Stabilization of the Bio-Oil Organic Phase via Solvent-Assisted Hydrotreating, Part 1: Investigating the Influence of Various Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fast Pyrolysis Procedure
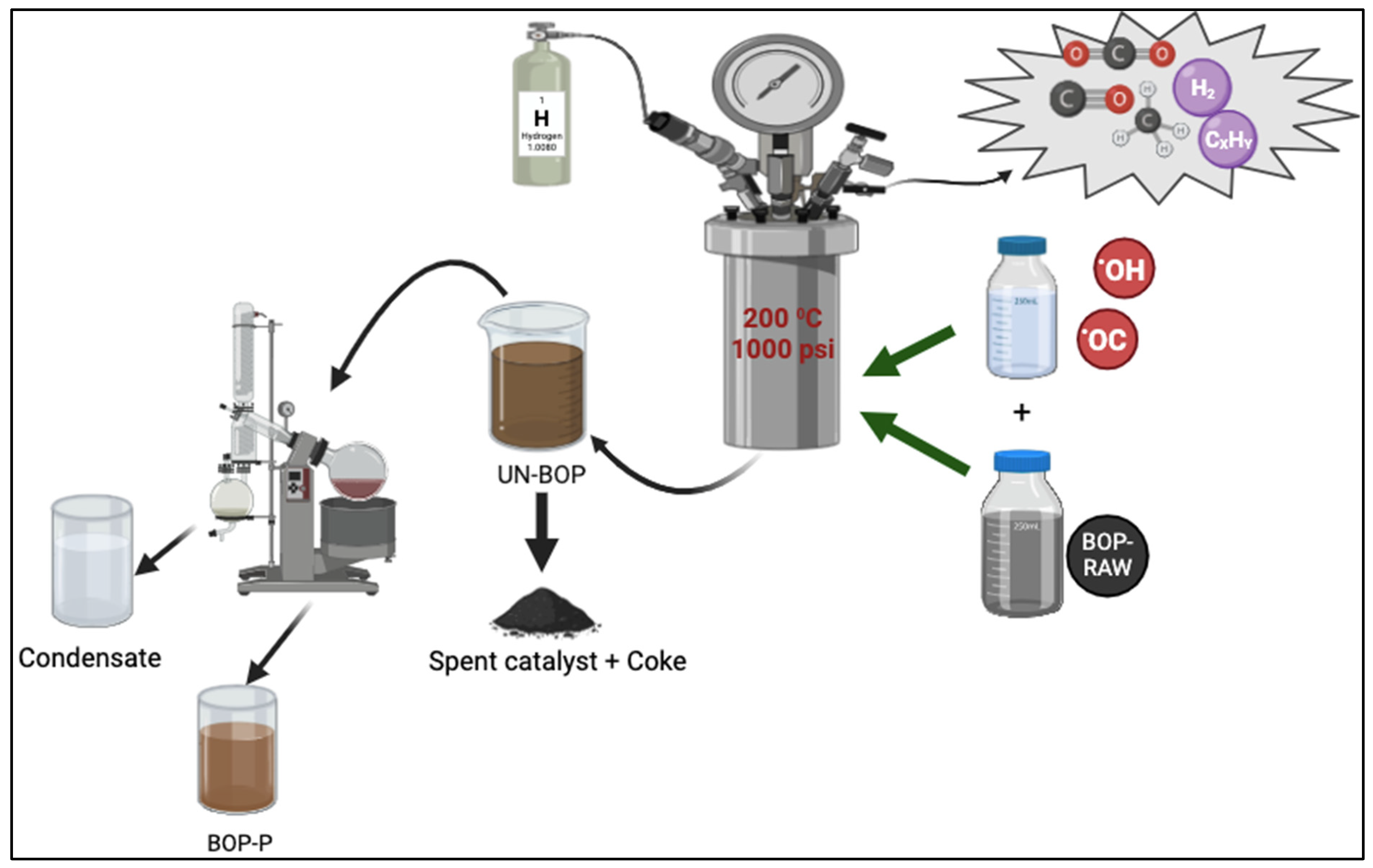
2.3. BOP Stabilization Procedure
2.4. Product Characterization
3. Results and Discussion
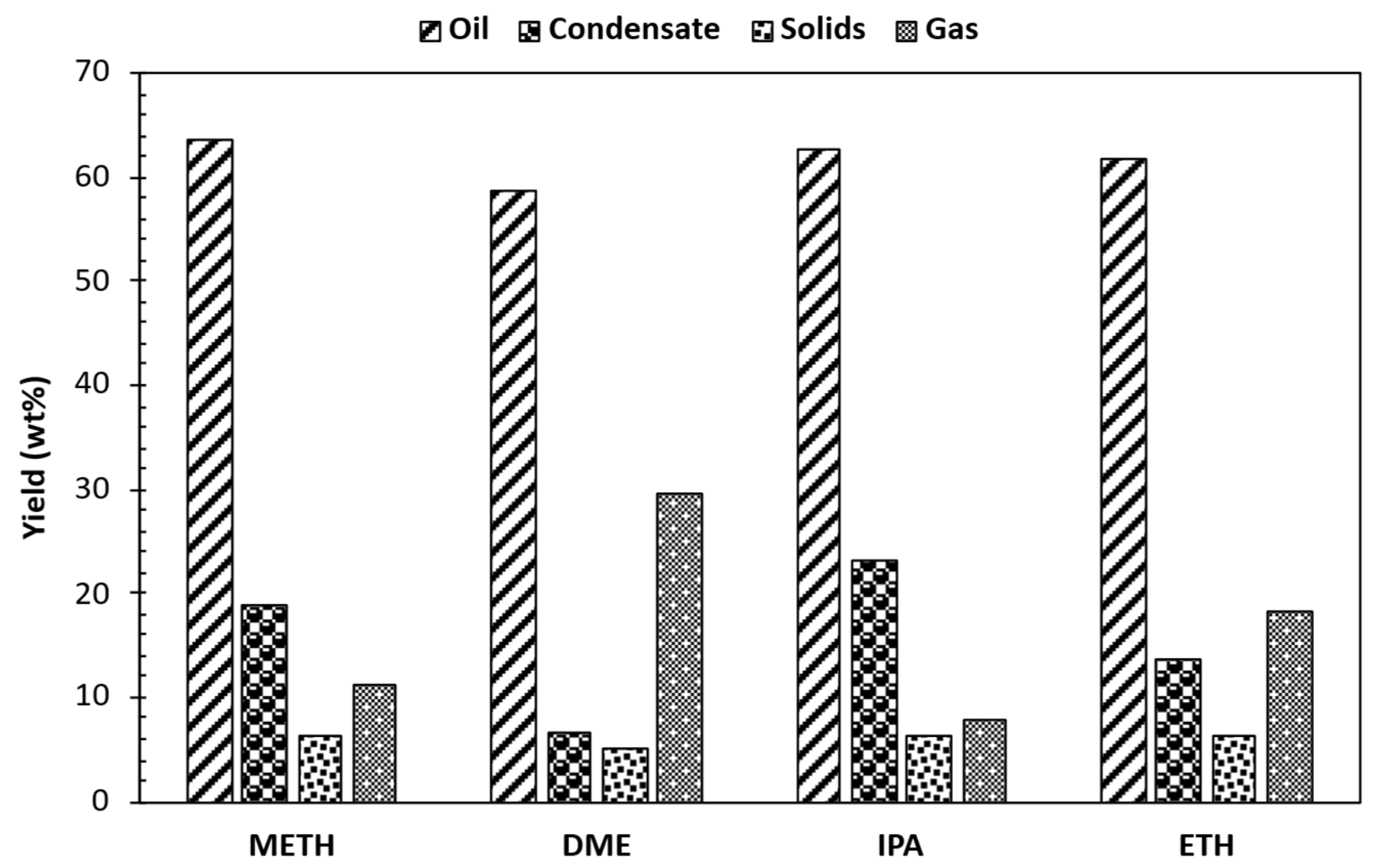
3.1. Product Yields
3.2. Physicochemical Properties of Raw, Blended, and Stabilized BOP
3.3. Energy Density and Moisture Content Reduction
3.4. Degree of Deoxygenation and Carbon Recovery
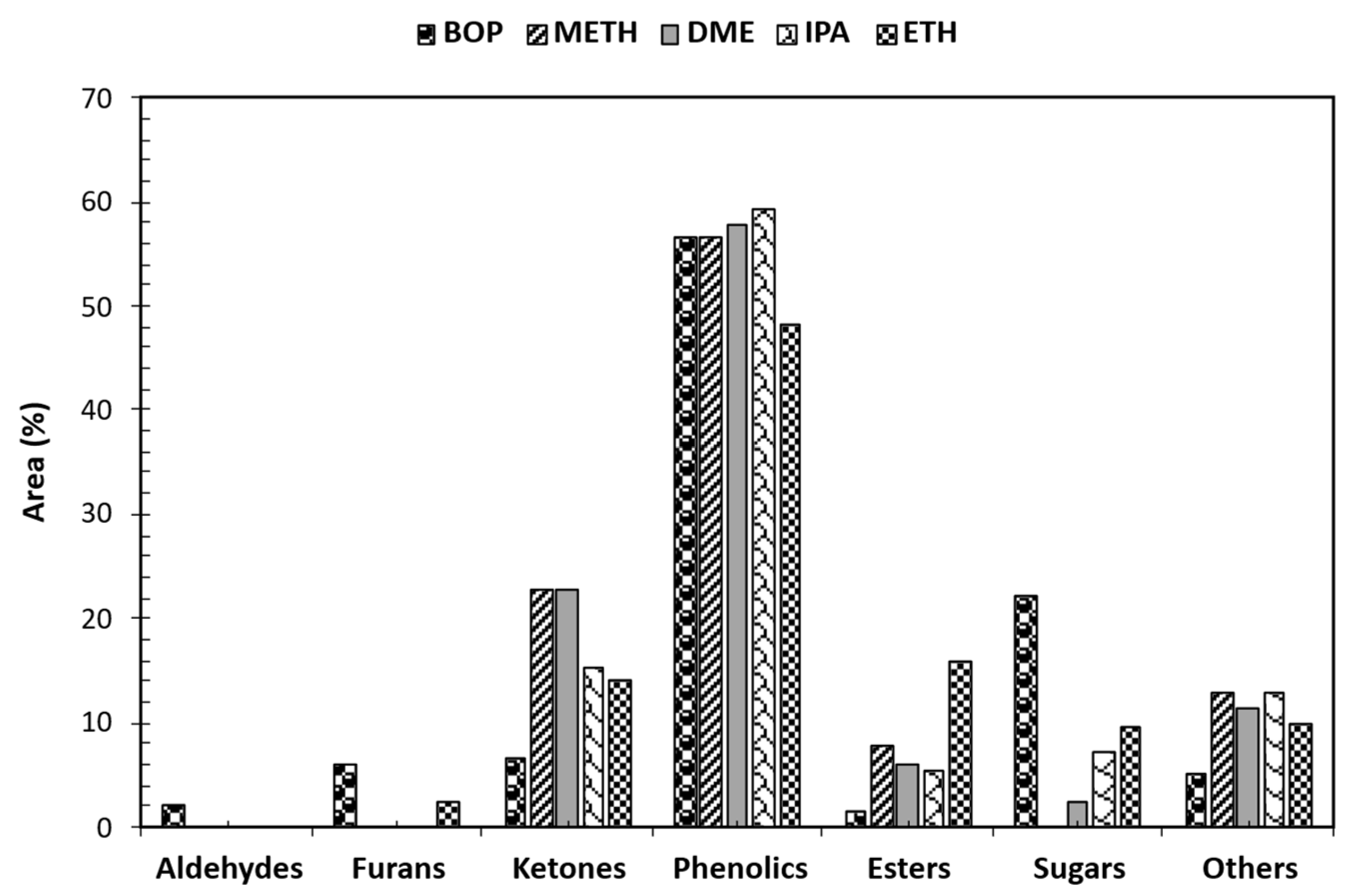
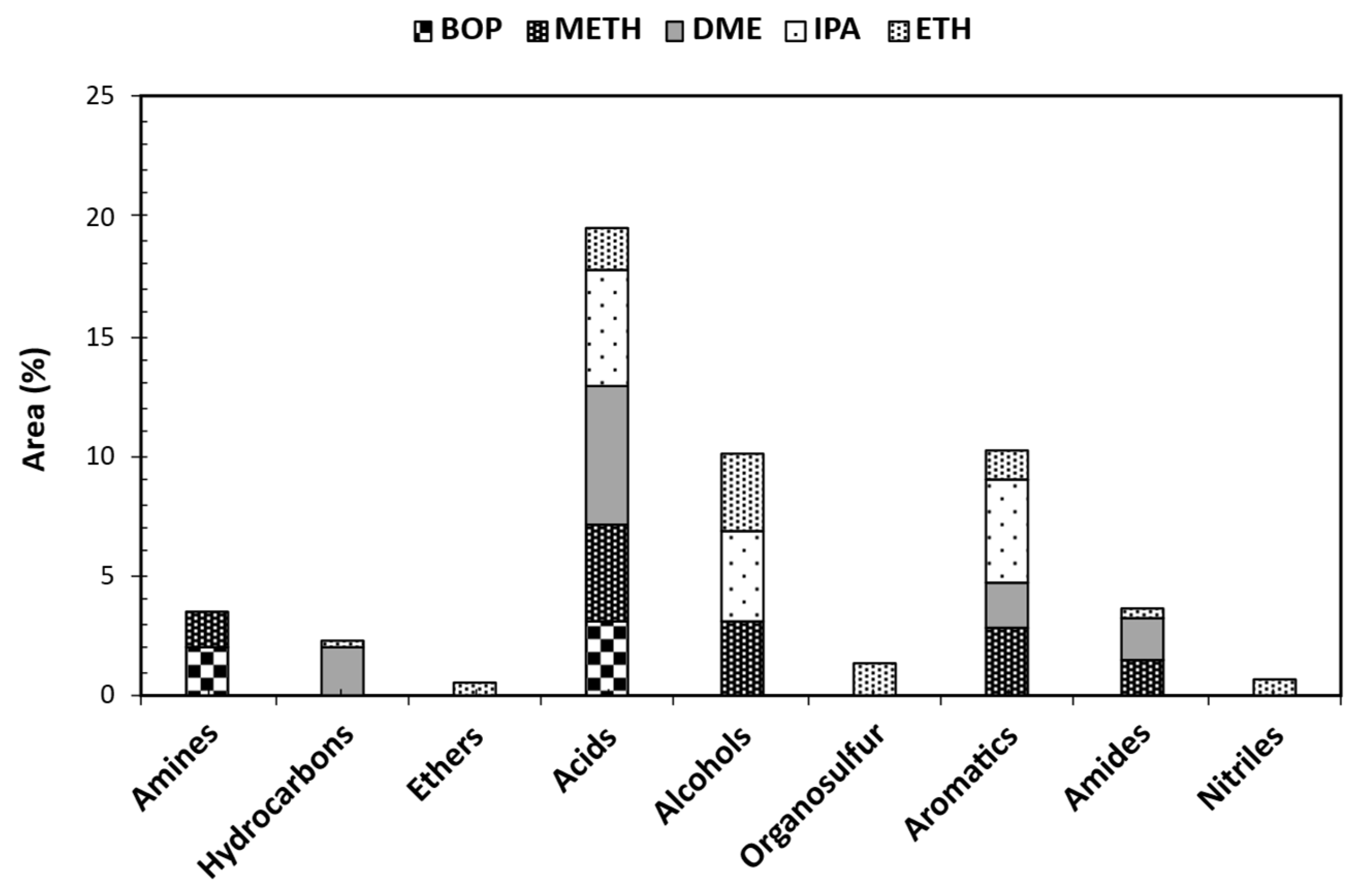
3.5. GCMS Analysis of Stabilized BOP
3.6. Van Krevelen Plot of Raw, Blended, and Stabilized BOP
3.7. Statistical Comparison of Treatment Effects
3.8. Degree of Dehydration and Deoxygenation
3.9. Implications for Bio-Oil Upgrading
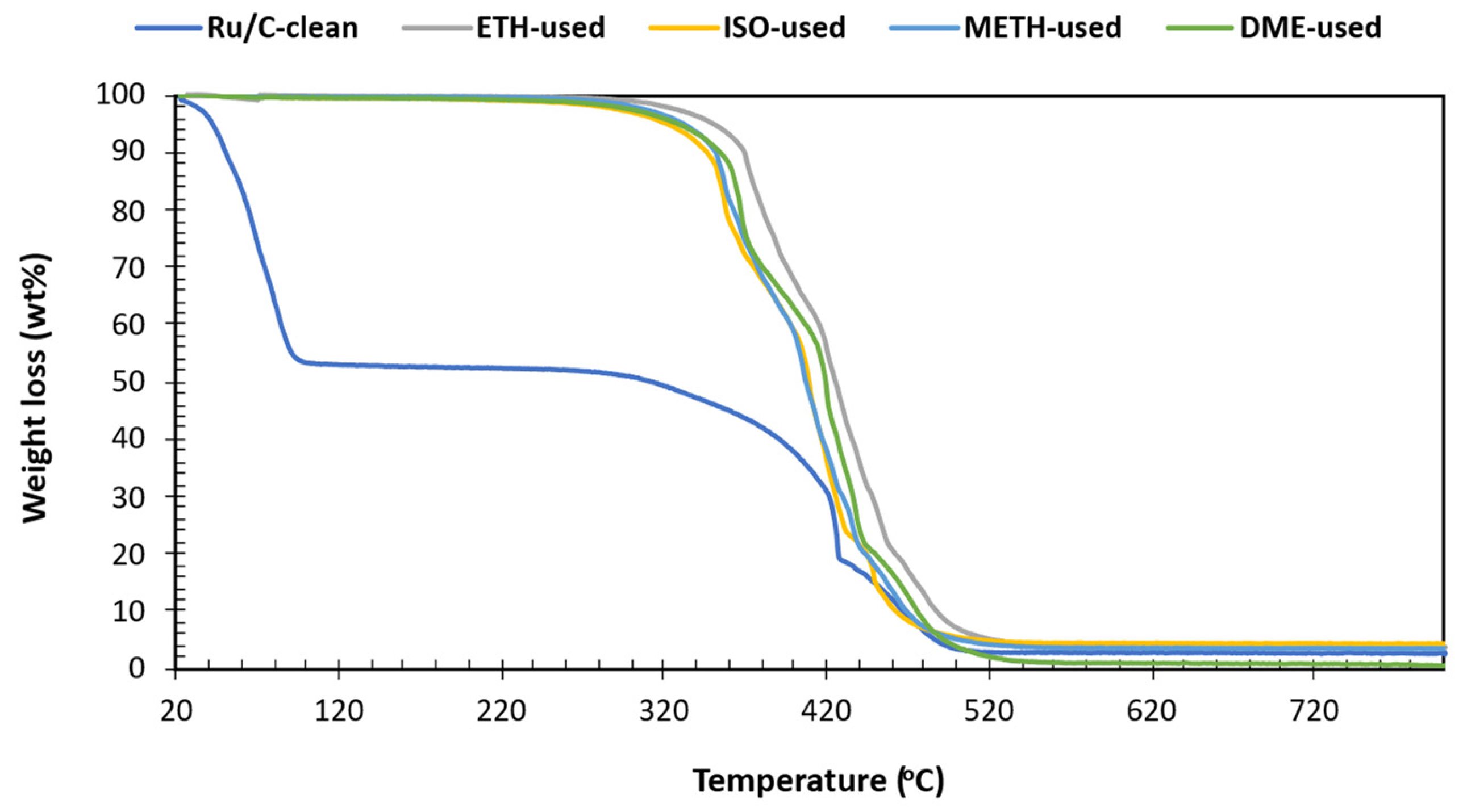
3.10. Turnover Frequency Thermogravimetric Analysis of Catalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez, J.; Curt, M.D.; Robert, N.; Fernández, J. Biomass resources. In The Role of Bioenergy in the Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–111. [Google Scholar]
- Çağlar, A.; Demirbaş, A. Conversion of cotton cocoon shell to hydrogen rich gaseous products by pyrolysis. Energy Convers. Manag. 2002, 43, 489–497. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sar, T.; Gowd, S.C.; Rajendran, K.; Kumar, V.; Sarsaiya, S.; Li, Y.; Sindhu, R.; Binod, P.; Zhang, Z.; et al. A comprehensive review on thermochemical, and biochemical conversion methods of lignocellulosic biomass into valuable end product. Fuel 2023, 342, 127790. [Google Scholar] [CrossRef]
