Physicomechanical Properties of Tissue Conditioner Reinforced with Glass Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Fabrication of Experimental Tissue Conditioner Samples
2.2. Fourier Transform Infrared Spectroscopy
2.3. Surface Roughness Test
2.4. Contact Angle Evaluation
2.5. Tensile Strength Test
2.6. Linear Dimensional Stability Test
2.7. Water Sorption and Solubility Tests
2.8. Statistical Analysis
3. Results
3.1. FTIR
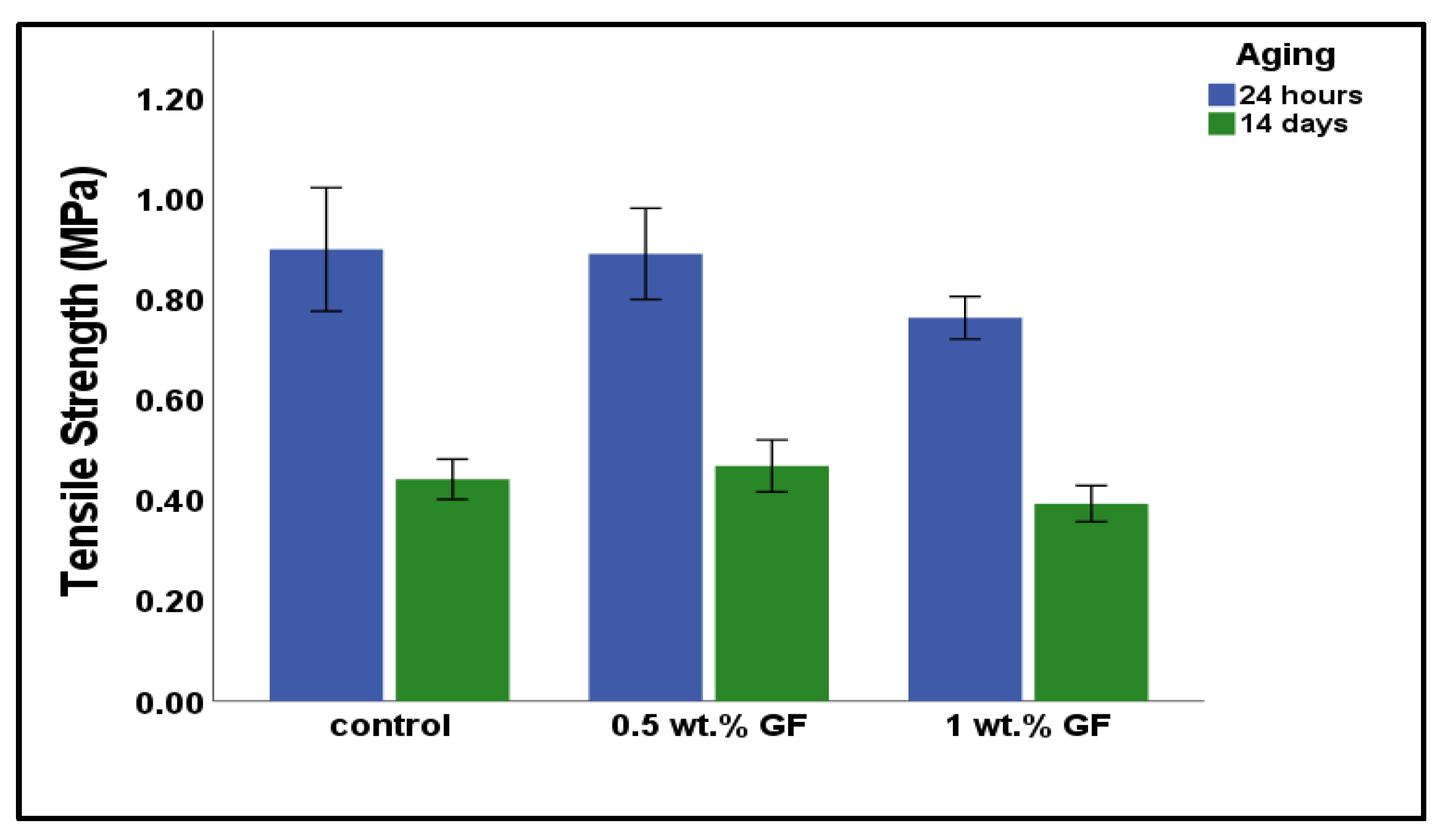
3.2. Tensile Strength
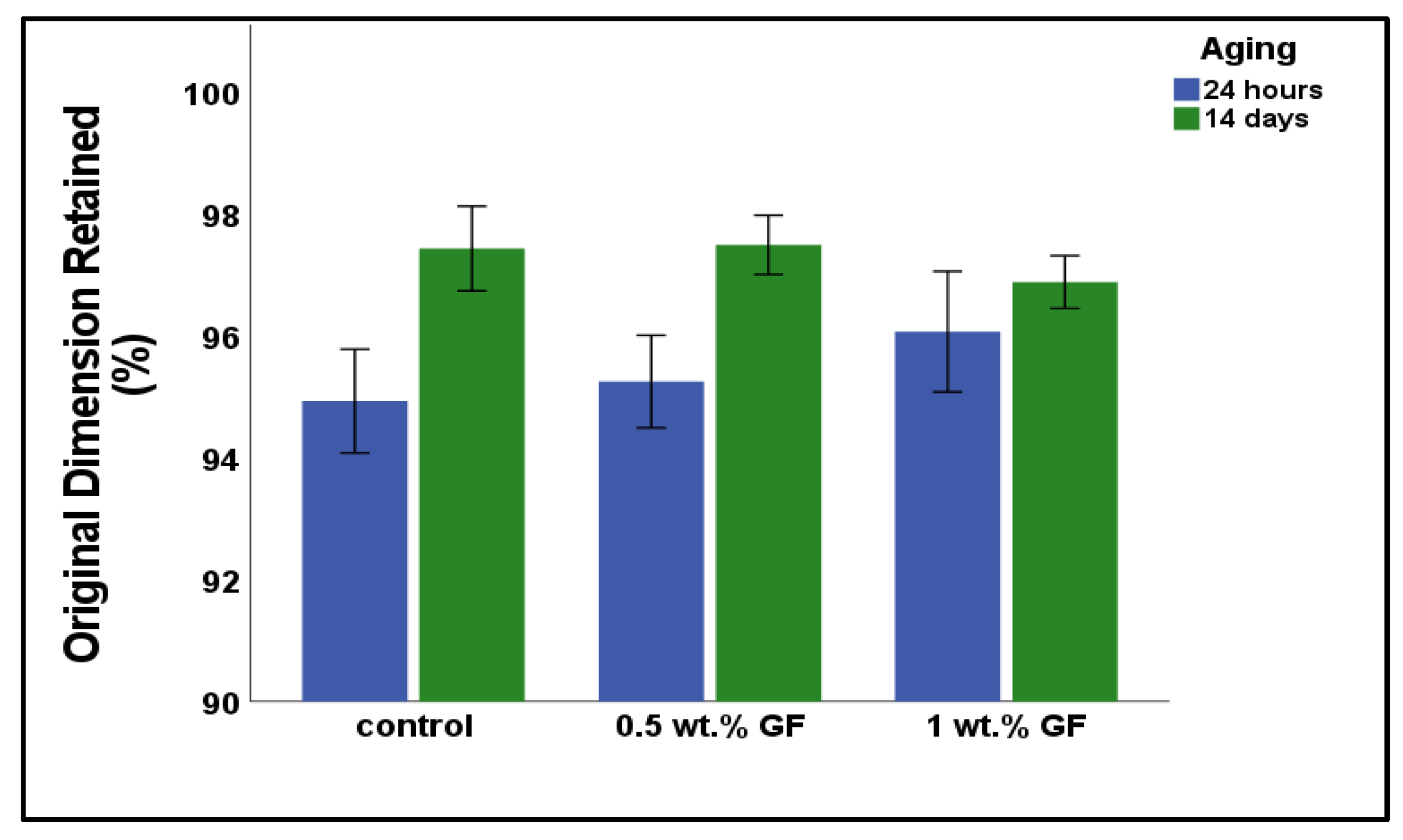
3.3. Linear Dimensional Stability
3.4. Surface Roughness
3.5. Water Contact Angle
3.6. Water Sorption and Solubility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammed, G.A. Studying the Anti Candidal-Activity of Different Herbal Oils Incorporated into Tissue Conditioner: (A Comparative study). Jordan J. Pharm. Sci. 2023, 16, 871–879. [Google Scholar] [CrossRef]
- Homsiang, W.; Kamonkhantikul, K.; Arksornnukit, M.; Takahashi, H. Effect of zinc oxide nanoparticles incorporated into tissue conditioner on antifungal, physical, and mechanical properties. Dent. Mater. J. 2021, 40, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Zafar, M.S. Role of antifungal medicaments added to tissue conditioners: A systematic review. J. Prosthodont. Res. 2016, 60, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Hegde, V. Comparative evaluation of antifungal activity of melaleuca oil and fluconazole when incorporated in tissue conditioner: An in vitro study. J. Prosthodont. 2014, 23, 367–373. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Narayana, A.I.; Peralam, P.Y.; Balkrishanan, D. To study the effect of Cocos nucifera oil when incorporated into tissue conditioner on its tensile strength and antifungal activity: An in vitro study. J. Indian Prosthodont. Soc. 2019, 19, 225–232. [Google Scholar]
- Lee, H.-L.; Wang, R.-S.; Hsu, Y.-C.; Chuang, C.-C.; Chan, H.-R.; Chiu, H.-C.; Wang, Y.-B.; Chen, K.-Y.; Fu, E. Antifungal effect of tissue conditioners containing poly (acryloyloxyethyltrimethyl ammonium chloride)-grafted chitosan on Candida albicans growth in vitro. J. Dent. Sci. 2018, 13, 160–166. [Google Scholar] [CrossRef]
