Influence of Running Surface Differences on Physiological and Biomechanical Responses During Specific Sports Loading
Abstract
1. Introduction
2. Methods
2.1. Subjects
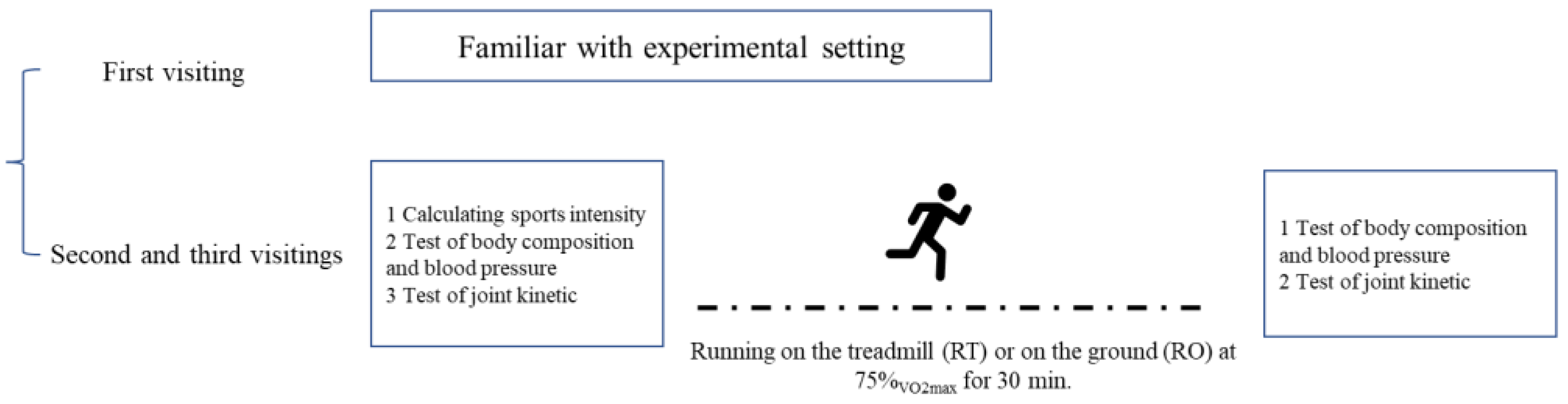
2.2. Experimental Design
2.3. Standardized Sports Loading Protocol
2.4. Body Composition Test, Blood Pressure, and Joint Kinetics Test
2.5. Statistical Analysis
3. Results
3.1. Running Performance Under Specific Sports Loading
3.2. Surface-Specific Differences in Body Composition
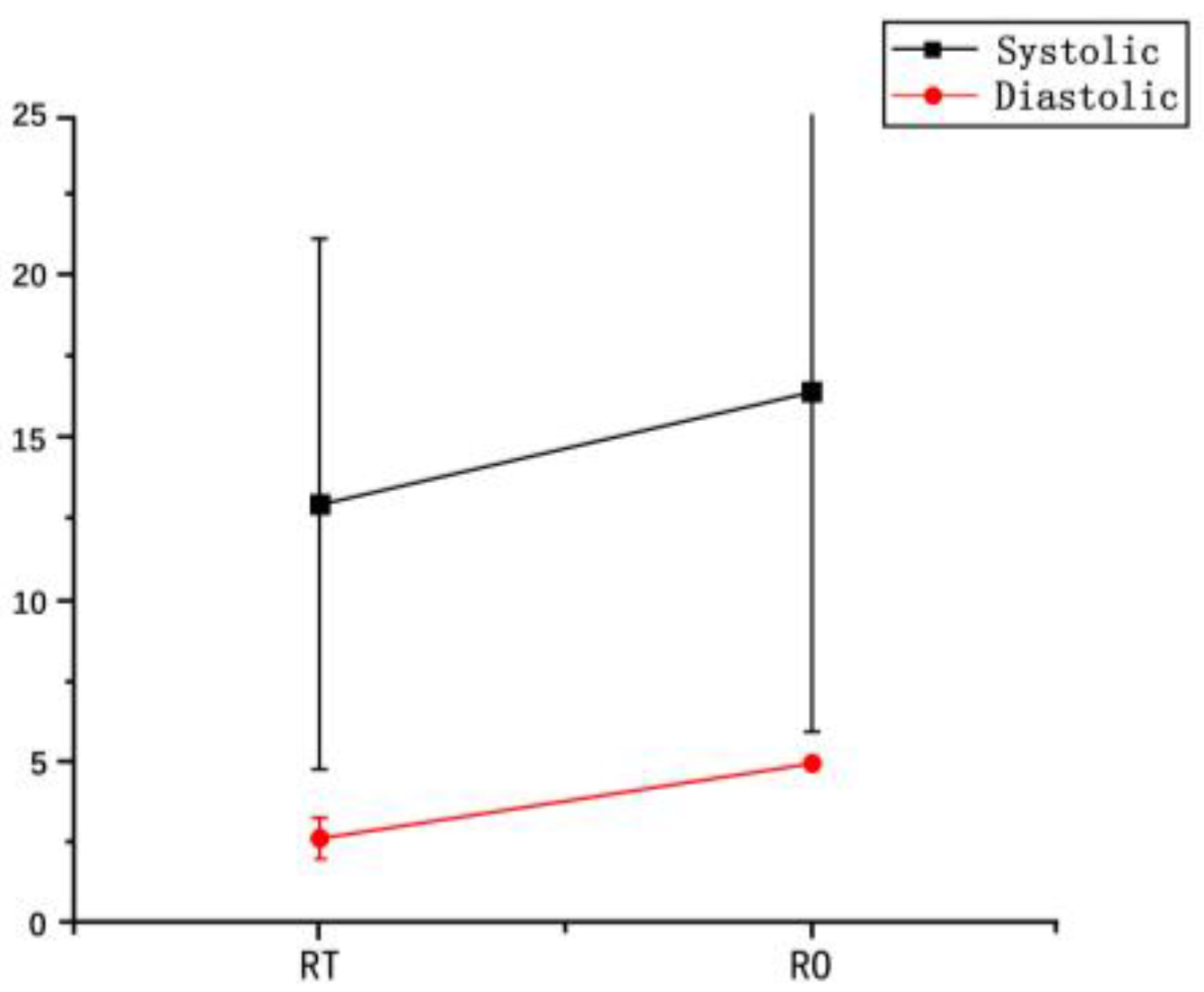
3.3. Physiological Strain and Recovery
3.4. Knee Joint Kinetic Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TR | Treadmill running |
| OR | Overground running |
| FFW | Fat-free weight |
| MV | Muscle volume |
| BM | Body moisture |
| EF | Extracellular fluid |
| P | Protein |
| BD | Bone density |
| FV | Fat volume |
| BFR | Body fat rate |
| BMI | Body mass index |
| TWME | Total work of muscle extensor |
References
- Jiang, L.; Ma, J.; Zhang, Y.; Zhou, C.N.; Zhang, L.; Chao, F.L.; Chen, L.M.; Jiang, R.; Wu, H.; Tang, Y. Effect of running exercise on the number of the neurons in the hippocampus of young transgenic APP/PS1 mice. Brain Res. 2018, 1692, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Lee, D.C.; Sui, X.; Arena, R.; O’Keefe, J.H.; Church, T.S.; Milani, R.V.; Blair, S.N. Effects of Running on Chronic Diseases and Cardiovascular and All-Cause Mortality. Mayo Clin. Proc. 2015, 90, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Brellenthin, A.G.; Thompson, P.D.; Sui, X.; Lee, I.M.; Lavie, C.J. Running as a Key Lifestyle Medicine for Longevity. Prog. Cardiovasc. Dis. 2017, 60, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Semaan, M.B.; Wallard, L.; Ruiz, V.; Gillet, C.; Leteneur, S.; Simoneau-Buessinger, E. Is treadmill walking biomechanically comparable to overground walking? A systematic review. Gait Posture 2022, 92, 249–257. [Google Scholar] [CrossRef]
- Firminger, C.R.; Vernillo, G.; Savoldelli, A.; Stefanyshyn, D.J.; Millet, G.Y.; Edwards, W.B. Joint kinematics and ground reaction forces in overground versus treadmill graded running. Gait Posture 2018, 63, 109–113. [Google Scholar] [CrossRef]
