, C.F. Alteration of Brainstem Raphe Measured by Transcranial Sonography in Depression Patients with or Without Parkinson’s Disease. Neurol. Sci. 2016, 37, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Pérez, E.S.; Delgado-Mederos, R.; Rubiera, M.; Delgado, P.; Ribó, M.; Maisterra, O.; Ortega, G.; Álvarez-Sabin, J.; Molina, C.A. Transcranial duplex sonography for monitoring hyperacute intracerebral hemorrhage. Stroke 2009, 40, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Toia, F.; Gagliardo, A.; D’Arpa, S.; Gagliardo, C.; Gagliardo, G.; Cordova, A. Preoperative Evaluation of Peripheral Nerve Injuries: What Is the Place for Ultrasound? J. Neurosurg. 2016, 125, 603–614. [Google Scholar] [CrossRef]
- Aaslid, R.; Markwalder, T.M.; Nornes, H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg. 1982, 57, 769–774. [Google Scholar] [CrossRef]
- Gunda, S.T.; Ng, T.K.V.; Liu, T.Y.; Chen, Z.; Han, X.; Chen, X.; Pang, M.Y.; Ying, M.T. A Comparative Study of Transcranial Color-Coded Doppler (TCCD) and Transcranial Doppler (TCD) Ultrasonography Techniques in Assessing the Intracranial Cerebral Arteries Haemodynamics. Diagnostics 2024, 14, 387. [Google Scholar] [CrossRef]
- Bonow, R.H.; Young, C.C.; Bass, D.I.; Moore, A.; Levitt, M.R. Transcranial Doppler ultrasonography in neurological surgery and neurocritical care. Neurosurg. Focus 2019, 47, E2. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, D.W.; Kwon, S.U. Intracranial atherosclerosis: Incidence, diagnosis and treatment. J. Clin. Neurol. 2005, 1, 1–7. [Google Scholar] [CrossRef]
- Leng, X.; Hurford, R.; Feng, X.; Chan, K.L.; Wolters, F.J.; Li, L.; Soo, Y.O.; Wong, K.S.L.; Mok, V.C.; Leung, T.W.; et al. Intracranial arterial stenosis in Caucasian versus Chinese patients with TIA and minor stroke: Two contemporaneous cohorts and a systematic review. J. Neurol. Neurosurg. Psychiatry 2021, 92, 590–597. [Google Scholar] [CrossRef]
- Wang, L.; Xing, Y.; Li, Y.; Han, K.; Chen, J. Evaluation of flow velocity in unilateral middle cerebral artery stenosis by Transcranial Doppler. Cell Biochem. Biophys. 2014, 70, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Perren, F.; Vargas, M.I.; Kargiotis, O. Etiology of Intracranial Arterial Stenosis: Are Transcranial Color-Coded Duplex Ultrasound and 3T Black Blood MR Imaging Complementary? J. Neuroimaging 2016, 26, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Best, L.M.; Webb, A.C.; Gurusamy, K.S.; Cheng, S.F.; Richards, T. Transcranial Doppler Ultrasound Detection of Microemboli as a Predictor of Cerebral Events in Patients with Symptomatic and Asymptomatic Carotid Disease: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Martin, A.; Cosgrove, M.; Norris, J.W. The NASCET-ACAS plaque project. North American Symptomatic Carotid Endarterectomy Trial. Asymptomatic Carotid Atherosclerosis Study. Stroke 1993, 24 (Suppl. S12), I24–I25; discussion I31–I32. [Google Scholar]
- Markus, H.S.; King, A.; Shipley, M.; Topakian, R.; Cullinane, M.; Reihill, S.; Bornstein, N.M.; Schaafsma, A. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): A prospective observational study. Lancet Neurol. 2010, 9, 663–671. [Google Scholar] [CrossRef]
- Bonati, L.H.; Kakkos, S.; Berkefeld, J.; de Borst, G.J.; Bulbulia, R.; Halliday, A.; van Herzeele, I.; Koncar, I.; McCabe, D.J.; Lal, A.; et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur. Stroke J. 2021, 6, I–XLVII. [Google Scholar] [CrossRef]
- Kakkos, S.K.; Griffin, M.B.; Nicolaides, A.N.; Kyriacou, E.; Sabetai, M.M.; Tegos, T.; Makris, G.C.; Thomas, D.J.; Geroulakos, G.; Risk of Stroke (ACSRS) Study Group. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J. Vasc. Surg. 2013, 57, 609–618. [Google Scholar] [CrossRef]
- Telman, G.; Yalonetsky, S.; Kouperberg, E.; Sprecher, E.; Lorber, A.; Yarnitsky, D. Size of PFO and amount of microembolic signals in patients with ischaemic stroke or TIA. Eur. J. Neurol. 2008, 15, 969–972. [Google Scholar] [CrossRef]
- Demarin, V.; Vuković, V.; Škoro, I. Limitations and advantages of transcranial Doppler sonography in the assessment of cerebrovascular disorders. Acta Clin. Croat. 2012, 51, 533–541. [Google Scholar]
- Purkayastha, S.; Sorond, F. Transcranial Doppler: Technique and application. Semin. Neurol. 2012, 32, 411–420. [Google Scholar] [CrossRef]
- Alexandrov, A.; Joseph, M. Transcranial Doppler: An Overview of its Clinical Applications. Internet J. Neuromonitoring 1999, 1, 1. [Google Scholar]
- Rubiera, M.; Cava, L.; Tsivgoulis, G.; Patterson, D.E.; Zhao, L.; Zhang, Y.; Anderson, A.M.; Robinson, A.; Harrigan, M.R.; Underwood, E.; et al. Diagnostic criteria and yield of real-time transcranial Doppler monitoring of intra-arterial reperfusion procedures. Stroke 2010, 41, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.T.; Chen, L.; Yue, W.W.; Xu, H.X. Artificial intelligence in ultrasound. Eur. J. Radiol. 2021, 139, 109717. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Lavin, P.T.; Birch, M.; Shah, N.; Folk, J.C. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. npj Digit. Med. 2018, 1, 39. [Google Scholar] [CrossRef]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef]
- Al-Antari, M.A. Artificial Intelligence for Medical Diagnostics-Existing and Future AI Technology! Diagnostics 2023, 13, 688. [Google Scholar] [CrossRef]
- Jayaraman, P.; Desman, J.; Sabounchi, M.; Nadkarni, G.N.; Sakhuja, A. A Primer on Reinforcement Learning in Medicine for Clinicians. npj Digit. Med. 2024, 7, 337. [Google Scholar] [CrossRef]
- Mumuni, A.; Mumuni, F. Automated data processing and feature engineering for deep learning and big data applications: A survey. J. Inf. Intell. 2025, 3, 113–153. [Google Scholar] [CrossRef]
- Kufel, J.; Bargieł-Łączek, K.; Kocot, S.; Koźlik, M.; Bartnikowska, W.; Janik, M.; Czogalik, Ł.; Dudek, P.; Magiera, M.; Lis, A.; et al. What Is Machine Learning, Artificial Neural Networks and Deep Learning?—Examples of Practical Applications in Medicine. Diagnostics 2023, 13, 2582. [Google Scholar] [CrossRef]
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaria, J.; Fadhel, M.A.; Al-Amidie, M.; Farhan, L. Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions. J. Big Data 2021, 8, 53. [Google Scholar] [CrossRef]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Wang, C. Trends in using deep learning algorithms in biomedical prediction systems. Front. Neurosci. 2023, 17, 1256351. [Google Scholar] [CrossRef]
- Gaffney, H.; Mirza, K.M. Pathology in the artificial intelligence era: Guiding innovation and implementation to preserve human insight. Acad. Pathol. 2025, 12, 100166. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Turan, T.N.; Hoh, B.L.; Chimowitz, M.I. Intracranial atherosclerotic stenosis: Risk factors, diagnosis, and treatment. Lancet Neurol. 2022, 21, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Li, X.; Hou, X.; Yang, B.; Xia, C.; Ntaios, G.; Chen, H.S. Intracranial atherosclerotic plaque as a potential cause of embolic stroke of undetermined source. J. Am. Coll. Cardiol. 2021, 77, 680–691. [Google Scholar] [CrossRef]
- Sacco, R.; Kargman, D.; Gu, Q.; Zamanillo, M. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: The Northern Manhattan Stroke Study. Stroke 1995, 26, 14–20. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 guidelines for the early Management of Patients with Acute Ischemic Stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e99. [Google Scholar] [CrossRef]
- Naqvi, J.; Yap, K.H.; Ahmad, G.; Ghosh, J. Transcranial doppler ultrasound: A review of the physical principles and major applications in critical care. Int. J. Vasc. Med. 2013, 2013, 629378. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, S.; Ghosh, S.K.; Collier, A. Role of transcranial doppler ultrasonography in stroke. Postgrad. Med. J. 2007, 83, 683–689. [Google Scholar] [CrossRef]