- Pawar, A.; Panwar, N.; Salvi, B. Comprehensive review on pyrolytic oil production, upgrading and its utilization. J. Mater. Cycles Waste Manag. 2020, 22, 1712–1722. [Google Scholar] [CrossRef]
- Lohitharn, N.; Shanks, B.H. Upgrading of bio-oil: Effect of light aldehydes on acetic acid removal via esterification. Catal. Commun. 2009, 11, 96–99. [Google Scholar] [CrossRef]
- Auersvald, M.; Shumeiko, B.; Staš, M.; Kubička, D.; Chudoba, J.; Šimáček, P. Quantitative Study of straw bio-oil hydrodeoxygenation over a sulfided nimo catalyst. ACS Sustain. Chem. Eng. 2019, 7, 7080–7093. [Google Scholar] [CrossRef]
- Oasmaa, A.; Kuoppala, E.; Elliott, D.C. Development of the Basis for an analytical protocol for feeds and products of bio-oil hydrotreatment. Energy Fuels 2012, 26, 2454–2460. [Google Scholar] [CrossRef]
- Garba, M.; Musa, U.; Olugbenga, A.; Mohammad, Y.S.; Yahaya, M.; Ibrahim, A. Catalytic upgrading of bio-oil from bagasse: Thermogravimetric analysis and fixed bed pyrolysis. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 776–781. [Google Scholar] [CrossRef]
- Gollakota, A.R.; Reddy, M.; Subramanyam, M.D.; Kishore, N. A review on the upgradation techniques of pyrolysis oil. Renew. Sustain. Energy Rev. 2016, 58, 1543–1568. [Google Scholar] [CrossRef]
- Roy, P.; Jahromi, H.; Rahman, T.; Baltrusaitis, J.; Hassan, E.B.; Torbert, A.; Adhikari, S. Hydrotreatment of pyrolysis bio-oil with non-edible carinata oil and poultry fat for producing transportation fuels. Fuel Process. Technol. 2023, 245, 107753. [Google Scholar] [CrossRef]