- Ganokwalai, N.; Chotprasert, N.; Choonharuangdej, S.; Shrestha, B.; Srithavaj, T. Mechanical properties of dental tissue conditioner containing lemongrass essential oil. J. Prosthet. Dent. 2024, 132, 1068.e1–1068.e8. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Yoshida, K.; Takase, K.; Valanezhad, A.; Watanabe, I.; Kojio, K.; Murata, H. Evaluation of viscoelastic properties, hardness, and glass transition temperature of soft denture liners and tissue conditioner. Odontology 2020, 108, 366–375. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K.; Raszewski, Z. Comparison of mechanical properties of three tissue conditioners: An evaluation in vitro study. Medicina 2023, 59, 1359. [Google Scholar] [CrossRef]
- Hashem, M.I. Advances in soft denture liners: An update. J. Contemp. Dent. Pract. 2015, 16, 314–318. [Google Scholar]
- Neppelenbroek, K.H. Sustained drug-delivery system: A promising therapy for denture stomatitis? J. Appl. Oral Sci. 2016, 24, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Lee, C.J. Characterization of silver nanoparticles incorporated acrylic-based tissue conditioner with antimicrobial effect and cytocompatibility. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 335–348. [Google Scholar]
- Khan, A.A.; Zafar, M.S.; Fareed, M.A.; AlMufareh, N.A.; Alshehri, F.; AlSunbul, H.; Lassila, L.; Garoushi, S.; Vallittu, P.K. Fiber-reinforced composites in dentistry—An insight into adhesion aspects of the material and the restored tooth construct. Dent. Mater. 2023, 39, 141–151. [Google Scholar] [CrossRef]
- Väkiparta, M.; Koskinen, M.; Vallittu, P.; Närhi, T.; Yli-Urpo, A. In vitro cytotoxicity of E-glass fiber weave preimpregnated with novel biopolymer. J. Mater. Sci. Mater. Med. 2004, 15, 69–72. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef]
