Integrating Artificial Intelligence in Orthopedic Care: Advancements in Bone Care and Future Directions
Abstract
1. Introduction
2. Methods
3. Discussion
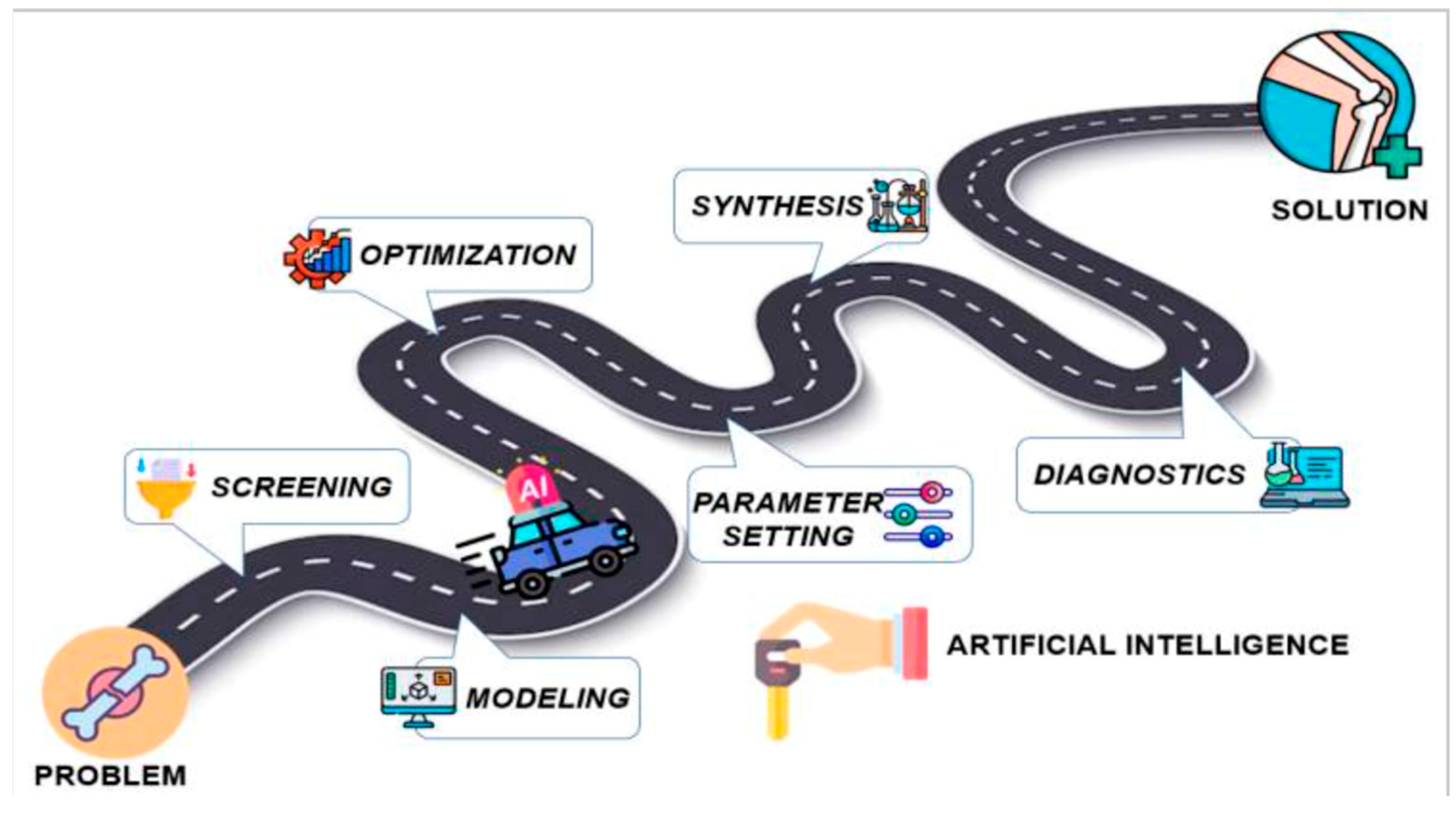
3.1. AI’s Transformative Role in Orthopedic Bioengineering
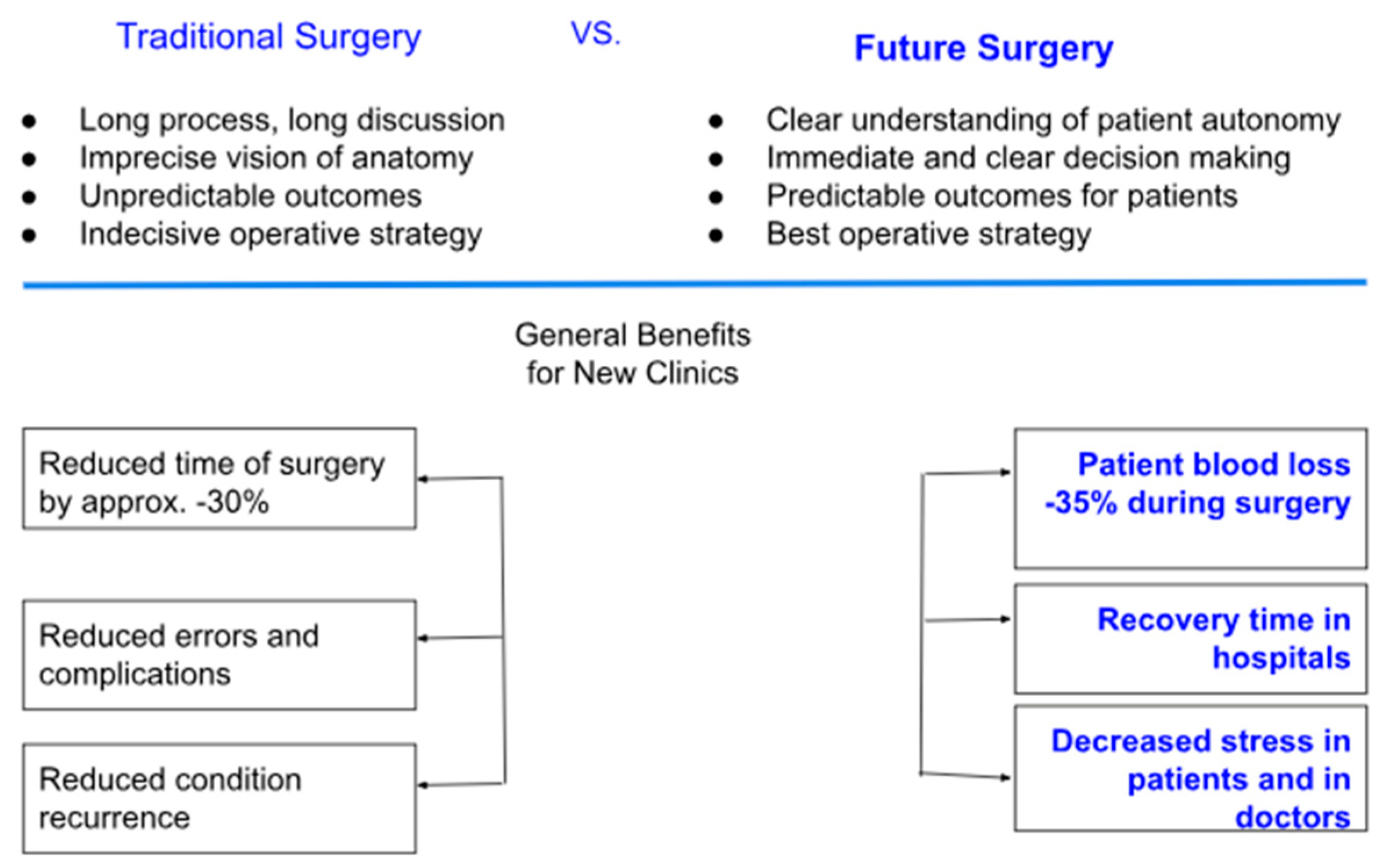
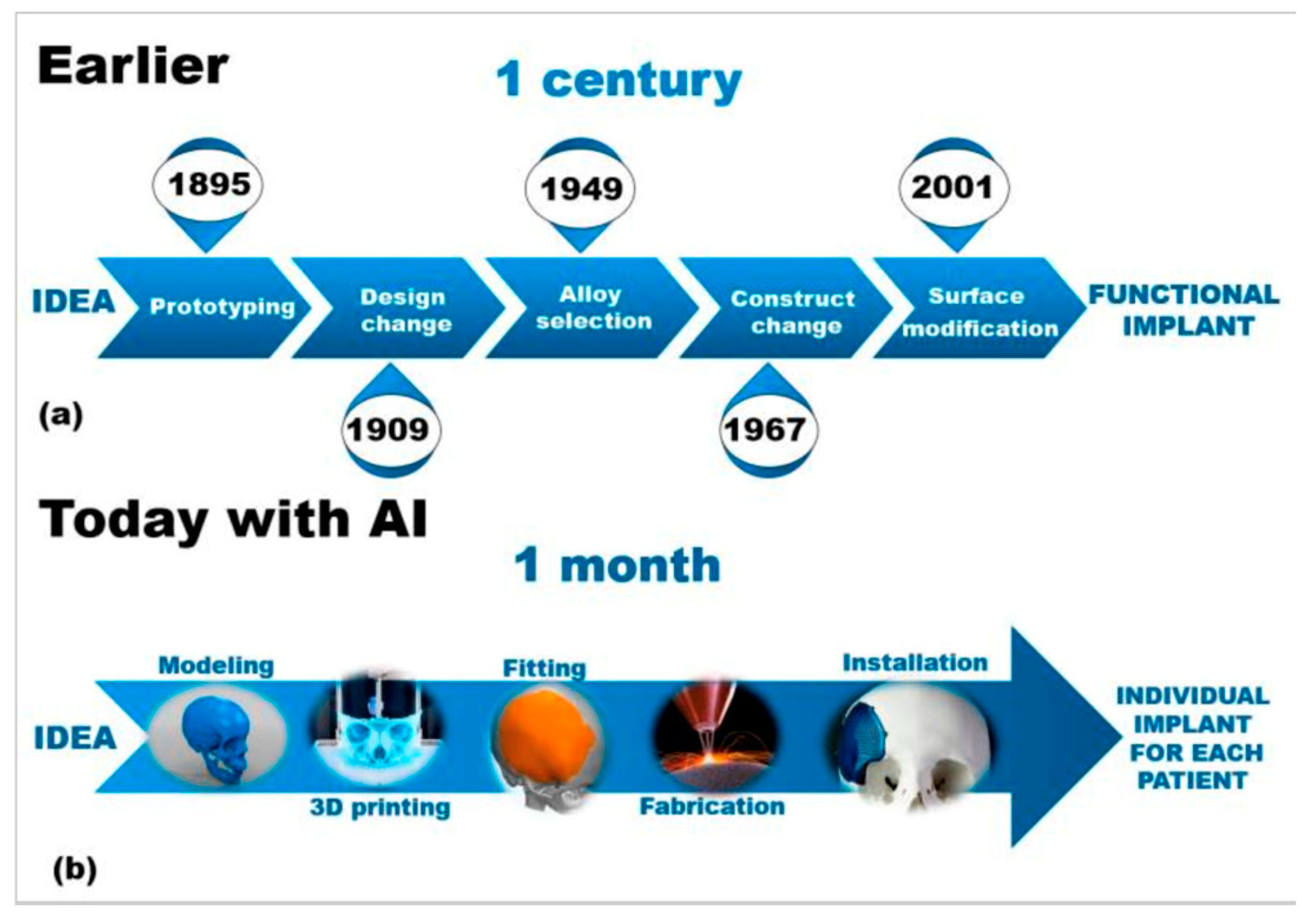
3.2. AI in Preoperative Planning and Surgical Optimization
3.3. AI in Bone Grafting and Biomaterial Innovation
3.4. Machine Learning and Neural Networks in Implantology
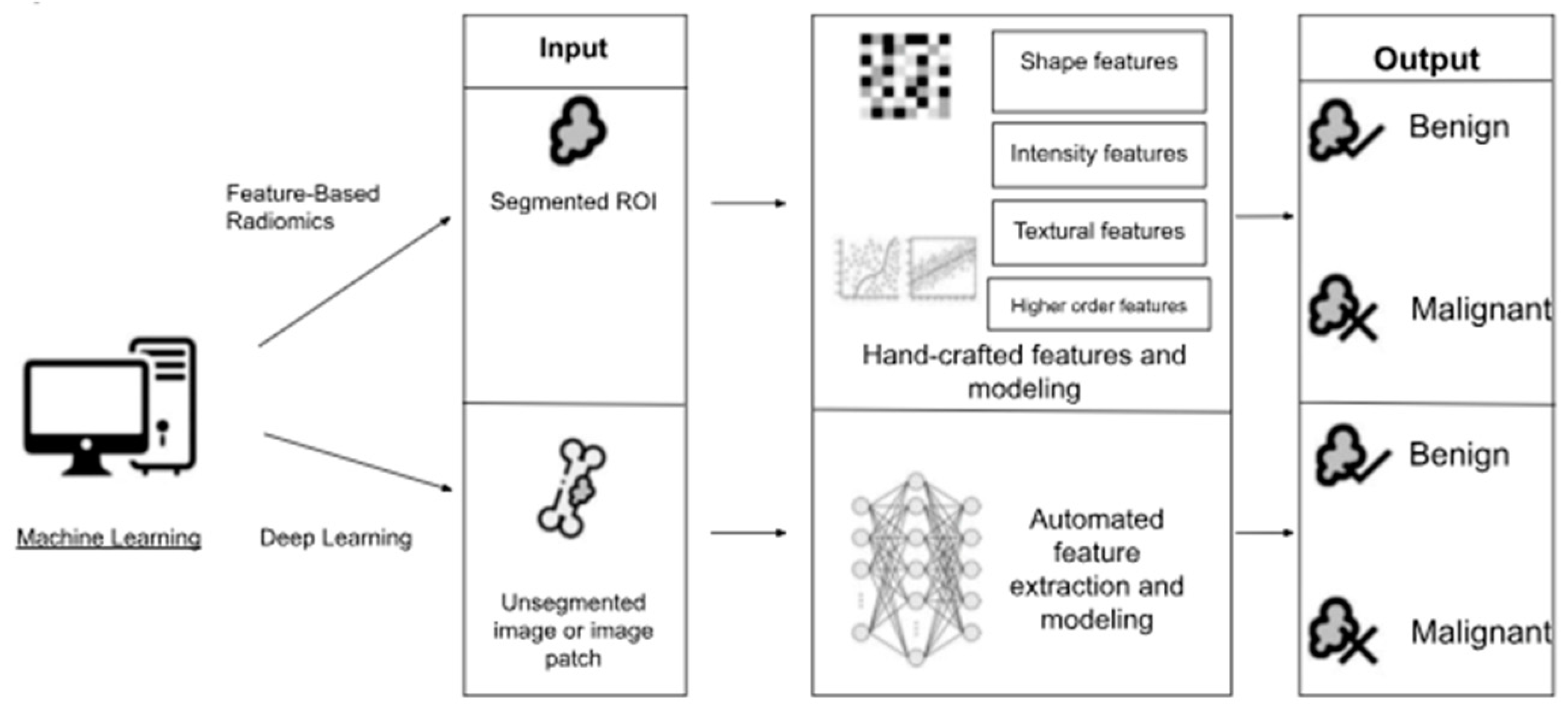
3.5. AI in Bone Tumor Diagnosis and Treatment