- Capunitan, J.A.; Capareda, S.C. Hydrotreatment of corn stover bio-oil using noble metal catalysts. Fuel Process. Technol. 2014, 125, 190–199. [Google Scholar] [CrossRef]
- Shumeiko, B.; Auersvald, M.; Straka, P.; Šimáček, P.; Vrtiška, D.; Kubička, D. Efficient one-stage bio-oil upgrading over sulfided catalysts. ACS Sustain. Chem. Eng. 2020, 8, 15149–15167. [Google Scholar] [CrossRef]
- Han, Y.; Gholizadeh, M.; Tran, C.-C.; Kaliaguine, S.; Li, C.-Z.; Olarte, M.; Garcia-Perez, M. Hydrotreatment of pyrolysis bio-oil: A review. Fuel Process. Technol. 2019, 195, 106140. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.-D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Mullen, C.A.; Pighinelli, A.L.; Boateng, A.A. Hydrodeoxygenation of fast-pyrolysis bio-oils from various feedstocks using carbon-supported catalysts. Fuel Process. Technol. 2014, 123, 11–18. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Gunawan, R.; Hu, X.; Kadarwati, S.; Westerhof, R.; Chaiwat, W.; Hasan, M.; Li, C.-Z. Importance of hydrogen and bio-oil inlet temperature during the hydrotreatment of bio-oil. Fuel Process. Technol. 2016, 150, 132–140. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Rodríguez-Mirasol, J.; Cordero, T.; Bilbao, J. Advances and Challenges in the valorization of bio-oil: Hydrodeoxygenation using carbon-supported catalysts. Energy Fuels 2021, 35, 17008–17031. [Google Scholar] [CrossRef]