- Altinbas, N.K.; Ustuner, E.; Ozcan, H.; Bilgic, S.; Sancak, T.; Dusunceli, E. Effect of carotid artery stenting on ophthalmic artery flow patterns. J. Ultrasound Med. 2014, 33, 629–638. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Zhang, J.; Chen, X.; Lin, Z.; Nie, S.; Shi, M.; Gao, X.; Huang, Y. Electroacupuncture improves cerebral vasospasm and functional outcome of patients with aneurysmal subarachnoid hemorrhage. Front. Neurosci. 2018, 12, 724. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.B.; Englund, E.K.; Chen, S.; Frank, L.R.; Ward, S.R. Medical imaging of tissue engineering and regenerative medicine constructs. Biomater. Sci. 2021, 9, 301–314. [Google Scholar] [CrossRef]
- Pinillos, O.M.; Rodríguez, C.N.; Hakimi, R. Transcranial Doppler ultrasound pulsatility index: Utility and clinical interpretation. In Neurosonology in Critical Care: Monitoring Neurological Impact of the Critical Pathology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 357–376. [Google Scholar] [CrossRef]
- Rainov, N.G.; Weise, J.B.; Burkert, W. Transcranial Doppler sonography in adult hydrocephalic patients. Neurosurg. Rev. 2000, 23, 34–38. [Google Scholar] [CrossRef]
- Wijnhoud, A.D.; Franckena, M.; Van Der Lugt, A.; Koudstaal, P.J. Inadequate acoustical temporal bone window in patients with a transient ischemic attack or minor stroke: Role of skull thickness and bone density. Ultrasound Med. Biol. 2008, 34, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Aghoram, R.; Narayan, S.K. Patterns of transcranial Doppler flow velocities in recent ischemic stroke patients. Ann. Indian Acad. Neurol. 2018, 21, 193–196. [Google Scholar] [CrossRef]
- Du, L.; Hua, Y.; Jia, L.; Yang, J.; Jiao, L.; Liu, J. Analysis of blood flow velocity variability in middle cerebral artery stenosis using transcranial color-coded sonography. J. Clin. Neurosci. 2025, 133, 111020. [Google Scholar] [CrossRef]
- Zhao, L.; Barlinn, K.; Sharma, V.K.; Tsivgoulis, G.; Cava, L.F.; Vasdekis, S.N.; Teoh, L.H.; Triantafyllou, N.; Chan, B.P.L.; Sharma, A.; et al. Velocity criteria for intracranial stenosis revisited: An international multicenter study of transcranial Doppler and digital subtraction angiography. Stroke 2011, 42, 3429–3434. [Google Scholar] [CrossRef]
- Kushner, M.J.; Zanette, E.M.; Bastianello, S.; Mancini, G.; Sacchetti, M.L.; Carolei, A.; Bozzao, L. Transcranial Doppler in acute hemispheric brain infarction. Neurology 1991, 41, 109. [Google Scholar] [CrossRef] [PubMed]
- Solti, I.; Cooke, C.R.; Xia, F.; Wurfel, M.M. Automated classification of radiology reports for acute lung injury: Comparison of keyword and machine learning based natural language processing approaches. In Proceedings of the 2009 IEEE International Conference on Bioinformatics and Biomedicine Workshop, Washington, DC, USA, 1–4 November 2009; pp. 314–319. [Google Scholar] [CrossRef]
- Güler, I.; Hardalaç, F.; Kaymaz, M. Comparison of FFT and adaptive ARMA methods in transcranial Doppler signals recorded from the cerebral vessels. Comput. Biol. Med. 2002, 32, 445–453. [Google Scholar] [CrossRef]
- Karaboga, N.; Latifoglu, F. Elimination of noise on transcranial Doppler signal using IIR filters designed with artificial bee colony—ABC-algorithm. Digit. Signal Process. 2013, 23, 1051–1058. [Google Scholar] [CrossRef]
- Serhatlioğlu, S.; Hardalaç, F.; Güler, I. Classification of transcranial Doppler signals using artificial neural network. J. Med. Syst. 2003, 27, 205–214. [Google Scholar] [CrossRef]
- Uğuz, H. A hybrid system based on information gain and principal component analysis for the classification of transcranial Doppler signals. Comput. Methods Programs Biomed. 2012, 107, 598–609. [Google Scholar] [CrossRef]
- Uğuz, H.; Arslan, A. A new approach based on discrete hidden Markov model using Rocchio algorithm for the diagnosis of the brain diseases. Digit. Signal Process. 2010, 20, 923–934. [Google Scholar] [CrossRef]
- Seera, M.; Lim, C.P.; Tan, K.S.; Liew, W.S. Classification of transcranial Doppler signals using individual and ensemble recurrent neural networks. Neurocomputing 2017, 249, 337–344. [Google Scholar] [CrossRef]
- Myrden, A.; Kushki, A.; Sejdić, E.; Chau, T. Towards increased data transmission rate for a three class metabolic brain-computer interface based on transcranial Doppler ultrasound. Neurosci. Lett. 2012, 528, 99–103. [Google Scholar] [CrossRef]
- Goyal, A.; Samadani, A.A.; Guerguerian, A.M.; Chau, T. An online three-class Transcranial Doppler ultrasound brain computer interface. Neurosci. Res. 2016, 107, 47–56. [Google Scholar] [CrossRef]
- Khalaf, A.; Sybeldon, M.; Sejdic, E.; Akcakaya, M. A brain-computer interface based on functional transcranial doppler ultrasound using wavelet transform and support vector machines. J. Neurosci. Methods 2018, 293, 174–182. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Lin, C.-H.; Johnson, K.R.; Liu, C.-H.; Chang, T.-Y.; Huang, K.-L.; Fann, Y.-C.; Lee, T.-H. Autodetect Extracranial and Intracranial Artery Stenosis by Machine Learning Using Ultrasound. Comput. Biol. Med. 2020, 116, 103569. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Dorn, A.Y.; Ranjbaran, M.; Nie, Z.; Scheidt, M.; Mirnateghi, N.; Radhakrishnan, S.; Hamilton, R.B. Fully Automated Transcranial Doppler Ultrasound for Middle Cerebral Artery Insonation. J. Neurosonol. Neuroimaging 2022, 14, 27–34. [Google Scholar]
- Mei, Y.J.; Hu, R.T.; Lin, J.; Xu, H.Y.; Wu, L.Y.; Li, H.P.; Ye, Z.M.; Qin, C. Diagnosis of middle cerebral artery stenosis using transcranial Doppler images based on convolutional neural network. World Neurosurg. 2022, 161, e118–e125. [Google Scholar] [CrossRef]
- Yeh, C.Y.; Lee, H.H.; Islam, M.M.; Chien, C.H.; Atique, S.; Chan, L.; Lin, M.C. Development and Validation of Machine Learning Models to Classify Artery Stenosis for Automated Generating Ultrasound Report. Diagnostics 2022, 12, 3047. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, H.; Su, F.; Qiu, S.; Tong, H.; Huang, M.; Yao, J. Identification of middle cerebral artery stenosis in transcranial Doppler using a modified VGG-16. Front. Neurol. 2024, 15, 1394435. [Google Scholar] [CrossRef] [PubMed]
- Nisha, N.N.; Podder, K.K.; Chowdhury, M.E.H.; Rabbani, M.; Wadud, M.S.I.; Al-Maadeed, S.; Mahmud, S.; Khandakar, A.; Zughaier, S.M. A Deep Learning Framework for the Detection of Abnormality in Cerebral Blood Flow Velocity Using Transcranial Doppler Ultrasound. Diagnostics 2023, 13, 2000. [Google Scholar] [CrossRef] [PubMed]
- Übeyli, E.D.; Güler, I. Wavelet-based neural network analysis of internal carotid arterial Doppler signals. J. Med. Syst. 2006, 30, 221–229. [Google Scholar] [CrossRef]
- Uguz, H. Detection of carotid artery disease by using learning vector quantization neural network. J. Med. Syst. 2012, 36, 533–540. [Google Scholar] [CrossRef]
- Guan, J.; Zhou, Q.; Ouyang, H.; Zhang, S.; Lu, Z. The diagnostic accuracy of TCD for intracranial arterial stenosis/occlusion in patients with acute ischemic stroke: The importance of time interval between detection of TCD and CTA. Neurol. Res. 2013, 35, 930–936. [Google Scholar] [CrossRef]
- Kilic, M.; Wendl, C.; Wilfling, S.; Olmes, D.; Linker, R.A.; Schlachetzki, F. Acute Middle Cerebral Artery Occlusion Detection Using Mobile Non-Imaging Brain Perfusion Ultrasound—First Case. J. Clin. Med. 2022, 11, 3384. [Google Scholar] [CrossRef]
- Rim, S.J.; Leong-Poi, H.; Lindner, J.R.; Couture, D.; Ellegala, D.; Mason, H.; Durieux, M.; Kassel, N.F.; Kaul, S. Quantification of cerebral perfusion with “Real-Time” contrast-enhanced ultrasound. Circulation 2001, 104, 2582–2587. [Google Scholar] [CrossRef]
- Antipova, D.; Eadie, L.; Makin, S.; Shannon, H.; Wilson, P.; Macaden, A. The use of transcranial ultrasound and clinical assessment to diagnose ischaemic stroke due to large vessel occlusion in remote and rural areas. PLoS ONE 2020, 15, e0239653. [Google Scholar] [CrossRef]