3.6. Intraoperative Robotics and Precision Surgery
3.7. Smart Implants and Remote Monitoring
3.8. AI-Driven Bone Regeneration and Neuroprosthetics
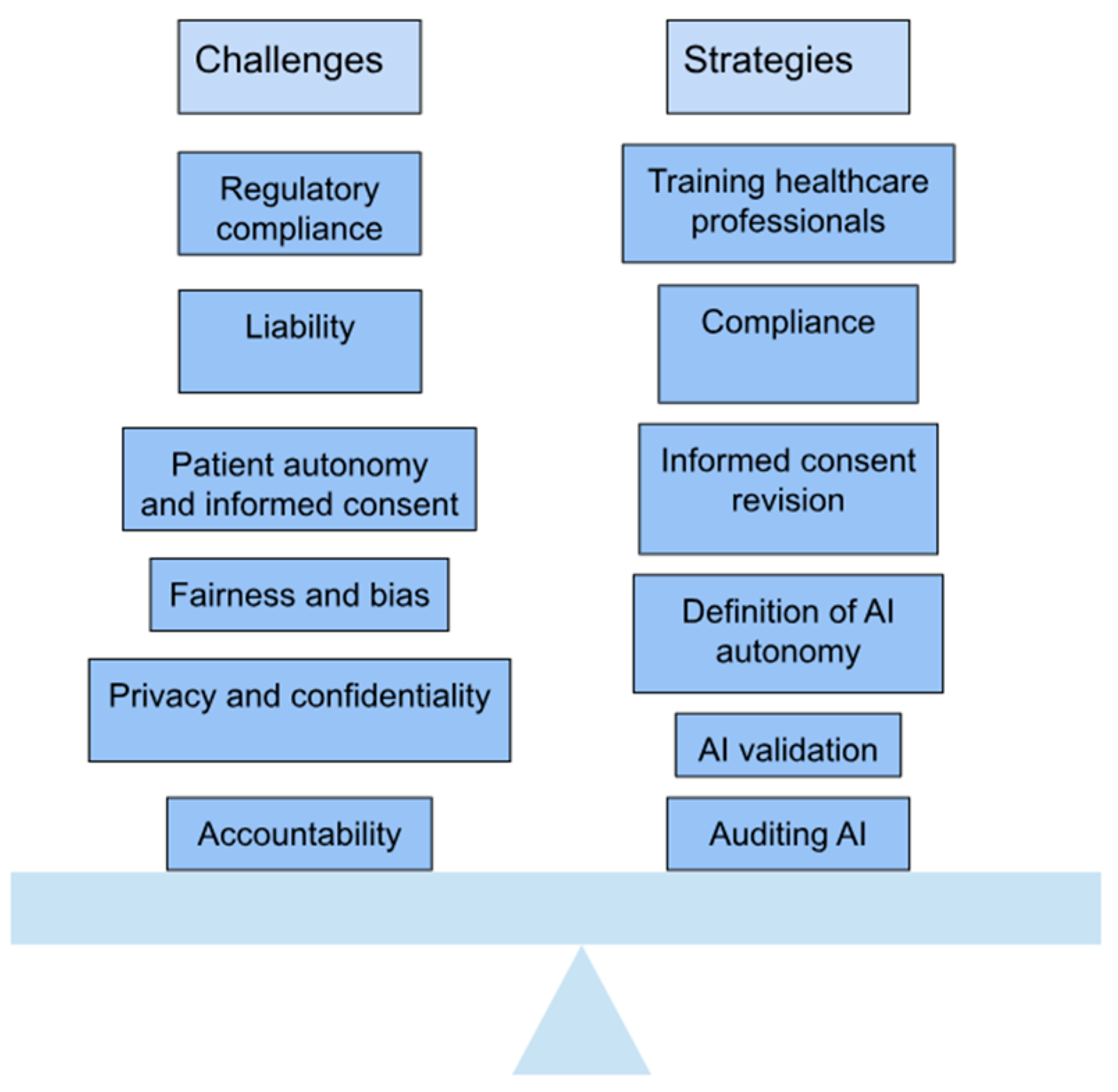
4. Ethical and Practical Considerations
5. Future Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farhadi, F.; Barnes, M.R.; Sugito, H.R.; Sin, J.M.; Henderson, E.R.; Levy, J.J. Applications of artificial intelligence in orthopaedic surgery. Front. Med. Technol. 2022, 4, 995526. [Google Scholar] [CrossRef] [PubMed]
- Lisacek-Kiosoglous, A.B.; Powling, A.S.; Fontalis, A.; Gabr, A.; Mazomenos, E.; Haddad, F.S. Artificial intelligence in orthopaedic surgery. Bone Jt. Res. 2023, 12, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Bicer, E.K.; Fangerau, H.; Sur, H. Artifical intelligence use in orthopedics: An ethical point of view. EFORT Open Rev. 2023, 8, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Ashkani-Esfahani, S. Artificial Intelligence Improves Orthopedic Diagnosis. Mass General Advances in Motion. Published 4 January 2021. Available online: https://advances.massgeneral.org/ortho/article.aspx?id=1330 (accessed on 28 March 2025).