- Ardiyanti, A.; Gutierrez, A.; Honkela, M.; Krause, A.; Heeres, H. Hydrotreatment of wood-based pyrolysis oil using zirconia-supported mono-and bimetallic (Pt, Pd, Rh) catalysts. Appl. Catal. A Gen. 2011, 407, 56–66. [Google Scholar] [CrossRef]
- Shezi, M.; Kiambi, S.L. Isothermal Pyrolysis of Bamboo and Pinewood Biomass: Product Characterization and Comparative Study in a Fluidized Bed Reactor. Bioengineering 2025, 12, 99. [Google Scholar] [CrossRef]
- ASTM D5373; Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke. ASTM: West Conshohocken, PA, USA, 2021.
- ASTM D5291; Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Petroleum Products and Lubricants. ASTM: West Conshohocken, PA, USA, 2021.
- ASTM D664-07; Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM: West Conshohocken, PA, USA, 2010.
- Ahamed, T.S.; Anto, S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Upgrading of bio-oil from thermochemical conversion of various biomass–Mechanism, challenges and opportunities. Fuel 2021, 287, 119329. [Google Scholar] [CrossRef]
- Omar, S.; Alsamaq, S.; Yang, Y.; Wang, J. Production of renewable fuels by blending bio-oil with alcohols and upgrading under supercritical conditions. Front. Chem. Sci. Eng. 2019, 13, 702–717. [Google Scholar] [CrossRef]
- Yu, F.; Deng, S.; Chen, P.; Liu, Y.; Wan, Y.; Olson, A.; Kittelson, D.; Ruan, R. Physical and chemical properties of bio-oils from microwave pyrolysis of corn stover. In Applied Biochemistry and Biotecnology: The Twenty-Eighth Symposium, Proceedings of the Twenty-Eight Symposium on Biotechnology for Fuels and Chemicals, Nashville, TN, USA, 30 April–3 May 2006; Springer: Berlin/Heidelberg, Germany, 2007; pp. 957–970. [Google Scholar]