- Brozici, M.; van der Zwan, A.; Hillen, B. Anatomy and functionality of leptomeningeal anastomoses: A review. Stroke 2003, 34, 2750–2762. [Google Scholar] [CrossRef]
- Benemerito, I.; Narata, A.P.; Narracott, A.; Marzo, A. Determining Clinically-Viable Biomarkers for Ischaemic Stroke Through a Mechanistic and Machine Learning Approach. Ann. Biomed. Eng. 2022, 50, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Sin, D.-S.; Park, H.-Y.; Park, M.-S.; Cho, K.-H. Relationship between Flow Diversion on Transcranial Doppler Sonography and Leptomeningeal Collateral Circulation in Patients with Middle Cerebral Artery Occlusive Disorder. J. Neuroimaging 2009, 19, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Meyer, J.S.; Garami, Z.; Molina, C.A.; Pavlovic, A.M.; Alexandrov, A.V. Flow diversion in transcranial Doppler ultrasound is associated with better improvement in patients with acute middle cerebral artery occlusion. Cerebrovasc. Dis. 2006, 21, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Ghozy, S.; Azzam, A.Y.; Kallmes, K.M.; Matsoukas, S.; Fifi, J.T.; Luijten, S.P.; van der Lugt, A.; Adusumilli, G.; Heit, J.J.; Kadirvel, R.; et al. The diagnostic performance of artificial intelligence algorithms for identifying M2 segment middle cerebral artery occlusions: A systematic review and meta-analysis. J. Neuroradiol. 2023, 50, 449–454. [Google Scholar] [CrossRef]
- Kim, P.E.; Yang, H.; Kim, D.; Sunwoo, L.; Kim, C.K.; Kim, B.J.; Kim, J.-T.; Ryu, W.-S.; Kim, H.S. Automated Prediction of Proximal Middle Cerebral Artery Occlusions in Noncontrast Brain Computed Tomography. Stroke 2024, 55, 1609–1618. [Google Scholar] [CrossRef]
- Fasen, B.A.C.M.; Berendsen, R.C.M.; Kwee, R.M. Artificial intelligence software for diagnosing intracranial arterial occlusion in patients with acute ischemic stroke. Neuroradiology 2022, 64, 1579–1583. [Google Scholar] [CrossRef]
- Reinhard, M.; Schwarzer, G.; Briel, M.; Altamura, C.; Palazzo, P.; King, A.; Bornstein, N.M.; Petersen, N.; Motschall, E.; Hetzel, A.; et al. Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 2014, 83, 1424–1431. [Google Scholar] [CrossRef]
- Gupta, A.; Chazen, J.L.; Hartman, M.; Delgado, D.; Anumula, N.; Shao, H.; Mazumdar, M.; Segal, A.Z.; Kamel, H.; Leifer, D.; et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: A systematic review and meta-analysis. Stroke 2012, 43, 2884–2891. [Google Scholar] [CrossRef]
- Diehl, R.R.; Berlit, P. Funktionelle Doppler-Sonographie der zerebralen Autoregulation: Grundlagen und klinische Anwendung. Klin. Neurophysiol. 1998, 29, 222–227. [Google Scholar] [CrossRef]
- Blaber, A.P.; Bondar, R.L.; Stein, F.; Dunphy, P.T.; Moradshahi, P.; Kassam, M.S.; Freeman, R. Transfer function analysis of cerebral autoregulation dynamics in autonomic failure patients. Stroke 1997, 28, 1686–1692. [Google Scholar] [CrossRef]
- Diehl, R.R.; Linden, D.; Lücke, D.; Berlit, P. Spontaneous blood pressure oscillations and cerebral autoregulation. Clin. Auton. Res. 1998, 8, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.B.; Chern, C.M.; Sheng, W.Y.; Wong, W.J.; Hu, H.H. Frequency domain analysis of cerebral blood flow velocity and its correlation with arterial blood pressure. J. Cereb. Blood Flow Metab. 1998, 18, 311–318. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Z.-N.; Xing, Y.; Ma, H.; Jin, H.; Liu, J.; Yang, Y. Dynamic Cerebral Autoregulation in Asymptomatic Patients With Unilateral Middle Cerebral Artery Stenosis. Medicine 2015, 94, e2234. [Google Scholar] [CrossRef]
- Haubrich, C.; Kruska, W.; Diehl, R.R.; Möller-Hartmann, W.; Klötzsch, C. Dynamic Autoregulation Testing in Patients with Middle Cerebral Artery Stenosis. Stroke 2003, 34, 1881–1885. [Google Scholar] [CrossRef]
- Latka, M.; Turalska, M.; Glaubic-Latka, M.; Kolodziej, W.; Latka, D.; West, B.J. Phase dynamics in cerebral autoregulation. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H2272–H2279. [Google Scholar] [CrossRef] [PubMed]
- Panerai, R.B.; Chacon, M.; Pereira, R.; Evans, D.H. Neural network modelling of dynamic cerebral autoregulation: Assessment and comparison with established methods. Med. Eng. Phys. 2004, 26, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and metaanalysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Etminan, N.; Chang, H.S.; Hackenberg, K.; de Rooij, N.K.; Vergouwen, M.D.I.; Rinkel, G.J.E.; Algra, A. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: A systematic review and meta-analysis. JAMA Neurol. 2019, 76, 588–597. [Google Scholar] [CrossRef]
- Platz, J.; Güresir, E.; Wagner, M.; Seifert, V.; Konczalla, J. Increased risk of delayed cerebral ischemia in subarachnoid hemorrhage patients with additional intracerebral hematoma. J. Neurosurg. 2017, 126, 504–510. [Google Scholar] [CrossRef]
- Koide, M.; Nishizawa, S.; Ohta, S.; Yokoyama, T.; Namba, H. Chronological changes of the contractile mechanism in prolonged vasospasm after subarachnoid hemorrhage: From protein kinase C to protein tyrosine kinase. Neurosurgery 2002, 51, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Schwartz, J.; Vajkoczy, P.; Macdonald, R.L.; Pluta, R.M.; Zhang, J.H. Cerebral vasospasm: Looking beyond vasoconstriction. Trends Pharmacol. Sci. 2007, 28, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.S.; Oldfield, E.H.; Harvey-White, J.; Espey, M.G.; Zimmermann, M.; Seifert, V.; Pluta, R.M. Association of an endogenous inhibitor of nitric oxide synthase with cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2007, 107, 945–950. [Google Scholar] [CrossRef]
- Mascia, L.; Fedorko, L.; Stewart, D.J.; Mohamed, F.; terBrugge, K.; Ranieri, V.M.; Wallace, M.C. Temporal relationship between endothelin-1 concentrations and cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Stroke 2001, 32, 1185–1190. [Google Scholar] [CrossRef]
- Dumont, A.S.; Dumont, R.J.; Chow, M.M.; Lin, C.L.; Calisaneller, T.; Ley, K.F.; Kassell, N.F.; Lee, K.S. Cerebral vasospasm after subarachnoid hemorrhage: Putative role of inflammation. Neurosurgery 2003, 53, 123–135. [Google Scholar] [CrossRef]
- Fisher, C.M.; Roberson, G.H.; Ojemann, R.G. Cerebral vasospasm with ruptured saccular aneurysm—The clinical manifestations. Neurosurgery 1977, 1, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Otawara, Y.; Ogasawara, K.; Ogawa, A.; Sasaki, M.; Takahashi, K. Evaluation of vasospasm after subarachnoid hemorrhage by use of multislice computed tomographic angiography. Neurosurgery 2002, 51, 939–943. [Google Scholar] [CrossRef][Green Version]
- Sharma, S.; Lubrica, R.J.; Song, M.; Vandse, R.; Boling, W.; Pillai, P. The role of transcranial Doppler in cerebral vasospasm: A literature review. Acta Neurochir. Suppl. 2020, 127, 201–205. [Google Scholar] [CrossRef]
- Grosset, D.G.; Straiton, J.; du Trevou, M.; Bullock, R. Prediction of symptomatic vasospasm after subarachnoid hemorrhage by rapidly increasing transcranial Doppler velocity and cerebral blood flow changes. Stroke 1992, 23, 674–679. [Google Scholar] [CrossRef]
- Sviri, G.E.; Ghodke, B.; Britz, G.W.; Douville, C.M.; Haynor, D.R.; Mesiwala, A.H.; Lam, A.M.; Newell, D.W. Transcranial Doppler grading criteria for basilar artery vasospasm. Neurosurgery 2006, 59, 360–366. [Google Scholar] [CrossRef]
- Lindegaard, K.F.; Bakke, S.J.; Sorteberg, W.; Nakstad, P.; Nornes, H. A non-invasive Doppler ultrasound method for the evaluation of patients with subarachnoid hemorrhage. Acta Radiol. Suppl. 1986, 369, 96–98. [Google Scholar] [PubMed]