- Ong, W.; Zhu, L.; Tan, Y.L.; Teo, E.C.; Tan, J.H.; Kumar, N.; Vellayappan, B.A.; Ooi, B.C.; Quek, S.T.; Makmur, A.; et al. Application of Machine Learning for Differentiating Bone Malignancy on Imaging: A Systematic Review. Cancers 2023, 15, 1837. [Google Scholar] [CrossRef]
- Papalia, G.F.; Brigato, P.; Sisca, L.; Maltese, G.; Faiella, E.; Santucci, D.; Pantano, F.; Vincenzi, B.; Tonini, G.; Papalia, R.; et al. Artificial Intelligence in Detection, Management, and Prognosis of Bone Metastasis: A Systematic Review. Cancers 2024, 16, 2700. [Google Scholar] [CrossRef]
- Kamal, A.H.; Zakaria, O.M.; Majzoub, R.A.; Nasir, E.W.F. Artificial intelligence in orthopedics: A qualitative exploration of the surgeon perspective. Medicine 2023, 102, e34071. [Google Scholar] [CrossRef]
- Rupp, M.; Moser, L.B.; Hess, S.; Angele, P.; Aurich, M.; Dyrna, F.; Nehrer, S.; Neubauer, M.; Pawelczyk, J.; Izadpanah, K.; et al. Orthopaedic surgeons display a positive outlook towards artificial intelligence: A survey among members of the AGA Society for Arthroscopy and Joint Surgery. J. Exp. Orthop. 2024, 11, e12080. [Google Scholar] [CrossRef]
- Enhatch Marketing. Intelligent Surgery: The Power of Preoperative Planning. 2023. Available online: https://blog.enhatch.com/intelligent-surgery-pre-op-planning-software (accessed on 28 March 2025).
- Surgiprint 3D Intelligence. SurgiPrint-3D Intelligence. 2022. Available online: https://www.surgiprint.com/ (accessed on 28 March 2025).
- Portnoy, Y.; Koren, J.; Khoury, A.; Factor, S.; Dadia, S.; Ran, Y.M.; Benady, A. Three-dimensional technologies in presurgical planning of bone surgeries: Current evidence and future perspectives. Int. J. Surg. 2023, 109, 3–10. [Google Scholar] [CrossRef]
- Ryan, M.L.; Wang, S.; Pandya, S.R. Integrating Artificial Intelligence Into the Visualization and Modeling of Three-Dimensional Anatomy in Pediatric Surgical Patients. J. Pediatr. Surg. 2024, 59, 161629. [Google Scholar] [CrossRef]
- Roelofs, L.J.M.; Assink, N.; Kraeima, J.; Duis, K.T.; Doornberg, J.N.; de Vries, J.-P.P.M.; Meesters, A.M.L.; Ijpma, F.F.A. Clinical Application of 3D-Assisted Surgery Techniques in Treatment of Intra-Articular Distal Radius Fractures: A Systematic Review in 718 Patients. J. Clin. Med. 2024, 13, 7296. [Google Scholar] [CrossRef]
- 3D Systems, Inc. Orthopaedic Solutions|3D Systems. 3D Systems. Published 2025. Available online: https://www.3dsystems.com/healthcare/orthopaedic-solutions (accessed on 1 May 2025).
- Orthopaedic Surgery.JPG. Wikimedia Commons. Published 25 October 2020. Available online: https://commons.wikimedia.org/w/index.php?title=File:Orthopaedic_Surgery.JPG&oldid=501156089 (accessed on 1 May 2025).
- Wang, X.; Zhang, Y.; Lou, L.; Xu, L.; Fei, W.; Dai, J.; Wang, J. Robotic-assisted systems for the safe and reliable treatment of femoral neck fractures: Retrospective cohort study. J. Orthop. Surg. Res. 2023, 18, 633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, H.; Liu, H. Progress in clinical application of computer-assisted orthopedic surgery. Digit. Med. 2023, 9, e00002. [Google Scholar] [CrossRef]
- Thomas, D. How AI and convolutional neural networks can revolutionize orthopaedic surgery. J. Clin. Orthop. Trauma 2023, 40, 102165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riem, L.; Feng, X.; Cousins, M.; DuCharme, O.; Leitch, E.B.; Werner, B.C.; Sheean, A.J.; Hart, J.; Antosh, I.J.; Blemker, S.S. A Deep Learning Algorithm for Automatic 3D Segmentation of Rotator Cuff Muscle and Fat from Clinical MRI Scans. Radiol. Artif. Intell. 2023, 5, 220132. [Google Scholar] [CrossRef]
- Patel, R.; Thong, E.H.E.; Batta, V.; Bharath, A.A.; Francis, D.; Howard, J. Automated Identification of Orthopedic Implants on Radiographs Using Deep Learning. Radiol. Artif. Intell. 2021, 3, e200183. [Google Scholar] [CrossRef]
- Harake, E.S.; Linzey, J.R.; Jiang, C.; Joshi, R.S.; Zaki, M.M.; Jones, J.C.; Khalsa, S.S.S.; Lee, J.H.; Wilseck, Z.; Joseph, J.R.; et al. Development and validation of an artificial intelligence model to accurately predict spinopelvic parameters. J. Neurosurg. Spine 2024, 41, 88–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lalehzarian, S.P.; Gowd, A.K.; Liu, J.N. Machine learning in orthopaedic surgery. World J. Orthop. 2021, 12, 685–699. [Google Scholar] [CrossRef]
- Kolomenskaya, E.; Butova, V.; Poltavskiy, A.; Soldatov, A.; Butakova, M. Application of Artificial Intelligence at All Stages of Bone Tissue Engineering. Biomedicines 2023, 12, 76. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Logeshwaran, A.; Elsen, R.; Nayak, S. Artificial Intelligence-Based 3D Printing Strategies for Bone Scaffold Fabrication and Its Application in Preclinical and Clinical Investigations. ACS Biomater. Sci. Eng. 2024, 10, 677–696. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Ratna, H.V.K.; Jeyaraman, N.; Venkatesan, A.; Ramasubramanian, S.; Yadav, S. Leveraging Artificial Intelligence and Machine Learning in Regenerative Orthopedics: A Paradigm Shift in Patient Care. Cureus 2023, 15, e49756. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Liu, G.; Gao, Q.; Zhang, S.; Wang, Q.; Wu, L.; He, P.; Yu, Q. A random forest algorithm-based prediction model for moderate to severe acute postoperative pain after orthopedic surgery under general anesthesia. BMC Anesthesiol. 2023, 23, 1–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Exxact. AutoML: An Introduction Using Auto-Sklearn and Auto-PyTorch. Published 14 April 2021. Available online: https://www.exxactcorp.com/blog/Deep-Learning/automl-an-introduction-using-auto-sklearn-and-auto-pytorch (accessed on 24 April 2025).