- Pidtasang, B.; Sukkasi, S.; Pattiya, A. Effect of in-situ addition of alcohol on yields and properties of bio-oil derived from fast pyrolysis of eucalyptus bark. J. Anal. Appl. Pyrolysis 2016, 120, 82–93. [Google Scholar] [CrossRef]
- Broumand, M.; Albert-Green, S.; Yun, S.; Hong, Z.; Thomson, M.J. Spray combustion of fast pyrolysis bio-oils: Applications, challenges, and potential solutions. Prog. Energy Combust. Sci. 2020, 79, 100834. [Google Scholar] [CrossRef]
- Kumar, A.; Chakraborty, J.; Singh, R. Bio-oil: The future of hydrogen generation. Biofuels 2016, 8, 663–674. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Xu, X.; Ding, Y.; Guo, Z. Production of conjugated linoleic acid-rich cottonseed oil by supported Ru catalyzed isomerization. Ind. Crops Prod. 2017, 97, 10–20. [Google Scholar] [CrossRef]
- Chia, J.W.; Sawai, O.; Nunoura, T. Reaction pathway of poly(ethylene) terephthalate carbonization: Decomposition behavior based on carbonized product. Waste Manag. 2020, 108, 62–69. [Google Scholar] [CrossRef]
- Nolte, M.W.; Shanks, B.H. A Perspective on catalytic strategies for deoxygenation in biomass pyrolysis. Energy Technol. 2016, 5, 7–18. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Remón, J.; Arauzo, J.; García, L.; Arcelus-Arrillaga, P.; Millan, M.; Suelves, I.; Pinilla, J. Bio-oil upgrading in supercritical water using Ni-Co catalysts supported on carbon nanofibres. Fuel Process. Technol. 2016, 154, 178–187. [Google Scholar] [CrossRef]