- Ratsep, T.; Asser, T. Cerebral hemodynamic impairment after aneurysmal subarachnoid hemorrhage as evaluated using transcranial doppler ultrasonography: Relationship to delayed cerebral ischemia and clinical outcome. J. Neurosurg. 2001, 95, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, N.R.; Boscardin, W.J.; Glenn, T.; Vinuela, F.; Martin, N.A. Vasospasm probability index: A combination of transcranial Doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2007, 107, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhou, H.; Yan, T.; Miu, H.; Xiao, F.; Zhu, X.; Shu, L.; Yang, S.; Jin, R.; Dou, W.; et al. Deep learning-assisted identification and quantification of aneurysmal subarachnoid hemorrhage in non-contrast CT scans: Development and external validation of Hybrid 2D/3D UNet. NeuroImage 2023, 279, 120321. [Google Scholar] [CrossRef]
- Xue, J.; Zheng, H.; Lai, R.; Zhou, Z.; Zhou, J.; Chen, L.; Wang, M. Comprehensive Management of Intracranial Aneurysms Using Artificial Intelligence: An Overview. World Neurosurg. 2025, 193, 209–221. [Google Scholar] [CrossRef]
- Elzaafarany, K.; Aly, M.H.; Kumar, G.; Nakhmani, A. Cerebral Artery Vasospasm Detection Using Transcranial Doppler Signal Analysis. J. Ultrasound Med. 2018, 38, 2191–2202. [Google Scholar] [CrossRef]
- Kumar, G.; Elzaafrani, K.; Nakhmani, A. Machine Learning Approach to Automate Detection of Cerebral Vasospasm Using Transcranial Doppler Monitoring (S23.004). Neurology, 2017; 88, (Suppl. S16), S23-004. [Google Scholar] [CrossRef]
- Esmaeeli, S.; Hrdlicka, C.M.; Bastos, A.B.; Wang, J.; Gomez-Paz, S.; Hanafy, K.A.; Lioutas, V.-A.; Ogilvy, C.S.; Thomas, A.J.; Shaefi, S.; et al. Robotically assisted transcranial Doppler with artificial intelligence for assessment of cerebral vasospasm after subarachnoid hemorrhage. J. Neurocrit. Care 2020, 13, 32–40. [Google Scholar] [CrossRef]
- Clare, K.; Stein, A.; Damodara, N.; Feldstein, E.; Alshammari, H.; Ali, S.; Kurian, C.; Rosenberg, J.; Bauerschmidt, A.; Kaur, G.; et al. Safety and efficacy of a novel robotic transcranial doppler system in subarachnoid hemorrhage. Sci. Rep. 2022, 12, 2266. [Google Scholar] [CrossRef]
- Lysakowski, C.; Walder, B.; Costanza, M.C.; Tramèr, M.R. Transcranial doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm. Stroke 2001, 32, 2292–2298. [Google Scholar] [CrossRef]
- Kassab, M.Y.; Majid, A.; Farooq, M.U.; Azhary, H.; Hershey, L.A.; Bednarczyk, E.M.; Graybeal, D.F.; Johnson, M.D. Transcranial doppler: An introduction for primary care physicians. J. Am. Board Fam. Med. 2007, 20, 65–71. [Google Scholar] [CrossRef]
- Kim, Y.J.; Go, D.W.; Kim, I.; Kim, J.; Park, Y. Abstract TMP107: Detection of Cerebral Artery Vasospasm in Transcranial Doppler Using Artificial Intelligence Based on Convolutional Neural Network. Stroke 2023, 54 (Suppl. S1), ATMP107. [Google Scholar] [CrossRef]
- McGrath, M.; Sen, R.; Wang, L.; Meyer, R.; Shenoy, V.; Kim, L.; Levitt, M.; Sekhar, L. E-200 Use of Artificial Intelligence in Patients with Aneurysmal Subarachnoid Hemorrhage to Predict Outcomes and Complications. In Proceedings of the SNIS 20th Annual Meeting Electronic Poster Abstracts, San Diego, CA, USA, 31 July–4 August 2023. [Google Scholar] [CrossRef]
- Mohammadzadeh, I.; Niroomand, B.; Eini, P.; Khaledian, H.; Choubineh, T.; Luzzi, S. Leveraging machine learning algorithms to forecast delayed cerebral ischemia following subarachnoid hemorrhage: A systematic review and meta-analysis of 5115 participants. Neurosurg. Rev. 2025, 48, 26. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Cobb, J.D.; D’Agostino, R. Epidemiology of stroke. In Stroke: Pathophysiology, Diagnosis and Management; Elsevier: Amsterdam, The Netherlands, 1992; pp. 4–6. [Google Scholar]
- Guo, Y.; Zhang, J.; Lip, G.Y.H. Embolic Stroke of Undetermined Source: The Need for an Integrated and Holistic Approach to Care. Thromb. Haemost. 2021, 121, 251–254. [Google Scholar] [CrossRef]
- Bakola, E.; Palaiodimou, L.; Kargiotis, O.; Safouris, A.; Psychogios, K.; Karapanayiotides, T.; Moschovos, C.; Sharma, V.K.; Rubin, M.N.; Freitas, J.S.; et al. Microembolic signal detection in acute ischemic stroke: Clinical relevance and impact on treatment individualization-A narrative review. Eur. J. Neurol. 2025, 32, e16584. [Google Scholar] [CrossRef] [PubMed]
- Kemény, V.; Droste, D.W.; Hermes, S.; Nabavi, D.G.; Schulte-Altedorneburg, G.; Siebler, M.; Bernd Ringelstein, E. Automatic Embolus Detection by a Neural Network. Stroke 1999, 30, 807–810. [Google Scholar] [CrossRef]
- Van Zuilen, E.V.; Mess, W.H.; Jansen, C.; Van Der Tweel, I.; Van Gijn, J.; Ackerstaff, R.G.A. Automatic embolus detection compared with human experts: A Doppler ultrasound study. Stroke 1996, 27, 1840–1843. [Google Scholar] [CrossRef]
- Fan, L.; Boni, E.; Tortoli, P.; Evans, D.H. Multigate Transcranial Doppler Ultrasound System with Real-Time Embolic Signal Identification and Archival. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 1853–1861. [Google Scholar] [CrossRef]
- Vindas, Y.; Guepie, B.K.; Almar, M.; Roux, E.; Delachartre, P. An hybrid CNN-Transformer model based on multi-feature extraction and attention fusion mechanism for cerebral emboli classification. In Proceedings of the 7th Machine Learning for Healthcare Conference, Durham, NC, USA, 5–6 August 2022; Volume 182, pp. 270–296. [Google Scholar]
- Sposato, L.A.; Albin, C.S.W.; Elkind, M.S.V.; Kamel, H.; Saver, J.L. Patent Foramen Ovale Management for Secondary Stroke Prevention: State-of-the-Art Appraisal of Current Evidence. Stroke 2024, 55, 236–247. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Psaltopoulou, T.; Sergentanis, T.N.; Frogoudaki, A.; Vrettou, A.R.; Ikonomidis, I.; Paraskevaidis, I.; Parissis, J.; Bogiatzi, C.; Zompola, C.; et al. Transcranial Doppler versus transthoracic echocardiography for the detection of patent foramen ovale in patients with cryptogenic cerebral ischemia: A systematic review and diagnostic test accuracy meta-analysis. Ann. Neurol. 2016, 79, 625–635. [Google Scholar] [CrossRef]
- Spencer, M.P.; Moehring, M.A.; Jesurum, J.; Gray, W.A.; Olsen, J.V.; Reisman, M. Power m-mode transcranial Doppler for diagnose of patent foramen ovale and assessing transcatheter closure. J. Neuroimaging 2004, 14, 342–349. [Google Scholar] [CrossRef]
- Jauss, M.; Zanette, E. Detection of right-to-left shunt with ultrasound contrast agent and transcranial Doppler sonography. Cerebrovasc. Dis. 2000, 10, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.N.; Shah, R.; Devlin, T.; Youn, T.S.; Waters, M.F.; Volpi, J.J.; Stayman, A.; Douville, C.M.; Lowenkopf, T.; Tsivgoulis, G.; et al. Robot-assisted transcranial Doppler versus transthoracic echocardiography for right to left shunt detection. Stroke 2023, 54, 2842–2850. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, L.R. Robot-Assisted TCD for Detection of Right to Left Shunt: Teaching an Old Device New Tricks. Stroke 2023, 54, 2851–2852. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Pirahanchi, Y.; Izaguirre, S.; Rodriguez, R.; Wicknick, A. Incorporation of Robotic Automated Transcranial Doppler to Screen for Patent Foramen Ovale (PFO) and Quantify Right to Left Shunt Severity in the Evaluation of Ischemic Stroke Patients for Etiology and PFO Management. Front. Neurol. Sec. Stroke 2025, 15, 1481814. [Google Scholar] [CrossRef]
- Keunen, R.W.M.; Temmink, H.; Schipper, M.; Romers, G.J.; van Kampen, P.M.; Daal, S. Validation of an algorithm that separates gaseous micro-embolic signals and artifacts during transcranial Doppler persistent foramen ovale examinations. WFUMB Ultrasound Open 2024, 2, 100067. [Google Scholar] [CrossRef]
- Shah, R.; Devlin, C.; Gao, L.; Ledford, S.; Ramjee, V.; Madan, V.; Patterson, J.; Daniel, L.; Devlin, T. Enhancing the Diagnostic Efficacy of Right-to-Left Shunt Using Robot- Assisted Transcranial Doppler: A Quality Improvement Project. Front. Neurol. Sec. Stroke 2025, 16, 1512061. [Google Scholar] [CrossRef]
- Wu, X.; Klomparens, K.; Chen, Z.; Zhang, M.; Song, S.; Ding, Y.; Ji, X.; Meng, R. Different patterns of white matter lesions among patent foramen ovale, atherosclerotic cerebral small vessel disease and cerebral venous thrombosis. J. Thromb. Thrombolysis 2022, 53, 911–925. [Google Scholar] [CrossRef]