- Hatamleh, M.M.; Maryan, C.J.; Silikas, N.; Watts, D.C. Effect of net fiber reinforcement surface treatment on soft denture liner retention and longevity. J. Prosthodont. Implant Esthet. Reconstr. Dent. 2010, 19, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Sharma, S.K.; Negi, P.; Kumar, M.; Rajpurohit, D.; Khobre, P. Comparative evaluation of flexural strength of heat polymerised denture base resins after reinforcement with glass fibres and nylon fibres: An in vitro study. Adv. Hum. Biol. 2016, 6, 91–94. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef]
- Alsunbul, H.; Khan, A.A.; Alqahtani, Y.M.; Hassan, S.A.b.; Asiri, W.; Saadaldin, S.; Alharthi, R.; Aldegheishem, A. Using functionalized micron-sized glass fibres for the synergistic effect of glass ionomer on luting material. J. Funct. Biomater. 2023, 14, 550. [Google Scholar] [CrossRef]
- ISO E 10139-2: 2016; Dentistry—Soft Lining Materials for Removable Dentures—Part 2: Materials for Long-Term Use. ISO International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO I 10139-1: 2018; Dentistry—Soft Lining Materials for Removable Dentures—Part 1: Materials for Short-Term Use. ISO International Organization for Standardization: Geneva, Switzerland, 2018.
- Chladek, G.; Żmudzki, J.; Kasperski, J. Long-term soft denture lining materials. Materials 2014, 7, 5816–5842. [Google Scholar] [CrossRef]
- Prasad, A.; Prasad, B.R.; Shetty, V.; Shastry, C. Tissue conditioners: A review. J. Health Allied Sci. NU 2014, 4, 152–157. [Google Scholar]
- Khan, A.A.; Bari, A.; Abdullah Al-Kheraif, A.; Alsunbul, H.; Alhaidry, H.; Alharthi, R.; Aldegheishem, A. Oxidized natural biopolymer for enhanced surface, physical and mechanical properties of glass ionomer luting cement. Polymers 2023, 15, 2679. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Kawamura, M.; Hamada, T.; Saleh, S.; Kresnoadi, U.; Toki, K. Dimensional stability and weight changes of tissue conditioners. J. Oral Rehabil. 