- Lo, S.-L.; Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H. Microwave Pyrolysis of lignocellulosic biomass. Energy Procedia 2017, 105, 41–46. [Google Scholar] [CrossRef]
- Kim, J.-S.; Choi, G.-G. Pyrolysis of lignocellulosic biomass for biochemical production. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 323–348. [Google Scholar]
- Bu, Q.; Lei, H.; Ren, S.; Wang, L.; Zhang, Q.; Tang, J.; Ruan, R. Production of phenols and biofuels by catalytic microwave pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2012, 108, 274–279. [Google Scholar] [CrossRef]
- Pecha, M.B.; Garcia-Perez, M. Pyrolysis of lignocellulosic biomass: Oil, char, and gas. In Bioenergy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 581–619. [Google Scholar]
- Zacher, A.H.; Olarte, M.V.; Santosa, D.M.; Elliott, D.C.; Jones, S.B. A review and perspective of recent bio-oil hydrotreating research. Green Chem. 2014, 16, 491–515. [Google Scholar] [CrossRef]




| Parameters | BOP Blends | BOP-MILD-HDO | BOP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| METH | DME | IPA | ETH | METH | DME | IPA | ETH | ||
| Proximate Analysis (wt%) | |||||||||
| Moisture Content b | 9.08 ± 0.36 | 9.09 ± 0.00 | 9.96 ± 0.03 | 9.94 ± 0.02 | 3.91 ± 0.02 | 2.60 ± 0.01 | 2.15 ± 0.23 | 3.38 ± 0.04 | 9.79 ± 0.180 |
| Ultimate Analysis (wt%) db | |||||||||
| Carbon | 59.41 ± 0.69 | 64.83 ± 0.26 | 64.07 ± 0.74 | 59.83 ± 2.61 | 65.78 ± 0.01 | 67.12 ± 0.02 | 66.57 ± 0.15 | 67.44 ± 0.04 | 64.54 ± 0.45 |
| Hydrogen | 8.50 ± 0.12 | 8.50 ± 0.02 | 8.91 ± 0.19 | 8.30 ± 0.40 | 8.10 ± 0.01 | 7.77 ± 0.01 | 7.99 ± 0.00 | 8.30 ± 0.02 | 7.820 ± 0.02 |