- Liu, J.R.; Plotz, B.M.; Rohr, A.; Stingele, R.; Jansen, O.; Alfke, K. Association of right-to-left shunt with frontal white matter lesions in T2-weighted MR imaging of stroke patients. Neuroradiology 2009, 51, 299–304. [Google Scholar] [CrossRef]
- Signoriello, E.; Cirillo, M.; Puoti, G.; Signoriello, G.; Negro, A.; Koci, E.; Melone, M.A.B.; Rapacciuolo, A.; Maresca, G.; Lus, G. Migraine as possible red flag of PFO presence in suspected demyelinating disease. J. Neurol. Sci. 2018, 390, 222–226. [Google Scholar] [CrossRef]
- Badea, R.S.; Mihăilă-Bâldea, S.; Ribigan, A.; Negrilă, A.; Grecu, N.; Marinescu, A.N.; Antochi, F.; Tiu, C.; Vinereanu, D.; Popescu, B.O. PFO-spectrum disorder: Two different cerebrovascular diseases in patients with PFO as detected by AI brain imaging software. Front. Neurol. 2024, 15, 1357348. [Google Scholar] [CrossRef]
- Lopez, O.L.; Jagust, W.J.; Dulberg, C.; Becker, J.T.; DeKosky, S.T.; Fitzpatrick, A.; Breitner, J.; Lyketsos, C.; Jones, B.; Kawas, C.; et al. Risk factors for mild cognitive impairment in the cardiovascular health study cognition study: Part 2. Arch. Neurol. 2003, 60, 1394–1399. [Google Scholar] [CrossRef]
- Vermeer, S.E.; Prins, N.D.; den Heijer, T.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Silent brain infarcts and the risk of dementia and cognitive decline. N. Engl. J. Med. 2003, 348, 1215–1222. [Google Scholar] [CrossRef]
- Klötzsch, C.; Sliwka, U.; Berlit, P.; Noth, J. An Increased Frequency of Patent Foramen Ovale in Patients with Transient Global Amnesia Analysis of 53 Consecutive Patients. Arch. Neurol. 1996, 53, 504–508. [Google Scholar] [CrossRef]
- Cao, W.; Shen, Y.; Zhong, J.; Chen, Z.; Wang, N.; Yang, J. The Patent Foramen Ovale and Migraine: Associated Mechanisms and Perspectives from MRI Evidence. Brain Sci. 2022, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Galadanci, N.A.; Johnson, W.; Carson, A.; Hellemann, G.; Howard, V.; Kanter, J. Association Between Patent Foramen Ovale and Overt Ischemic Stroke in Children with Sickle Cell Disease. Front. Neurol. 2021, 12, 761443. [Google Scholar] [CrossRef]
- Davlouros, P.A.; Mplani, V.C.; Koniari, I.; Tsigkas, G.; Hahalis, G. Transcatheter aortic valve replacement and stroke: A comprehensive review. J. Geriatr. Cardiol. 2018, 15, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.; Agarwal, S.; Miller, D.C.; Webb, J.G.; Mack, M.; Ellis, S.; Herrmann, H.C.; Pichard, A.D.; Tuzcu, E.M.; Svensson, L.G.; et al. Insights into timing, risk factors, and outcomes of stroke and transient ischemic attack after transcatheter aortic valve replacement in the PARTNER trial (placement of aortic transcatheter valves). Circ. Cardiovasc. Interv. 2016, 9, e002981. [Google Scholar] [CrossRef] [PubMed]
- Diaz, V.A.J.; Kapadia, S.R.; Linke, A.; Mylotte, D.; Lansky, A.J.; Grube, E.; Settergren, M.; Puri, R. Cerebral embolic protection during transcatheter heart interventions. EuroIntervention 2023, 19, 549–570. [Google Scholar] [CrossRef]
- Baig, A.; Manion, C.; Iyer, V.; Khawar, W.; Donnelly, B.; Monteiro, A.; Levy, E.; Siddiqui, A. E-142 Robotic Transcranial Doppler with Artificial Intelligence to Identify Cerebral Emboli During Transcatheter Aortic Valve Replacement—A Novel Neuromonitoring Tool. In Proceedings of the SNIS 19th Annual Meeting Electronic Poster Abstracts, Toronto, ON, Canada, 25–28 July 2022; p. 253. [Google Scholar] [CrossRef]
- Abdul-Rahim, A.H.; Fulton, R.L.; Frank, B.; Tatlisumak, T.; Paciaroni, M.; Caso, V.; Diener, H.-C.; Lees, K.R.; VISTA collaborators. Association of improved outcome in acute ischaemic stroke patients with atrial fibrillation who receive early antithrombotic therapy: Analysis from VISTA. Eur. J. Neurol. 2015, 22, 1048–1055. [Google Scholar] [CrossRef]
- Castro, P.; Ferreira, J.; Malojcic, B.; Bazadona, D.; Baracchini, C.; Pieroni, A.; Skoloudik, D.; Azevedo, E.; Kaps, M. Detection of microemboli in patients with acute ischaemic stroke and atrial fibrillation suggests poor functional outcome. Eur. Stroke J. 2024, 9, 409–417. [Google Scholar] [CrossRef]
- Meszaros, H.; Vamosi, P.; Komlosi, F.; Toth, P.; Szegedi, N.; Sallo, Z.; Perge, P.; Geller, L.; Simon, Z.S.; Nardai, S.; et al. Comparing cerebral microembolisation patterns between high power short duration and pulsed-field ablation techniques using artificial intelligence based robotic transcranial doppler monitoring. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666-384. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Natale, A.; Stroker, E.; Sieira, J.; Vanbinst, A.-M.; Ceccarelli, A.; Pannone, L.; Del Monte, A.; Bala, G.; Tanaka, K.; et al. PO-01-121 Microembolic Signal Evaluation During Pulsed Field Ablation: Preliminary Results Using an Autonomous, Robotically-Assisted Transcranial Doppler. Heart Rhythm 2023, 20, 244. [Google Scholar] [CrossRef]
- Markus, H.S.; MacKinnon, A. Asymptomatic embolization detected by Doppler ultrasound predicts stroke risk in symptomatic carotid artery stenosis. Stroke 2005, 36, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.; Cappuzzo, J.M.; Monteiro, A.; Khawar, W.; Waqas, M.; Donnelly, B.; Davies, J.; Levy, E.I.; Siddiqui, A.H. 470 Robotic Transcranial Doppler with Artificial Intelligence for Real-Time Intraoperative Neuromonitoring During Endovascular Carotid Revascularization—A Novel and Autonomous Neuromonitoring Tool. Neurosurgery 2023, 69 (Suppl. S1), 99. [Google Scholar] [CrossRef]
- Izaguirre, S.; Chang, I.; Hamilton, R. Abstract Number-244: Feasibility of Detecting Carotid Stenosis Microemboli Using Autonomous Robotic TCD at a Large Stroke Center. Stroke Vasc. Interv. Neurol. 2023, 3, e12667. [Google Scholar] [CrossRef]
- Fattorello Salimbeni, A.; Kulyk, C.; Favruzzo, F.; De Rosa, L.; Viaro, F.; Pieroni, A.; Mozzetta, S.; Vosko, M.R.; Baracchini, C. Robotic Assisted Transcranial Doppler Monitoring in Acute Neurovascular Care: A Feasibility and Safety Study. Neurocrit. Care 2024, 42, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Husvethova, D.; Bardoczi, A.; Haddad, P.; Bavare, C.S.; Lumsden, A.B.; Garami, Z. Real-time monitoring of middle cerebral artery blood flow using intraoperative transcranial doppler during trans-carotid artery revascularization. Ann. Vasc. Surg.-Brief Rep. Innov. 2024, 4, 100322. [Google Scholar] [CrossRef]
- Ntaios, G.; Perlepe, K.; Lambrou, D.; Sirimarco, G.; Strambo, D.; Eskandari, A.; Karagkiozi, E.; Vemmou, A.; Koroboki, E.; Manios, E.; et al. Prevalence and overlap of potential embolic sources in patients with embolic stroke of undetermined source. J. Am. Heart Assoc. 2019, 8, e012858. [Google Scholar] [CrossRef]
- Navi, B.B.; Kawaguchi, K.; Hriljac, I.; Lavi, E.; DeAngelis, L.M.; Jamieson, D.G. Multifocal stroke from tumor emboli. Arch. Neurol. 2009, 66, 1174–1175. [Google Scholar] [CrossRef]
- Bang, O.Y.; Chung, J.W.; Lee, M.J.; Kim, S.J.; Cho, Y.H.; Kim, G.M.; Chung, C.S.; Lee, K.H.; Ahn, M.J.; Moon, G.J.; et al. Cancer cell-derived extracellular vesicles are associated with coagulopathy causing ischemic stroke via tissue factor-independent way: The OASIS-CANCER study. PLoS ONE 2016, 11, e0159170. [Google Scholar] [CrossRef]
- Chung, J.W.; Cho, Y.H.; Ahn, M.J.; Lee, M.J.; Kim, G.M.; Chung, C.S.; Bang, O.Y. Association of cancer cell type and extracellular vesicles with coagulopathy in patients with lung cancer and stroke. Stroke 2018, 49, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cao, Y. Radiation-induced carotid artery stenosis: A comprehensive review of the literature. Interv. Neurol. 2014, 2, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Maekawa, K.; Kobayashi, K.; Sano, T.; Yabana, T.; Shibata, M.; Miya, F. Tumor Embolism Through Right-to-Left Shunt Due to Venous Invasion of Esophageal Carcinoma. J. Stroke Cerebrovasc. Dis. 2020, 29, 105352. [Google Scholar] [CrossRef]