- Yaserifar, M.; Souza Oliveira, A. Surface EMG variability while running on grass, concrete and treadmill. J. Electromyogr Kinesiol. 2022, 62, 102624–102631. [Google Scholar] [CrossRef]
- Alton, F.; Al, E. A kinematic comparison of overground and treadmill walking. Clin. Biomech. 2014, 17, A774. [Google Scholar] [CrossRef]
- Whyte, D.G.; Tofari, P.J.; Cormack, S.J.; Edwards, R.B. Metabolic Cost of Overground, Motorized Treadmill and Non-motorized Treadmill Running. Med. Sci. Sports Exerc. 2015, 47, 195. [Google Scholar] [CrossRef]
- Santarpia, L.; Contaldo, F.; Pasanisi, F. Body composition changes after weight-loss interventions for overweight and obesity. Clin. Nutr. 2013, 32, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Theodorakopoulos, C.; Jones, J.; Bannerman, E.; Greig, C.A. Effectiveness of nutritional and exercise interventions to improve body composition and muscle strength or function in sarcopenic obese older adults: A systematic review. Nutr. Res. 2017, 43, 3–15. [Google Scholar] [CrossRef]
- Baechle, T.R.; Earle, R.W. Weight Training 4th Edition: Steps to Success; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Martin, R.A.; Viggars, M.R.; Sanford, J.A.; Taylor, Z.W.; Hansen, J.R.; Clair, G.C.; Adkins, J.N.; Douglas, C.M.; Esser, K.A. Alterations of the skeletal muscle nuclear proteome after acute exercise reveals a post-transcriptional influence. bioRxiv 2024. [Google Scholar] [CrossRef]
- Iwayama, K.; Tanabe, Y.; Yajima, K.; Tanji, F.; Onishi, T.; Takahashi, H. Preexercise High-Fat Meal Following Carbohydrate Loading Attenuates Glycogen Utilization During Endurance Exercise in Male Recreational Runners. J. Strength Cond. Res. 2023, 37, 661–668. [Google Scholar] [CrossRef]
- Li, S.; Xue, J.-J.; Hong, P.; Song, C.; He, Z.-H. Comparison of energy expenditure and substrate metabolism during overground and motorized treadmill running in Chinese middle-aged women. Sci. Rep. 2020, 10, 1815–1822. [Google Scholar] [CrossRef]
- Milanese, C.; Piscitelli, F.; Cavedon, V.; Zancanaro, C. Effect of distinct impact loading sports on body composition in pre-menarcheal girls. Sci. Sports 2014, 29, 10–19. [Google Scholar] [CrossRef]