- Yung, K.K.Y.; Wu, P.P.Y.; der Fünten, K.A.; Hecksteden, A.; Meyer, T. Using a Bayesian network to classify time to return to sport based on football injury epidemiological data. PLoS ONE 2025, 20, e0314184. [Google Scholar] [CrossRef]
- Endo, Y.; Giunta, E.; Mroueh, J.; McCarthy, W.; Graf, N. Editorial: The application of bioactive materials in bone repair. Front. Bioeng. Biotechnol. 2025, 12, 1539142. [Google Scholar] [CrossRef]
- Gupta, H.; Bindra, G. The Evolving Role of AI in Orthopedics Surgery. Published 30 September 2024. Available online: https://hospidio.com/medical-travel/the-evolving-role-of-ai-in-orthopedics-surgery (accessed on 28 March 2025).
- Fan, X.; Wang, Y.; Zhang, S.; Xing, Y.; Li, J.; Ma, X.; Ma, J. Orthopedic surgical robotic systems in knee arthroplasty: A comprehensive review. Front. Bioeng. Biotechnol. 2025, 13, 1523631. [Google Scholar] [CrossRef]
- Schwartz, A.B.; Cui, X.T.; Weber, D.J.; Moran, D.W. Brain-Controlled Interfaces: Movement Restoration with Neural Prosthetics. Neuron 2006, 52, 205–220. [Google Scholar] [CrossRef]
- Lopez Molina AI. LinkedIn. Published 10 December 2024. Available online: https://www.linkedin.com/pulse/neuroprosthetics-bone-regeneration-ai-future-smart-lopez-molina-eyh6e/ (accessed on 28 March 2025).
- Hofheinz, E.Ö. Alfred Mann Team Up on Mind-Controlled Prosthetics. Orthopedics This Week. Published 23 December 2019. Available online: https://ryortho.com/2019/12/ossur-alfred-mann-team-up-on-mind-controlled-prosthetics/? (accessed on 18 April 2025).
- Hofheinz, E. Amputees Controlling Bionic Legs with Their Thoughts. Orthopedics This Week. Published 27 May 2015. Available online: https://ryortho.com/2015/05/amputees-controlling-bionic-legs-with-their-thoughts/ (accessed on 17 April 2025).
- Zeilig, G.; Weingarden, H.; Zwecker, M.; Dudkiewicz, I.; Bloch, A.; Esquenazi, A. Safety and tolerance of the ReWalk™exoskeleton suit for ambulation by people with complete spinal cord injury: A pilot study. J. Spinal Cord Med. 2012, 35, 96–101. [Google Scholar] [CrossRef]
- Liu, D.-X.; Du, W.; Wu, X.; Wang, C.; Qiao, Y. Deep rehabilitation gait learning for modeling knee joints of lower-limb exoskeleton. In Proceedings of the 2016 IEEE International Conference On Robotics And Biomimetics (ROBIO), Qingdao, China, 3–7 December 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1058–1063. [Google Scholar]
- Musk, E. Neuralink An Integrated Brain-Machine Interface Platform with Thousands of Channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef]
- Rodiles, N.; Weispfenning, R.; Medtronic Earns U.S. FDA Approval for the World’s First Adaptive Deep Brain Stimulation System for People with Parkinson’s. Medtronic News. Published 24 February 2025. Available online: https://news.medtronic.com/2025-02-24-Medtronic-earns-U-S-FDA-approval-for-the-worlds-first-Adaptive-deep-brain-stimulation-system-for-people-with-Parkinsons (accessed on 17 April 2025).
- Phalguni Deswal. Medtronic Teases Positive Data Adaptive Deep Brain Stimulation Trial. Clinical Trials Arena. Published 18 September 2024. Available online: https://www.clinicaltrialsarena.com/news/medtronic-teases-positive-data-adaptive-deep-brain-stimulation-trial/?cf-view (accessed on 17 April 2025).
- Stanslaski, S.; Summers, R.L.S.; Tonder, L.; Tan, Y.; Case, M.; Raike, R.S.; Morelli, N.; Herrington, T.M.; Beudel, M.; Ostrem, J.L.; et al. Sensing data and methodology from the Adaptive DBS Algorithm for Personalized Therapy in Parkinson’s Disease (ADAPT-PD) clinical trial. Npj Park. Dis. 2024, 10, 174. [Google Scholar] [CrossRef]
- Bionik Laboratories Corp. Bionik Laboratories Advances InMotion AnkleBot into Com-Mercial Development. 2016. Available online: https://content.equisolve.net/bioniklabs/news/2016-12-20_Bionik_Laboratories_Advances_InMotion_AnkleBot_32.pdf (accessed on 17 April 2025).