2001, 28, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.F.; Maciel, J.G.; Arrais, C.A.; Porto, V.C.; Urban, V.M.; Neppelenbroek, K.H. Effect of incorporating antifungals on the water sorption and solubility of interim resilient liners for denture base relining. J. Prosthet. Dent. 2016, 115, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Zahid, S.; Sajid, M.; Ud Din, S.; Alam, M.K.; Chaudhary, F.A.; Kaleem, M.; Alswairki, H.J.; Abutayyem, H. Physico-mechanical properties of commercially available tissue conditioner modified with synthesized chitosan oligosaccharide. Polymers 2022, 14, 1233. [Google Scholar] [CrossRef]
- Urban, V.M.; De Souza, R.F.; Galvao Arrais, C.A.; Borsato, K.T.; Vaz, L.G. Effect of the association of nystatin with a tissue conditioner on its ultimate tensile strength. J. Prosthodont. 2006, 15, 295–299. [Google Scholar] [CrossRef]
- Khan, A.A.; De Vera, M.A.T.; Mohamed, B.A.; Javed, R.; Al-Kheraif, A.A. Enhancing the physical properties of acrylic resilient denture liner using graphene oxide nanosheets. J. Vinyl Addit. Technol. 2022, 28, 487–493. [Google Scholar] [CrossRef]
- Khan, A.A.; Al-Khureif, A.A.; Al-Mutairi, M.; Al-Majed, I.; Aftab, S. Physical and mechanical characterizations of experimental pit and fissure sealants based on bioactive glasses. J. Clin. Pediatr. Dent. 2024, 48, 69–77. [Google Scholar]


| Aging. | Group | Surface Roughness (µm) | Water Sorption (%) | Water Solubility (%) | Water Contact Angle (°) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Baseline (24 h) | Control | 0.95 ± 0.04 a | −2.39 ± 0.50 a | 1.43 ± 0.31 a | 88.59 ± 0.60 ab |
| 0.5% wt. GF | 0.97 ± 0.05 bc | −2.32 ±0.29 bc | 1.39 ± 0.25 bce | 90.09 ± 0.92 bc | |
| 1% wt. GF | 1.01 ± 0.08 b | −2.25 ± 0.30 bde | 1.19 ± 0.11 bde | 92.20 ± 0.43 abcd | |
| 14 days | Control | 1.17 ± 0.06 ab | 3.63 ± 0.86 ab | 2.05 ± 0.14 ab | 71.25 ± 0.89 ab |
| 0.5% wt. GF | 1.01 ± 0.11 | 2.96± 0.34 acd | 2.01 ± 0.18 acd | 82.25 ± 0.83 abcd | |
| 1% wt. GF | 1.15 ± 0.09 ac | 2.78 ± 0.33 ace | 1.95 ± 0.16 e | 90.11 ± 0.65 abd |
| Parameters | Sum of Squares | df | Mean Square | f-Value | p-Value |
|---|---|---|---|---|---|
| Material | 0.0192 | 2 | 0.01 | 6.95 | 0.22 |
| Aging | 0.168 | 1 | 0.168 | 27.874 | <0.001 |