- Martins, P.C.; Hansen, F.; Silva, A.M.; Silva, D.A.S. Fluid distribution and cell integrity indicators evaluated by bioelectrical im-pedance in university athletes: Comparison between team sports and individual sports. Physiol. Meas. 2019, 40, 15004–15019. [Google Scholar] [CrossRef]
- Sardinha, L.B. Physiology of exercise and phase angle: Another look at BIA. Eur. J. Clin. Nutr. 2018, 72, 1323–1327. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W. Dehydration: Physiology, assessment, and performance effects. Compr. Physiol. 2014, 4, 257–285. [Google Scholar] [CrossRef]
- Hanley, B.; Mohan, A.K. Changes in Gait During Constant Pace Treadmill Running. J. Strength Cond. Res. 2014, 28, 1219–1225. [Google Scholar] [CrossRef]
- Franks, K.A.; Brown, L.E.; Coburn, J.W.; Kersey, R.D.; Bottaro, M. Effects of motorized vs non-motorized treadmill training on ham-string/quadriceps strength ratios. J. Sports Sci. Med. 2012, 11, 71–76. [Google Scholar]
- Jang, J.-S.; Kim, S.-Y.; Hyeong, J.-H.; Roh, J.-R.; Park, G.-D. Comparison of Muscle Activity and Muscle Fatigue during Running Exercise on Non-motorized Treadmill, Motorized Treadmill and Overground. Korean J. Sports Sci. 2019, 28, 987–1000. [Google Scholar] [CrossRef]
- Rivera, P.M.; Hill, E.C.; Proppe, C.E.; Beltran, E. Acute Effects of Fatiguing Low-Load Leg Extension Muscle Actions on Maximal Strength and Neuromuscular Function. J. Sci. Sport Exerc. 2024, 6, 14–21. [Google Scholar] [CrossRef]
- Cronin, J.; Sleivert, G. Challenges in understanding the influence of maximal power training on improving athletic per-formance. Sports Med. 2005, 35, 213–234. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.-C.; Lomasney, C. Walking Stability and Kinematic Variability Following Motor Fatigue Induced by Incline Treadmill Walking. Sensors 2025, 25, 1489. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wu, J.; Jiang, J.; Wang, G. Neuromuscular and Biomechanical Adaptations of the Lower Limbs During the Pre-Landing and Landing Phase of Running Under Fatigue Conditions. Appl. Sci. 2025, 15, 2449. [Google Scholar] [CrossRef]