- Toi, S.; Higuchi, E.; Hosoya, M.; Arai, S.; Ishizuka, K.; Mizuno, T.; Hoshino, T.; Tsutsumi, Y.; Kitagawa, K. Association of Transcranial Doppler Microembolic Signal with Short-Term Mortality in Acute Ischemic Stroke and Active Cancer. J. Am. Heart Assoc. 2024, 13, e033634. [Google Scholar] [CrossRef]
- Iguchi, Y.; Kimura, K.; Kobayashi, K.; Ueno, Y.; Inoue, T. Ischaemic stroke with malignancy may often be caused by paradoxical embolism. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1336–1339. [Google Scholar] [CrossRef]
- Viguier, A.; Pavy le Traon, A.; Massabuau, P.; Valton, L.; Larrue, V. Asymptomatic cerebral embolic signals in patients with acute cerebral ischaemia and severe aortic arch atherosclerosis. J. Neurol. 2001, 248, 768–771. [Google Scholar] [CrossRef] [PubMed]
- French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N. Engl. J. Med. 1996, 334, 1216–1221. [Google Scholar] [CrossRef]
- Mackensen, G.B.; Ti, L.K.; Phillips-Bute, B.G.; Mathew, J.P.; Newman, M.F.; Grocott, H.P. Cerebral embolization during cardiac surgery: Impact of aortic atheroma burden. Br. J. Anaesth. 2003, 91, 656–661. [Google Scholar] [CrossRef]
- Bismuth, J.; Garami, Z.; Anaya-Ayala, J.E.; Naoum, J.J.; El Sayed, H.F.; Peden, E.K.; Lumsden, A.B.; Davies, M.G. Transcranial Doppler findings during thoracic endovascular aortic repair. J. Vasc. Surg. 2011, 54, 364–369. [Google Scholar] [CrossRef]
- Robba, C.; Graziano, F.; Rebora, P.; Elli, F.; Giussani, C.; Oddo, M.; Meyfroidt, G.; Helbok, R.; Taccone, F.S.; Prisco, L.; et al. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): An international, prospective observation- al cohort study. Lancet Neurol. 2021, 20, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Farahvar, A.; Gerber, L.M.; Chiu, Y.-L.; Carney, N.; Härtl, R.; Ghajar, J. Increased mortality in patients with severe traumatic brain in- jury treated without intracranial pressure monitoring. J. Neurosurg. 2012, 117, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Thomale, U.W.; Graetz, D.; Vajkoczy, P.; Sarrafzadeh, A.S. Severe traumatic brain injury in children-a single center experience regarding therapy and long-term outcome. Child’s Nerv. Syst. 2010, 26, 1563–1573. [Google Scholar] [CrossRef]
- Pinto, V.L.; Tadi, P.; Adeyinka, A. Increased Intracranial Pressure; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482119/ (accessed on 27 April 2025).
- Schizodimos, T.; Soulountsi, V.; Iasonidou, C.; Kapravelos, N. An overview of management of intracranial hypertension in the intensive care unit. J. Anesth. 2020, 34, 741–757. [Google Scholar] [CrossRef]
- Carpenter, K.L.H.; Czosnyka, M.; Jalloh, I.; Newcombe, V.F.J.; Helmy, A.; Shannon, R.J.; Budohoski, K.P.; Kolias, A.G.; Kirkpatrick, P.J.; Carpenter, T.A.; et al. Guidelines for the management of severe traumatic brain injury 3rd Edition. J. Neurotrauma. 2007, 24 (Suppl. S1), S1–S106. [Google Scholar] [CrossRef]
- Idro, R.; Marsh, K.; John, C.C.; Newton, C.R.J. Cerebral malaria: Mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr. Res. 2010, 68, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Bellner, J.; Romner, B.; Reinstrup, P.; Kristiansson, K.A.; Ryding, E.; Brandt, L. Transcranial doppler sonography pulsatility index (pi) reflects intracranial pressure (icp). Surg. Neurol. 2004, 62, 45–51. [Google Scholar] [CrossRef]
- Hunter, G.; Voll, C.; Rajput, M. Utility of transcranial doppler in idiopathic intracranial hypertension. Can. J. Neurol. Sci. 2010, 37, 235–239. [Google Scholar] [CrossRef]
- Steinmeier, R.; Laumer, R.; Bondar, I.; Priem, R.; Fahlbusch, R. Cerebral hemodynamics in subarachnoid hemorrhage evaluated by transcranial Doppler sonography. Part 2. Pulsatility indices: Normal reference values and characteristics in subarachnoid hemorrhage. Neurosurgery 1993, 33, 10–19. [Google Scholar] [CrossRef]
- Voulgaris, S.G.; Partheni, M.; Kaliora, H.; Haftouras, N.; Pessach, I.S.; Polyzoidis, K.S. Early cerebral monitoring using the transcranial Doppler pulsatility index in patients with severe brain trauma. Med. Sci. Monit. 2005, 11, CR49-52. [Google Scholar] [PubMed]
- Steiger, H.J. Carotid Doppler hemodynamics in post- traumatic intracranial hypertension. Surg. Neurol. 1981, 16, 459–461. [Google Scholar] [CrossRef]
- Homburg, A.M.; Jakobsen, M.; Enevoldsen, E. Transcranial Doppler recordings in raised intracranial pressure. Acta Neurol. Scand. 1993, 87, 488–493. [Google Scholar] [CrossRef]
- Moreno, J.A.; Mesalles, E.; Gener, J.; Tomasa, A.; Ley, A.; Roca, J.; Fernández-Llamazares, J. Evaluating the outcome of severe head injury with transcranial Doppler ultrasonography. Neurosurg. Focus 2000, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.A.; Czosnyka, M.; Matta, B.F.; Gooskens, I.; Piechnik, S.; Pickard, J.D. Non-invasive cerebral perfusion pressure (nCPP): Evaluation of the monitoring methodology in head-injured patients. Acta Neurochir. Suppl. 2000, 76, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Czosnyka, M.; Carrera, E.; de Riva, N.; Pickard, J.D.; Smielewski, P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery 2012, 71, 853–861. [Google Scholar] [CrossRef]
- Behrens, A.; Lenfeldt, N.; Ambarki, K.; Malm, J.; Eklund, A.; Koskinen, L.O. Transcranial Doppler pulsatility index: Not an accurate method to assess intracranial pressure. Neurosurgery 2010, 66, 1050–1057. [Google Scholar] [CrossRef]
- Lee, K.Y.; Sohn, Y.H.; Baik, J.S.; Kim, G.W.; Kim, J.S. Arterial pulsatility as an index of cerebral microangiopathy in diabetes. Stroke 2000, 31, 1111–1115. [Google Scholar] [CrossRef]
- Ursino, M.; Giulioni, M.; Lodi, C.A. Relationships among cerebral perfusion pressure, autoregulation, and transcranial Doppler waveform: A modeling study. J. Neurosurg. 1998, 89, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hamilton, R.; Pineles, S.; Bergsneider, M.; Hu, X. Non-invasive Intracranial Hypertension Detection Utilizing Semisupervised Learning. IEEE Trans. Biomed. Eng. 2012, 60, 1126–1133. [Google Scholar] [CrossRef][Green Version]
- Hu, X.; Xu, P.; Scalzo, F.; Vespa, P.; Bergsneider, M. Morphological clustering and analysis of continuous intracranial pressure. IEEE Trans. Biomed. Eng. 2009, 56, 696–705. [Google Scholar] [CrossRef]
- Wei, M.; Mercer, R.; Lin, J.; Krakauskaite, S.; Bartusis, L.; Scalzo, F. Non-invasive Intracranial Hypertension Detection using Machine-learning of Cerebral Blood Flow Velocity Waveforms. SSRN 2025, 34, 50–59. [Google Scholar] [CrossRef]
- Megjhani, M.; Terilli, K.; Weinerman, B.; Nametz, D.; Kwon, S.B.; Velazquez, A.; Ghoshal, S.; Roh, D.J.; Agarwal, S.; Connolly, E.S., Jr.; et al. A deep learning framework for deriving non-invasive intracranial pressure waveforms from transcranial doppler. Ann. Neurol. 2023, 94, 196–202. [Google Scholar] [CrossRef]
- Krieg, S.M.; Schwendner, M.; Kram, L.; Zhang, H.; Liang, R.; Negwer, C.; Meyer, B. Transcranial Transmission Ultrasound for Reliable Non-invasive Exclusion of Intracranial Hypertension in Traumatic Brain Injury Patients: A Proof of Concept Study. J. Neurotrauma 2024, 41, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Frigieri, G.; Brasil, S.; Cardim, D.; Czosnyka, M.; Ferreira, M.; Paiva, W.S.; Hu, X. Machine learning approach for non-invasive intracranial pressure estimation using pulsatile cranial expansion waveforms. npj Digit. Med. 2025, 8, 57. [Google Scholar] [CrossRef]
- Bhargava, V.; Tawfik, D.; Tan, Y.J.; Dunbar, T.; Haileselassie, B.; Su, E. Ultrasonographic optic nerve sheath diameter measurement to detect intracranial hypertension in children with neurological injury: A systematic review. Pediatr. Crit. Care Med. 2020, 21, e858–e868. [Google Scholar] [CrossRef]