- Oña, E.D.; Cano-de La Cuerda, R.; Sánchez-Herrera, P.; Balaguer, C.; Jardón, A. A Review of Robotics in Neurorehabilitation: Towards an Automated Process for Upper Limb. J. Healthc. Eng. 2018, 2018, 9758939. [Google Scholar] [CrossRef]
- Anderson, D.N.; Charlebois, C.M.; Smith, E.H.; Davis, T.S.; Peters, A.Y.; Newman, B.J.; Arain, A.M.; Wilcox, K.S.; Butson, C.R.; Rolston, J.D. Closed-loop stimulation in periods with less epileptiform activity drives improved epilepsy outcomes. Brain 2023, 147, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.T.; Morrell, M.J. Closed-loop Neurostimulation: The Clinical Experience. Neurotherapeutics 2014, 11, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.R.; Laxer, K.D.; Weber, P.B.; Murro, A.M.; Park, Y.D.; Barkley, G.L.; Smith, B.J.; Gwinn, R.P.; Doherty, M.J.; Noe, K.H.; et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology 2020, 95, e1244–e1256. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Newlon, M.; Franchini, L. Boston Beijing Shenzhen Hangzhou Real-Time Brainwave Feedback and Visualization; the Future of Functional Prosthetics. 2016. Available online: https://www.mindtecstore.com/mediafiles/Sonstiges/Shop/Brainco/BrainCo_Introduction_12_EN.pdf?srsltid=AfmBOoq8QzHlyadPL2U7_Id2y5539A-t0Jq2pXby12luoZ9VMixTQWgz (accessed on 17 April 2025).
- Diaz-Rodriguez, P.; López-Álvarez, M.; Serra, J.; González, P.; Landín, M. Current Stage of Marine Ceramic Grafts for 3D Bone Tissue Regeneration. Mar. Drugs 2019, 17, 471. [Google Scholar] [CrossRef]
- Xtant Medical. OsteoVive® Viable Cell Allograft. Available online: https://xtantmedical.com/app/uploads/2016/09/OSTEOVIVE-Sell-Sheet_digital.pdf (accessed on 18 April 2025).
- Roh, J.S.; Yeung, C.A.; Field, J.S.; McClellan, R.T. Allogeneic morphogenetic protein vs. recombinant human bone morphogenetic protein-2 in lumbar interbody fusion procedures: A radiographic and economic analysis. J. Orthop. Surg. Res. 2013, 8, 49. [Google Scholar] [CrossRef]
- Field, J.; Yeung, C.; Roh, J. Clinical Evaluation of Allogeneic Growth Factor in Cervical Spine Fusion. J. Spine 2014, 3, 158. [Google Scholar] [CrossRef]
- DeVries, J.G.; Scharer, B. The Use of Allograft Bone Morphogenetic Protein in Foot and Ankle Arthrodesis. J. Orthop. Res. Physiother. 2016, 2, 23. [Google Scholar] [CrossRef]
- Nolte, P.A.; van der Krans, A.; Patka, P.; Janssen, I.M.; Ryaby, J.P.; Albers, G.H. Low-intensity pulsed ultrasound in the treatment of non-unions. J. Trauma 2001, 51, 693–703. [Google Scholar] [CrossRef]
- Heckman, J.D.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Kilcoyne, R.F. Acceleration of tibial frac-ture-healing by non-invasive, low intensity pulsed ultrasound. J. Bone Jt. Surg. Am. 1994, 76, 26–34. [Google Scholar] [CrossRef]
- Kristiansen, T.K.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Roe, L.R. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo controlled study. J. Bone Jt. Surg. 1997, 79, 961–973. [Google Scholar] [CrossRef]
- Frey, S.P.; Jansen, H.; Raschke, M.J.; Meffert, R.H.; Ochman, S. VEGF Improves Skeletal Muscle Regeneration After Acute Trauma and Reconstruction of the Limb in a Rabbit Model. Clin. Orthop. Relat. Res. 2012, 470, 3607–3614. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodruff, M.; Epari, D.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, M. RevBio Awarded a $3.4 Million NIH Grant for Its Novel Regenerative Bone Adhesive to Treat Complex Fractures. BusinessWire. Published 6 October 2023. Available online: https://www.businesswire.com/news/home/20231006480237/en/RevBio-Awarded-a-%243.4-Million-NIH-Grant-for-Its-Novel-Regenerative-Bone-Adhesive-to-Treat-Complex-Fractures (accessed on 18 April 2025).
- Hess, B. (Ed.) NIA Small Business Showcase: RevBio. National Institute on Aging. Published 8 January 2024. Available online: https://www.nia.nih.gov/research/sbir/nia-small-business-showcase/revbio (accessed on 17 April 2025).
- Hedelin, H.M.; Larnert, P.; Antonsson, P.; Lagerstrand, K.; Brisby, H.; Hebelka, H.; Laine, T. Stability in Pelvic Triple Osteotomies in Children Using Resorbable PLGA Screws for Fixation. J. Pediatr. Orthop. 2021, 41, e787–e792. [Google Scholar] [CrossRef] [PubMed]
- Fixation of Tibial Plateau Fracture Using ActivaScrew™ Cannulated-11.5-Yearold Child. Ph.D. Thesis, Unité Pédiatrique de Chirurgie Or-thopédiaque et Traumatologique (UPCOT) Hôpital de l’Enfance Ch. Montétan, Lausanne, Switzerland, 2017.
- Medial Malleolus Fracture of a 12-Year Old Girl with One ActivaScrew™ LAG Bioabsorbable Screw. Ph.D. Thesis, University of Nancy, Nancy, France, 2024.
- Cruz, E. Ekso Bionics: Acquired Brain Injury Rehabilitation Case. Published 26 July 2021. Available online: https://eksobionics.com/ekso-bionics-acquired-brain-injury-rehabilitation-case/ (accessed on 17 April 2025).
- Rodgers, H.; Bosomworth, H.; Krebs, H.I.; van Wijck, F.; Howel, D.; Wilson, N.; Aird, L.; Alvarado, N.; Andole, S.; Cohen, D.L.; et al. Robot assisted training for the upper limb after stroke (RATULS): A multicentre randomised controlled trial. Lancet 2019, 394, 51–62. [Google Scholar] [CrossRef]
- Lee, B.; Zubair, M.N.; Marquez, Y.D.; Lee, D.M.; Kalayjian, L.A.; Heck, C.N.; Liu, C.Y. A Single-Center Experience with the NeuroPace RNS System: A Review of Techniques and Potential Problems. World Neurosurg. 2015, 84, 719–726. [Google Scholar] [CrossRef]
- ssur. Össur Prosthetics. Available online: https://www.ossur.com/en-us/prosthetics (accessed on 17 April 2025).