| Material*Aging | 0.022 | 2 | 0.011 | 1.826 | 0.183 |
| Error | 0.145 | 24 | 0.006 |
| Parameters | Sum of Squares | df | Mean Square | f-Value | p-Value |
|---|---|---|---|---|---|
| Material | 634.359 | 2 | 317.18 | 574.974 | <0.001 |
| Aging | 619.983 | 1 | 619.983 | 1123.888 | <0.001 |
| Material*Aging | 296.81 | 2 | 148.405 | 269.204 | <0.001 |
| Error | 13.239 | 24 | 0.552 |
| Parameters | Sum of Squares | df | Mean Square | f-Value | p-Value |
|---|---|---|---|---|---|
| Material | 0.731 | 2 | 0.366 | 1.567 | 0.229 |
| Aging | 222.06 | 1 | 222.06 | 951.64 | <0.001 |
| Material*Aging | 1.314 | 2 | 0.657 | 2.816 | 0.080 |
| Error | 5.599 | 24 | 0.233 |
| Parameters | Sum of Squares | df | Mean Square | f-Value | p-Value |
|---|---|---|---|---|---|
| Groups | 0.375 | 2 | 0.188 | 5.257 | 0.013 |
| Aging | 2.803 | 1 | 2.803 | 78.58 | <0.001 |
| Groups*Aging | 0.001 | 2 | 0 | 0.012 | 0.988 |
| Error | 0.856 | 24 | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.A.; AlKhureif, A.A.; Mirza, E.H.; AlHassoun, R.K.; Wasi, A.; Matinlinna, J. Physicomechanical Properties of Tissue Conditioner Reinforced with Glass Fibers. Bioengineering 2025, 12, 515. https://doi.org/10.3390/bioengineering12050515
Khan AA, AlKhureif AA, Mirza EH, AlHassoun RK, Wasi A, Matinlinna J. Physicomechanical Properties of Tissue Conditioner Reinforced with Glass Fibers. Bioengineering. 2025; 12(5):515. https://doi.org/10.3390/bioengineering12050515
Chicago/Turabian StyleKhan, Aftab Ahmed, Abdulaziz Abdullah AlKhureif, Eraj Humayun Mirza, Raghad Khalid AlHassoun, Aisha Wasi, and Jukka Matinlinna. 2025. "Physicomechanical Properties of Tissue Conditioner Reinforced with Glass Fibers" Bioengineering 12, no. 5: 515. https://doi.org/10.3390/bioengineering12050515
APA StyleKhan, A. A., AlKhureif, A. A., Mirza, E. H., AlHassoun, R. K., Wasi, A., & Matinlinna, J. (2025). Physicomechanical Properties of Tissue Conditioner Reinforced with Glass Fibers. Bioengineering, 12(5), 515. https://doi.org/10.3390/bioengineering12050515