| Nitrogen | 0.18 ± 0.03 | 0.09 ± 0.01 | 0.16 ± 0.02 | 0.16 ± 0.01 | 0.08 ± 0.00 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.180 ± 0.07 |
| Sulfur | 0 | 0 | 0 | 0 | 0 | 0 | 0.04 ± 0.03 | 0.05 ± 0.07 | 0.040 ± 0.02 |
| Oxygen a | 31.9 ± 0.84 | 26.58 ± 0.24 | 26.85 ± 0.95 | 31.71 ± 3.00 | 26.04 ± 0.01 | 25.04 ± 0.02 | 25.32 ± 0.11 | 24.16 ± 0.14 | 27.42 ± 0.55 |
| C/H ratio | 6.99 ± 0.02 | 7.63 ± 0.01 | 7.19 ± 0.07 | 7.21 ± 0.03 | 8.12 ± 0.01 | 8.64 ± 0.01 | 8.33 ± 0.02 | 8.13 ± 0.02 | 8.252 ± 0.82 |
| H/C ratio | 0.143 ± 0.00 | 0.131 ± 0.00 | 0.139 ± 0.00 | 0.139 ± 0.00 | 0.123 ± 0.00 | 0.116 ± 0.00 | 0.120 ± 0.00 | 0.123 ± 0.00 | 0.121 ± 0.01 |
| O/C | 0.54 ± 0.00 | 0.41 ± 0.00 | 0.42 ± 0.01 | 0.53 ± 0.03 | 0.40 ± 0.00 | 0.37 ± 0.00 | 0.38 ± 0.00 | 0.36 ± 0.00 | 0.425 ± 0.43 |
| Density (g/cm3, 40 °C) ar | 1.09 ± 0.00 | 1.08 ± 0.00 | 1.09 ± 0.00 | 1.08 ± 0.00 | 1.13 ± 0.00 | 1.14 ± 0.00 | 1.13 ± 0.00 | 1.13 ± 0.00 | 1.18 ± 0.00 |
| K. Viscosity (mm2/s, 40 °C) ar | 13.0 ± 1.14 | 13.3 ± 1.25 | 29.8 ± 1.77 | 19.9 ± 1.55 | 4.82 ± 1.65 | 5.90 ± 1.70 | 15.18 ± 1.50 | 12.25 ± 1.25 | 67.85 ± 1.25 |
| D. Viscosity (mPa.s) ar | 14.15 ± 1.34 | 14.63 ± 1.50 | 32.32 ± 2.02 | 21.59 ± 1.75 | 5.45 ± 1.85 | 6.71 ± 1.95 | 17.16 ± 1.75 | 13.85 ± 1.45 | 80.20 ± 1.50 |
| TAN (mgKOH/g) ar | 62.55 ± 2.00 | 63.99 ±1.88 | 64.03 ± 1.55 | 62.56 ± 1.55 | 23.78 ± 2.88 | 36.98 ± 1.47 | 28.55 ± 2.44 | 19.56 ± 1.55 | 63.85 ± 2.45 |
| DOD-raw (%) b | n.d | n.d | n.d | n.d | 19.03 ± 0.05 | 24.47 ± 0.05 | 24.64 ± 0.05 | 23.66 ± 0.04 | n.d |