- Alimusaj, M.; Fradet, L.; Braatz, F.; Gerner, H.J.; Wolf, S.I. Kinematics and kinetics with an adaptive ankle foot system during stair ambulation of transtibial amputees. Gait Posture 2009, 30, 356–363. [Google Scholar] [CrossRef]
- White, T.; Justiz, R.; Almonte, W.; Micovic, V.; Shah, B.; Anderson, E.; Kapural, L.; Cordner, H.; El-Naggar, A.; Fishman, M.; et al. Twelve-month results from a randomized controlled trial comparing differential target multiplexed spinal cord stimulation and conventional spinal cord stimulation in subjects with chronic refractory axial low back pain not eligible for spine surgery. N. Am. Spine Soc. J. (NASSJ) 2024, 19, 100528. [Google Scholar] [CrossRef]
- Gainor, B.J.; Groh, G.I. Early Clinical Experience With Orthofix External Fixation of Complex Distal Radius Fractures. Orthopedics 1990, 13, 329–333. [Google Scholar] [CrossRef]
- Farazin, A.; Zhang, C.; Gheisizadeh, A.; Shahbazi, A. 3D bio-printing for use as bone replacement tissues: A review of biomedical application. Biomed. Eng. Adv. 2023, 5, 100075. [Google Scholar] [CrossRef]
- Ninarello, D.; Ballardini, A.; Morozzi, G.; La Barbera, L. A comprehensive systematic review of marketed bone grafts for load-bearing critical-sized bone defects. J. Mech. Behav. Biomed. Mater. 2024, 160, 106782. [Google Scholar] [CrossRef]
- Maas, B. Xtant Medical Expands Product Portfolio with Launch of Its Own Viable Bone Matrix, OsteoVive(R) Plus. BioSpace. Published 18 September 2024. Available online: https://www.biospace.com/press-releases/xtant-medical-expands-product-portfolio-with-launch-of-its-own-viable-bone-matrix-osteoviver-plus (accessed on 17 April 2025).
- Brusco, S. RevBio Gets FDA OK to Expand TETRANITE Bone Adhesive Trial, Earns CMS Coverage. ODT. Published 12 December 2024. Available online: https://www.odtmag.com/breaking-news/revbio-gets-fda-ok-to-expand-tetranite-bone-adhesive-trial-earns-cms-coverage/ (accessed on 17 April 2025).
- Naik, N.; Hameed, B.M.Z.; Shetty, D.K.; Swain, D.; Shah, M.; Paul, R.; Aggarwal, K.; Ibrahim, S.; Patil, V.; Smriti, K.; et al. Legal and Ethical Consideration in Artificial Intelligence in Healthcare: Who Takes Responsibility? Front. Surg. 2022, 9, 862322. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Lee, S.-S.; Hwang, H. The ethics of using artificial intelligence in medical research. Kosin Med. J. 2024, 39, 229–237. [Google Scholar] [CrossRef]
- Pressman, S.M.; Borna, S.; Gomez-Cabello, C.A.; Haider, S.A.; Haider, C.; Forte, A.J. AI and Ethics: A Systematic Review of the Ethical Considerations of Large Language Model Use in Surgery Research. Healthcare 2024, 12, 825. [Google Scholar] [CrossRef]
- Fangerau, H. Artifical intelligence in surgery: Ethical considerations in the light of social trends in the perception of health and medicine. EFORT Open Rev. 2024, 9, 323–328. [Google Scholar] [CrossRef]
- ElHassan, B.T.; Arabi, A.A. Ethical forethoughts on the use of artificial intelligence in medicine. Int. J. Ethic-Syst. 2024, 41, 35–44. [Google Scholar] [CrossRef]
- Chan, B. Black-box assisted medical decisions: AI power vs. ethical physician care. Med. Health Care Philos. 2023, 26, 285–292. [Google Scholar] [CrossRef]
- Durán, J.M.; Jongsma, K.R. Who is afraid of black box algorithms? On the epistemological and ethical basis of trust in medical AI. J. Med. Ethics 2021, 47, 329–335. [Google Scholar] [CrossRef]
- Editorial Staff. Navigating the Black Box AI Debate in Healthcare|TechTarget. Healthtech Analytics. Published 1 May 2024. Available online: https://www.techtarget.com/healthtechanalytics/feature/Navigating-the-black-box-AI-debate-in-healthcare (accessed on 28 March 2025).
- Loria, K. Game-Changing Advances in Orthopedics. Outpatient Surgery Magazine. Published 4 February 2025. Available online: https://www.aorn.org/outpatient-surgery/article/game-changing-advances-in-orthopedics (accessed on 28 March 2025).
- Admin.AOP. American Orthopedic Partners|Advancing Spine Health: Key Innovations in Orthopedic Care for World Spine Day. Published 10 December 2024. Available online: https://aorthopartners.com/orthopedic-innovations-to-watch-in-2025/ (accessed on 28 March 2025).
- Kumar, R.; Kumar, S. Measurement Of Background Radiation at Oracle Plastics and Sacks Company in Makurdi, Benue State. Recent Advancements in Orthopedic Implant Materials: A Short Review. J. Biomed. Eng. Res. 2024, 1, 1–6. Available online: https://www.wecmelive.com/open-access/recent-advancements-in-orthopedic-implant-materials-a-short-review.pdf (accessed on 28 March 2025).