- Jung, M.; Koo, S. Physical factors that differentiate body kinematics between treadmill and overground walking. Front Bioeng. Biotechnol. 2022, 10, 888691–888704. [Google Scholar] [CrossRef]
- Schücker, L.; Parrington, L. Thinking about your running movement makes you less efficient: Attentional focus effects on running economy and kinematics. J. Sports Sci. 2019, 37, 638–646. [Google Scholar] [CrossRef]
- Miller, J.R.; Van Hooren, B.; Bishop, C.; Buckley, J.D.; Willy, R.W.; Fuller, J.T. A Systematic Review and Meta-Analysis of Crossover Studies Comparing Physiological, Perceptual and Performance Measures Between Treadmill and Overground Running. Sports Med. 2019, 49, 763–782. [Google Scholar] [CrossRef]
- Hudson, S.; Barwood, M.; Low, C.; Wills, J.; Fish, M. A systematic review of the physiological and biomechanical differences between males and females in response to load carriage during walking activities. Appl. Ergon. 2024, 114, 104123–104135. [Google Scholar] [CrossRef]
- Poláčková Šolcová, I.; Lačev, A. Differences in male and female subjective experience and physiological reactions to emotional stimuli. Int. J. Psychophysiol. 2017, 117, 75–80. [Google Scholar] [CrossRef]
- Lewis, D.A.; Kamon, E.; Hodgson, J.L. Physiological differences between genders: Implications for sports conditioning. Sports Med. 1986, 3, 357–369. [Google Scholar] [CrossRef]
| MHR (Times) | RHR (Times) | Sports Intensity | ||
|---|---|---|---|---|
| Up Limit (Times) | Target HR (Times) | Down Limit (Times) | ||
| 195.5 ± 1.29 | 61.3 ± 0.58 | 166.67 ± 1.15 | 160.67 ± 1.15 | 154.67 ± 1.53 |
| Index | TR | OR | Difference | p | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | TR | OR | ||
| Weight (kg) | 67.38 ± 3.63 | 66.48 ± 4.04 | 68.43 ± 4.04 | 67.57 ± 4.14 | 0.90 ± 0.74 | 0.87 ± 0.35 | 0.946 |
| Fat-free weight (kg) | 58.20 ± 1.69 | 59.28 ± 1.83 | 59.37 ± 1.62 | 59.5 ± 1.75 | −1.08 ± 0.24 | −0.33 ± 0.035 | 0.008 * |
| Muscle volume (kg) | 55.20 ± 1.61 | 56.20 ± 1.75 | 56.33 ± 1.56 | 56.43 ± 1.70 | −1.00 ± 0.24 | −0.10 ± 0.30 | 0.014 * |
| Body moisture (kg) | 39.95 ± 1.62 | 42.05 ± 1.87 | 41.57 ± 1.07 | 42.23 ± 1.44 | −2.10 ± 0.37 | −0.67 ± 0.42 | 0.005 * |