| DOD-blend (%) b | 9.39 ± 0.03 f | 4.49 ± 0.05 | 1.95 ± 0.01 | 1.97 ± 0.04 f | 24.06 ± 0.05 | 18.86 ± 0.05 | 21.14 ± 0.05 | 29.17 ± 0.03 | n.d |
| DOD h (%) b | 7.25 ± 0.02 | 7.15 ± 0.03 | 1.74 ± 0.02 k | 1.53 ± 0.03 k | 56.99 ± 0.01 | 71.45 ± 0.06 | 78.46 ± 0.04 | 66.05 ± 0.01 | n.d |
| HHV (MJ/kg) | 24.02 ± 0.04 | 26.66 ± 0.05 | 26.26 ± 0.02 | 25.59 ± 0.06 | 28.23 ± 0.04 | 28.83 ± 0.08 | 29.05 ± 0.06 | 28.86 ± 0.06 | 21.56 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shezi, M.; Sakhakarmy, M.; Adhikari, S.; Kiambi, S.L. Stabilization of the Bio-Oil Organic Phase via Solvent-Assisted Hydrotreating, Part 1: Investigating the Influence of Various Solvents. Bioengineering 2025, 12, 537. https://doi.org/10.3390/bioengineering12050537
Shezi M, Sakhakarmy M, Adhikari S, Kiambi SL. Stabilization of the Bio-Oil Organic Phase via Solvent-Assisted Hydrotreating, Part 1: Investigating the Influence of Various Solvents. Bioengineering. 2025; 12(5):537. https://doi.org/10.3390/bioengineering12050537
Chicago/Turabian StyleShezi, Manqoba, Manish Sakhakarmy, Sushil Adhikari, and Sammy Lewis Kiambi. 2025. "Stabilization of the Bio-Oil Organic Phase via Solvent-Assisted Hydrotreating, Part 1: Investigating the Influence of Various Solvents" Bioengineering 12, no. 5: 537. https://doi.org/10.3390/bioengineering12050537
APA StyleShezi, M., Sakhakarmy, M., Adhikari, S., & Kiambi, S. L. (2025). Stabilization of the Bio-Oil Organic Phase via Solvent-Assisted Hydrotreating, Part 1: Investigating the Influence of Various Solvents. Bioengineering, 12(5), 537. https://doi.org/10.3390/bioengineering12050537