- Ashish Daniel, S.; Prem, S.; Jesuarockiam Naveen Khan, T.; Khahro, S.H. Advancement in biomedical implant materials—A mini review. Front. Bioeng. Biotechnol. 2024, 12, 1400918. [Google Scholar] [CrossRef]
- Salman, L.A. Transforming orthopedics: A glimpse into the future with artificial intelligence. J. Musculoskelet. Surg. Res. 2025, 9, 118–120. [Google Scholar] [CrossRef]
- Tripathi, D.; Hajra, K.; Mulukutla, A.; Shreshtha, R.; Maity, D. Artificial Intelligence in Biomedical Engineering and Its Influence on Healthcare Structure: Current and Future Prospects. Bioengineering 2025, 12, 163. [Google Scholar] [CrossRef] [PubMed]





| Traditional Surgery | Robotic-Assisted Surgery |
| Manual implant alignment | Algorithm-driven precision |
| Higher risk of human error | Reduced variability (<1° deviation) |
| Extended recovery periods | Faster mobilization (e.g., 20% shorter hospital stays) |
| Metric | Traditional TKA | AI–Robotic TKA |
|---|---|---|
| Implant alignment accuracy | 69.9% within target | 99.9% within target |
| Postoperative ROM accuracy | Slower | 20% faster recovery |
| Radiation exposure | High | Reduced by 70% |
| Company | Focus | AI Application |
|---|---|---|
| Össur [34,35,36] | Advanced neuroprosthetics and orthopedic technologies | Employs AI to improve the functionality of prosthetics, exemplified by the Proprio knee prosthesis, which dynamically adapts to terrain and walking speed. |
| ReWalk Robotics [34,37,38] | AI-developed exoskeletons for individuals with mobility impairments | Utilizes AI to interpret user intent and modulate exoskeleton movements, enabling more natural and responsive walking patterns. |
| Neuralink [34,39] | Brain–computer interface (BCIs) development for neurological disorders | Designs AI-driven BCIs for direct brain-to-device communication, targeting functional restoration in individuals with paralysis. |
| Medtronic [34,40,41,42] | Neurosurgical medical devices and surgical systems | Integrates AI to enhance precision in device implantation and tailor interventions for neurological injuries and diseases |
| Bionik Laboratories [34,43,44] | Rehabilitation technologies and neuroprosthetics | Develops AI-powered exoskeletons that respond adaptively to user movement, promoting mobility recovery for patients with paralysis. |
| NeuroPace [34,45,46,47] | Implantable neurostimulation devices | Uses AI to customize and adjust brain stimulation therapies in real time, optimizing treatment for individual patients. |
| BrainCo [34,48] | Cognitive and motor neurotechnology solutions | Leverages AI to support the development of neuroprosthetics and enhance brain function and control. |
| Company | Focus | AI Application |
|---|---|---|
| Xtant Medical [34,49,50] | Bone regeneration through surgical interventions | Applies AI in medical devices to improve implant integration and speed up the bone healing process |
| Bioventus [34,51,52,53,54,55,56] | Bone healing via stimulation technologies and cell therapies | Employs advanced AI to tailor therapies and enhance recovery for fractures and skeletal conditions |
| Orthofix [34,57,58] | Treatments for musculoskeletal disorders and bone repair | Integrates AI with electronic bone stimulation to support regeneration following surgical procedures |
| RevBio [34,59,60] | Biologically based therapies for bone repair | Uses AI to develop engineered biomaterials that facilitate healing in complex bone fractures |
| Bioretec [34,61,62,63] | AI-driven solutions for bone regeneration | Applies AI to design and optimize healing devices tailored to fracture-specific structural requirements |
| Health Challenge | Technology | Surgical Technique | Benefit |
|---|---|---|---|
| Spinal Cord Injuries (SCIs) [34,39,40,41,42,64] | Brain–computer interfaces (BCIs) by Neuralink, adaptive exoskeletons from ReWalk Robotics and Ekso Bionics | Exoskeletons require no surgery (external devices); BCIs involve surgical implantation of brain electrodes | Restoration of partial or full mobility, increased independence, and improved quality of life |
| Stroke (CVA) [34,43,44,65] | Smart prosthetics with adaptive control systems by Bionik Laboratories | Minimally invasive procedures for implanting sensors and electrodes in neuroprosthetics | Enhanced rehabilitation through prosthetics that adapt to user movement |
| Amyotrophic Lateral Sclerosis (ALS) [34,45,46,47,66] | Implantable brain stimulation devices from NeuroPace | Deep brain stimulation (DBS) surgery for device placement | Improved environmental interaction and device control via neural signals |
| Cerebral Palsy [34,40,41,42] | AI-powered prosthetics from Medtronic for tailored mobility solutions | Surgical implantation of neurostimulators to support motor control | Increased task performance and greater autonomy |
| Amputations [34,35,36,67,68,69] | Intelligent prosthetics like Össur’s Proprio system, responsive to terrain | Amputation surgery followed by prosthetic fitting | More precise motor functions and more natural gait simulation |
| Bone Regeneration Challenge | Technology | Surgical Technique | Benefit |
|---|---|---|---|
| Complex Fractures [34,57,58,70] | Predictive surgical planning models by Orthofix | Osteosynthesis using bone stimulators or AI-guided implant designs | Faster recovery through personalized treatment and enhanced fracture healing |
| Advanced Osteoporosis [34,61,62,63,71] | AI-driven, 3D-printed implants by Bioretec | Implant-based fracture fixation or joint replacement (osteosynthesis or arthroplasty) | Improved bone strength and reduced risk of recurrent fractures with biologically compatible implants |
| Bone Tumors [34,49,50,72,73] | Regenerative biomaterials and AI-assisted grafts by Xtant Medical | Oncologic surgery for bone tumor resection followed by AI-guided bone graft placement | Accurate anatomical post-tumor resection with enhanced structural integrity |
| Congenital Deformities [34,59,60,74] | AI-customized prosthetics | Corrective osteotomies with patient-specific prosthesis placement | Functional and aesthetic improvements via individualized surgical correction |
| Traumatic Injuries [34,51,52,53,54,55,56] | Bone stimulation and cell therapy from Bioventus | Surgical repair with bone stimulators and regenerative materials (osteosynthesis) | Effective regeneration of damaged bone using advanced biomaterials and stimulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Sporn, K.; Ong, J.; Waisberg, E.; Paladugu, P.; Vaja, S.; Hage, T.; Sekhar, T.C.; Vadhera, A.S.; Ngo, A.; et al. Integrating Artificial Intelligence in Orthopedic Care: Advancements in Bone Care and Future Directions. Bioengineering 2025, 12, 513. https://doi.org/10.3390/bioengineering12050513
Kumar R, Sporn K, Ong J, Waisberg E, Paladugu P, Vaja S, Hage T, Sekhar TC, Vadhera AS, Ngo A, et al. Integrating Artificial Intelligence in Orthopedic Care: Advancements in Bone Care and Future Directions. Bioengineering. 2025; 12(5):513. https://doi.org/10.3390/bioengineering12050513
Chicago/Turabian StyleKumar, Rahul, Kyle Sporn, Joshua Ong, Ethan Waisberg, Phani Paladugu, Swapna Vaja, Tamer Hage, Tejas C. Sekhar, Amar S. Vadhera, Alex Ngo, and et al. 2025. "Integrating Artificial Intelligence in Orthopedic Care: Advancements in Bone Care and Future Directions" Bioengineering 12, no. 5: 513. https://doi.org/10.3390/bioengineering12050513
APA StyleKumar, R., Sporn, K., Ong, J., Waisberg, E., Paladugu, P., Vaja, S., Hage, T., Sekhar, T. C., Vadhera, A. S., Ngo, A., Zaman, N., Tavakkoli, A., & Masalkhi, M. (2025). Integrating Artificial Intelligence in Orthopedic Care: Advancements in Bone Care and Future Directions. Bioengineering, 12(5), 513. https://doi.org/10.3390/bioengineering12050513