| Intracellular fluid (kg) | 25.28 ± 1.16 | 27.05 ± 1.38 | 26.43 ± 0.74 | 27.10 ± 1.04 | −1.78 ± 0.33 | −0.67 ± 0.32 | 0.008 * |
| Extracellular fluid (kg) | 14.75 ± 0.47 | 15.03 ± 0.53 | 15.13 ± 0.35 | 15.13 ± 0.40 | −0.28 ± 0.09 | 0.00 ± 0.10 | 0.015 * |
| Protein (kg) | 15.28 ± 0.80 | 14.23 ± 0.73 | 14.87 ± 0.64 | 14.26 ± 0.60 | 1.05 ± 0.24 | 0.60 ± 0.17 | 0.041 * |
| Bone density (kg) | 3.00 ± 0.08 | 3.08 ± 0.10 | 3.03 ± 0.06 | 3.07 ± 0.06 | −0.08 ± 0.05 | −0.33 ± 0.06 | 0.352 |
| Fat volume (kg) | 9.12 ± 2.36 | 7.23 ± 2.77 | 9.10 ± 2.63 | 8.10 ± 2.62 | 1.95 ± 0.72 | 1.00 ± 0.10 | 0.077 |
| Body fat rate (%) | 13.50 ± 2.69 | 10.68 ± 3.41 | 13.13 ± 2.99 | 11.80 ± 3.08 | 2.83 ± 1.09 | 1.33 ± 0.15 | 0.072 |
| Body mass index (m2/Kg) | 21.45 ± 1.48 | 21.18 ± 1.54 | 22.07 ± 1.17 | 21.80 ± 1.18 | 0.28 ± 0.28 | 0.27 ± 0.15 | 0.965 |
| Systolic pressure(KPa) | 122.67 ± 7.51 | 135.67 ± 2.08 | 127.50 ± 26.16 | 144.00 ± 15.56 | 13.00 ± 8.19 | 16.50 ± 10.61 | 0.700 |
| Diastolic pressure(KPa) | 69.33 ± 2.89 | 71.00 ± 2.65 | 66.00 ± 12.72 | 61.00 ± 11.31 | 2.67 ± 0.58 | 5.00 ± 1.42 | 0.722 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Shuo, Q.; Gao, C.; Lin, C.-T.; Fang, Y. Influence of Running Surface Differences on Physiological and Biomechanical Responses During Specific Sports Loading. Bioengineering 2025, 12, 534. https://doi.org/10.3390/bioengineering12050534
Liang Z, Shuo Q, Gao C, Lin C-T, Fang Y. Influence of Running Surface Differences on Physiological and Biomechanical Responses During Specific Sports Loading. Bioengineering. 2025; 12(5):534. https://doi.org/10.3390/bioengineering12050534
Chicago/Turabian StyleLiang, Zhiqiang, Qi Shuo, Chuang Gao, Chang-Te Lin, and Yufei Fang. 2025. "Influence of Running Surface Differences on Physiological and Biomechanical Responses During Specific Sports Loading" Bioengineering 12, no. 5: 534. https://doi.org/10.3390/bioengineering12050534
APA StyleLiang, Z., Shuo, Q., Gao, C., Lin, C.-T., & Fang, Y. (2025). Influence of Running Surface Differences on Physiological and Biomechanical Responses During Specific Sports Loading. Bioengineering, 12(5), 534. https://doi.org/10.3390/bioengineering12050534