- Dubourg, J.; Javouhey, E.; Geeraerts, T.; Messerer, M.; Kassai, B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2011, 37, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Yic, C.D.; Pontet, J.; Mercado, M.; Muñoz, M.; Biestro, A. Ultrasonographic measurement of the optic nerve sheath diameter to detect intracranial hypertension: An observational study. Ultrasound J. 2023, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Netteland, D.F.; Aarhus, M.; Smistad, E.; Sandset, E.C.; Padayachy, L.; Helseth, E.; Brekken, R. Non-invasive intracranial pressure assessment by optic nerve sheath diameter: Automated measurements as an alternative to clinician-performed measurements. Front. Neurol. 2023, 14, 1064492. [Google Scholar] [CrossRef]
- Kim, K.H.; Kang, H.K.; Koo, H.-W. Prediction of Intracranial Pressure in Patients with an Aneurysmal Subarachnoid Hemorrhage Using Optic Nerve Sheath Diameter via Explainable Predictive Modeling. J. Clin. Med. 2024, 13, 2107. [Google Scholar] [CrossRef]
- Fu, Z.; Peng, L.; Guo, L.; Qin, C.; Yu, Y.; Zhang, J.; Liu, Y. Ultrasound-based radiomics and clinical factors-based nomogram for early intracranial hypertension detection in patients with decompressive craniotomy. Front. Med. Technol. 2025, 7, 1485244. [Google Scholar] [CrossRef]
- Sahuquillo, P.; Tembl, J.I.; Parkhutik, V.; Vázquez, J.F.; Sastre, I.; Lago, A. The study of deep brain structures by transcranial duplex sonography and imaging resonance correlation. Ultrasound Med. Biol. 2013, 39, 226–232. [Google Scholar] [CrossRef]
- Yilmaz, R.; Berg, D. Transcranial B-Mode Sonography in Movement Disorders. Int. Rev. Neurobiol. 2018, 143, 179–212. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Godau, J.; Walter, U. Transcranial sonography in movement disorders. Lancet Neurol. 2008, 7, 1044–1055. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, L.; Liu, J.; Chen, H.; Wang, C.; Ding, H.; Zhang, Q. PADS-Net: GAN-based radiomics using multi-task network of denoising and segmentation for ultrasonic diagnosis of Parkinson disease. Comput. Med. Imaging Graph. 2025, 120, 102490. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Zhang, W.; Li, S.; Wang, X.; Sun, Y.; Sun, X.; Li, F.X.; Hou, C.; Lam, S.K.; Zheng, Y.P. A comprehensive benchmarking of a U-Net based model for midbrain auto-segmentation on transcranial sonography. Comput. Methods Programs Biomed. 2025, 258, 108494. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shi, Y.; Wan, L.; Liu, J.; Wan, Y.; Jiang, H.; Qiu, R. Attention-enhanced dilated convolution for Parkinson’s disease detection using transcranial sonography. Biomed. Eng. Online 2024, 23, 76. [Google Scholar] [CrossRef]
- Ding, C.W.; Ren, Y.K.; Wang, C.S.; Zhang, Y.C.; Zhang, Y.; Yang, M.; Mao, P.; Sheng, Y.J.; Chen, X.F.; Liu, C.F. Prediction of Parkinson’s disease by transcranial sonography-based deep learning. Neurol. Sci. 2024, 45, 2641–2650. [Google Scholar] [CrossRef]
- Fei, X.Y.; Dong, Y.; An, H.D.; Zhang, Q.; Zhang, Y.C.; Shi, J. Impact of region of interest size on transcranial sonography based computer-aided diagnosis for Parkinson’s disease. Math. Biosci. Eng. 2019, 16, 5640–5651. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, R.; Granert, O.; Schäffer, E.; Jensen-Kondering, U.; Schulze, S.; Bartsch, T.; Berg, D. Transcranial Sonography Findings in Alzheimer’s Disease: A New Imaging Biomarker. Ultraschall Med. 2021, 42, 623–633. [Google Scholar] [CrossRef]
- Allen, B.C.; Kapoor, S.; Anzalone, A.; Mayer, K.P.; Wolfe, S.Q.; Duncan, P.; Asimos, A.W.; D’Agostino, R., Jr.; Winslow, J.T.; Sarwal, A. Transcranial ultrasonography to detect intracranial pathology: A systematic review and meta-analysis. J. Neuroimaging 2023, 33, 333–358. [Google Scholar] [CrossRef]
- Meyer, K.; Seidel, G.; Knopp, U. Transcranial sonography of brain tumors in the adult: An in vitro and in vivo study. J. Neuroimaging 2001, 11, 287–292. [Google Scholar] [CrossRef]
- Della Pepa, G.M.; Menna, G.; Ius, T.; Di Bonaventura, R.; Altieri, R.; Marchese, E.; Olivi, A.; Sabatino, G.; La Rocca, G. Contrast enhanced ultrasound (CEUS) applications in neurosurgical and neurological settings—New scenarios for brain and spinal cord ultrasonography. A systematic review. Clin. Neurol. Neurosurg. 2020, 198, 106105. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; McDicken, W.N.; Skidmore, R.; Woodcock, J.P. Doppler Ultrasound: Physics, Instrumentation and Clinical Applications; Wiley: Hoboken, NJ, USA, 1989. [Google Scholar]
- Miao, J.; Benkeser, P.J.; Nichols, F.T. A computer-based statistical pattern recognition for Doppler spectral waveforms of intracranial blood flow. Comput. Biol. Med. 1996, 26, 53–63. [Google Scholar] [CrossRef]
- Jahren, T.S.; Steen, E.N.; Aase, S.A.; Solberg, A.H.S. Estimation of End-Diastole in Cardiac Spectral Doppler Using Deep Learning. IEEE Trans Ultrason. Ferroelectr. Freq. Control 2020, 67, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, J.H.; Lee, S.J. Artificial intelligence-based speckle featurization and localization for ultrasound speckle tracking velocimetry. Ultrasonics 2024, 138, 107241. [Google Scholar] [CrossRef] [PubMed]
- Ubeyli, E.D.; Güler, I. Feature extraction from Doppler ultrasound signals for automated diagnostic systems. Comput. Biol. Med. 2005, 35, 735–764. [Google Scholar] [CrossRef] [PubMed]
- Ubeyli, E.D. Usage of eigenvector methods to improve reliable classifier for Doppler ultrasound signals. Comput. Biol. Med. 2008, 38, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Nahas, H.; Yiu, B.Y.; Chee, A.J.; Au, J.S.; Yu, A.C. Deep-learning-assisted and GPU-accelerated vector Doppler imaging with aliasing-resistant velocity estimation. Ultrasonics 2023, 134, 107050. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.A.; Gough, N.A.; Rakebrandt, F.; Wahab, M.; Woodcock, J.P. Neural network analysis of Doppler ultrasound blood flow signals: A pilot study. Ultrasound Med. Biol. 1997, 23, 683–690. [Google Scholar] [CrossRef]
- Güler, İ.; Übeyli, E.D. Feature saliency using signal-to-noise ratios in automated diagnostic systems developed for Doppler ultrasound signals. Eng. Appl. Artif. Intell. 2006, 19, 53–63. [Google Scholar] [CrossRef]

| Reference | Year of Publication | Sample Size (N° of Patients) | Data-Source Origin | Algorithm | Validation Strategy | Performance Metrics | |
|---|---|---|---|---|---|---|---|
| ICAS | [69] Hsu, K-C et al. | 2020 | 8211 | Single-center cohort | SVM | Leave-one-out (LOO) cross-validation with ten bootstrap samplings |
Sensitivity: 71.7–100% Specificity: 88.9–100% |
| [71] Mei, Y. J. et al. | 2022 | 276 (203 healthy individuals and 73 patients with ICAS) | Single-center cohort | CNN | Comparison with healthy group | Sensitivity: 80% Specificity: 83% | |
| [72] Yeh, C.-Y. et al. | 2022 | 538 | Public registry (DICOM) | ML model | 10-fold cross-validation | Accuracy: 67–86% | |
| [73] Xu, D. et al. | 2024 | 1729 | Single-center cohort | DL (model VGG16) | Combined dataset consisting of TCD examination images from hospitalized patients (dataset1) and a population undergoing routine medical check-ups (dataset2) | Accuracy: 85.67 ± 0.43 Sensitivity: 83.60 ± 1.60 Specificity: 87.73 ± 1.47 | |
| [74] Nisha, N.N. et al. | 2023 | 18 (6 healthy individuals and 12 patients) | Public registry | Self-Organized Operational Neural Network (Self-ONN)-based deep learning model: Self-ResAttentioNet18 | 5-fold cross-validation |
Accuracy: 96.05% Specificity: 96% ROC curve: 0.99 |
| Reference | Year of Publication | Sample Size (N° of Patients) | Data-Source Origin | Algorithm | Validation Strategy | Performance Metrics | |
|---|---|---|---|---|---|---|---|
| SAH | [117] Elzaafarany, K. et al. | 2018 | 160 | Public registry | Pattern-recognition ML model | Error analysis was performed by using precision and recall measures | Sensitivity: 87.5% Specificity: 89.7% |
| [118] Kumar, G. et al. | 2017 | 267 | Public registry | ML model | Cross-validation technique used for training a classifier using 50% of the data | Sensitivity: 78% Specificity: 91% | |
| [120] Clare, K. Et al | 2022 | 12 | Single-center cohort | NovaGuide Model | Comparison with CTA studies | Sensitivity: 83% Negative predictive value: 90% Positive likelihood ratio: 8.75 | |
| [123] Kim, Y-G. et al. | 2023 | 19 | Single-center cohort | DL-based convolutional layer-based neural network | Training dataset comprised 1727 Doppler wave files; the remaining 565 files were evaluated for validation using the proposed classifier | Accuracy: 90% Sensitivity:90% | |
| [125] Mohammadzadeh, I. et al. | 2025 | Public registry (metanalysis of eight studies) | ML algorithm | Sensitivity: 79% Specificity: 78% AUC: 0.85 |
| Reference | Year of Publication | Sample Size (N° of Patients) | Data-Source Origin | Algorithm | Validation Strategy | Performance Metrics | |
|---|---|---|---|---|---|---|---|
| RTL SHUNT (patent foramen ovale) | [137] Rubin, M. N. et al. | 2023 | 129 | Multi-center cohort | Robot-assisted TCD (raTCD) | Comparison with TTE | Sensitivity: 64% |
| [139] Chang, I. et al. | 2024 | 212 | Single-center retrospective cohort study | raTCD | Comparison with TEE | Sensitivity: 91–100% Specificity: 78–100% | |
| [141] Shah, R. et al. | 2025 | 148 | Single-center cohort | raTCD | Comparison with TTE bubble studies, performed by certified ultrasonographers and read by blinded level III echocardiography board-certified cardiologists | Sensitivity: 95% Specificity: 88.9% | |
| RTL SHUNT (cardiac valve embolism) | [154] Baig, A. et al. | 2022 | 8 | Single-center cohort | TCD robot head-brace system | Linear regression model | - |
| RTL SHUNT (atrial fibrillation) | [157] Meszaros, H. et al. | 2024 | 26 | Single-center cohort | raTCD | Post-operative cranial MRI exams were performed; the MES load from the different pulmonary veins was compared using the Wilcoxon test and Bonferroni correction | Statistical difference comparing pulmonary veins, with p value < 0.01 |
| [158] Della Rocca, D. G. et al. | 2023 | 20 | Single-center cohort | raTCD | MRI sequences 24–48 h post-ablation | Statistical difference comparing procedures, with p value < 0.01 | |
| RTL SHUNT (carotid embolism) | [162] Fattorello Salimbeni, A. et al. | 2024 | 92 | Single-center cohort | NovaGuide™2 Intelligent Ultrasound | Comparison with parallel manual evaluation | High accuracy |
| Reference | Year of Publication | Sample Size (N° of Patients) | Data-Source Origin | Algorithm | Validation Strategy | Performance Metrics | |
|---|---|---|---|---|---|---|---|
| ICH | [197] Wei, M. et al. | 2025 | 89 | Single-center cohort | MOCAIP algorithm | 10-fold cross-validation | ROC curve (AUC) of 96% |
| [198] Megjhani, M. et al. | 2023 | 13 | Single-center cohort | ML model | Leave-one-session-out cross-validation technique | High accuracy | |
| [199] Krieg, S. M. et al. | 2024 | 25 | Single-center cohort | ML model | Comparison with invasive monitoring | Sensitivity: 100 Specificity: 47% NPV: 100% PPV: 14% | |
| [206] Fu, Z. et al. | 2025 | 199 | Retrospective observational single-cohort study | SVM | LASSO regression | AUC: 0.840 Accuracy: 0.853 Sensitivity: 0.69 Specificity: 0.89 PPV: 0.800 NPV: 0.858 |
| Aim of the Use of AI-Applied Methods | Parameters | Results | Benefits | Limitations | |
|---|---|---|---|---|---|
| ICAS | Evaluation of hemodynamic variables to indirectly identify intracranial stenosis to select the population needing to undergo further examinations | FV, PSV, EDV, MFV | Reduction in FV; increase in PSV, EDV and MFV; reduction in PI | Reducing the ionizing radiation exposure | Accuracy related to the degree of stenosis, with weak accuracy in the case of mild stenosis |
| SAH | Early detection of cerebral vasospasm by variations in cerebral circulation | MFV, intra-extracranial MFV ratio; PSV and EDV | MFV > 120 cm/s and MFV ratio > 3 is indicative of vasospasm; increase in PSV and EDV | Easy monitoring; high-grade vasospasm is demonstrated by TCD findings 24–48 h before the appearance of clinical symptoms | Inability to insonate intracranial vessels in 10% to 20% of patients, spatial resolution of TCD limited for the posterior circulation |
| RTL SHUNT (PFO) | Detection of HITSs for non-invasive diagnosis of PFO | Microembolic signals | High-intensity transient signals on the spectrogram | More feasible, superior sensitivity compared to TTE and TEE; more cost-effective than echocardiography; estimation of the PFO is largely on the basis of the HITS numbers; fewer false negatives; quantification of the RLS severity conducted by using the Spencer Logarithmic Grading Scale (SLS) | TCD with bubble injection is more sensitive but less specific than TTE and TEE with bubble studies |
| RTL SHUNT (cardiac valve embolism, atrial fibrillation, and carotid embolism) | Detection of cerebral HITSs to evaluate the risk of periprocedural stroke | Microembolic signals on the MCA | The number of HITSs is associated with the risk of symptomatic cerebral ischemia | Intraoperative non-invasive monitoring, assessment of the safety of the procedures | Expert operators, good temporal windows, maintaining the head position |
| ICH | Non-invasive detection and monitoring of ICH | Evaluation of the CBFV waveforms and PI; ONSD measure | Three-peak CBFV waveform and ONSD > 5 mm are abnormal | Feasibility in patients with contraindications to lumbar puncture, repeatable examination for continuous monitoring in ICU settings | Expert operators, good temporal windows, maintaining the head position |
| NEURO-DEGENERATIVE DEMENTIA | Recognizing specific patterns in the echogenicity of the brain structures to help diagnosis | SN hyper-echogenicity is present in up to 90% of PD patients | Autosegmentation of the cerebral regions with different echogenic patterns is associated with specific diseases | Non-invasive instrument for early diagnosis of neurological decline and cognitive impairment; other acoustic windows include the suboccipital and orbital windows, which allow for the visualization of the posterior fossa and optic nerve, respectively | Need for expert operators |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miceli, G.; Basso, M.G.; Cocciola, E.; Tuttolomondo, A., on behalf of the Italian Society of Neurosonology and Cerebral Hemodynamics (SINSEC) and SINSEC Study Group for Artificial Intelligence in Neurosonology. Artificial Intelligence in the Diagnostic Use of Transcranial Doppler and Sonography: A Scoping Review of Current Applications and Future Directions. Bioengineering 2025, 12, 681. https://doi.org/10.3390/bioengineering12070681
Miceli G, Basso MG, Cocciola E, Tuttolomondo A on behalf of the Italian Society of Neurosonology and Cerebral Hemodynamics (SINSEC) and SINSEC Study Group for Artificial Intelligence in Neurosonology. Artificial Intelligence in the Diagnostic Use of Transcranial Doppler and Sonography: A Scoping Review of Current Applications and Future Directions. Bioengineering. 2025; 12(7):681. https://doi.org/10.3390/bioengineering12070681
Chicago/Turabian StyleMiceli, Giuseppe, Maria Grazia Basso, Elena Cocciola, and Antonino Tuttolomondo on behalf of the Italian Society of Neurosonology and Cerebral Hemodynamics (SINSEC) and SINSEC Study Group for Artificial Intelligence in Neurosonology. 2025. "Artificial Intelligence in the Diagnostic Use of Transcranial Doppler and Sonography: A Scoping Review of Current Applications and Future Directions" Bioengineering 12, no. 7: 681. https://doi.org/10.3390/bioengineering12070681
APA StyleMiceli, G., Basso, M. G., Cocciola, E., & Tuttolomondo, A., on behalf of the Italian Society of Neurosonology and Cerebral Hemodynamics (SINSEC) and SINSEC Study Group for Artificial Intelligence in Neurosonology. (2025). Artificial Intelligence in the Diagnostic Use of Transcranial Doppler and Sonography: A Scoping Review of Current Applications and Future Directions. Bioengineering, 12(7), 681. https://doi.org/10.3390/bioengineering12070681







