Pathomechanics of Early-Stage Lumbar Intervertebral Disc Degradation Leading to Discogenic Pain—A Narrative Review
Abstract
1. Introduction
2. Methods
3. Results
3.1. Early-Stage Changes in Biology and Biochemistry Affect Disc Biomechanics
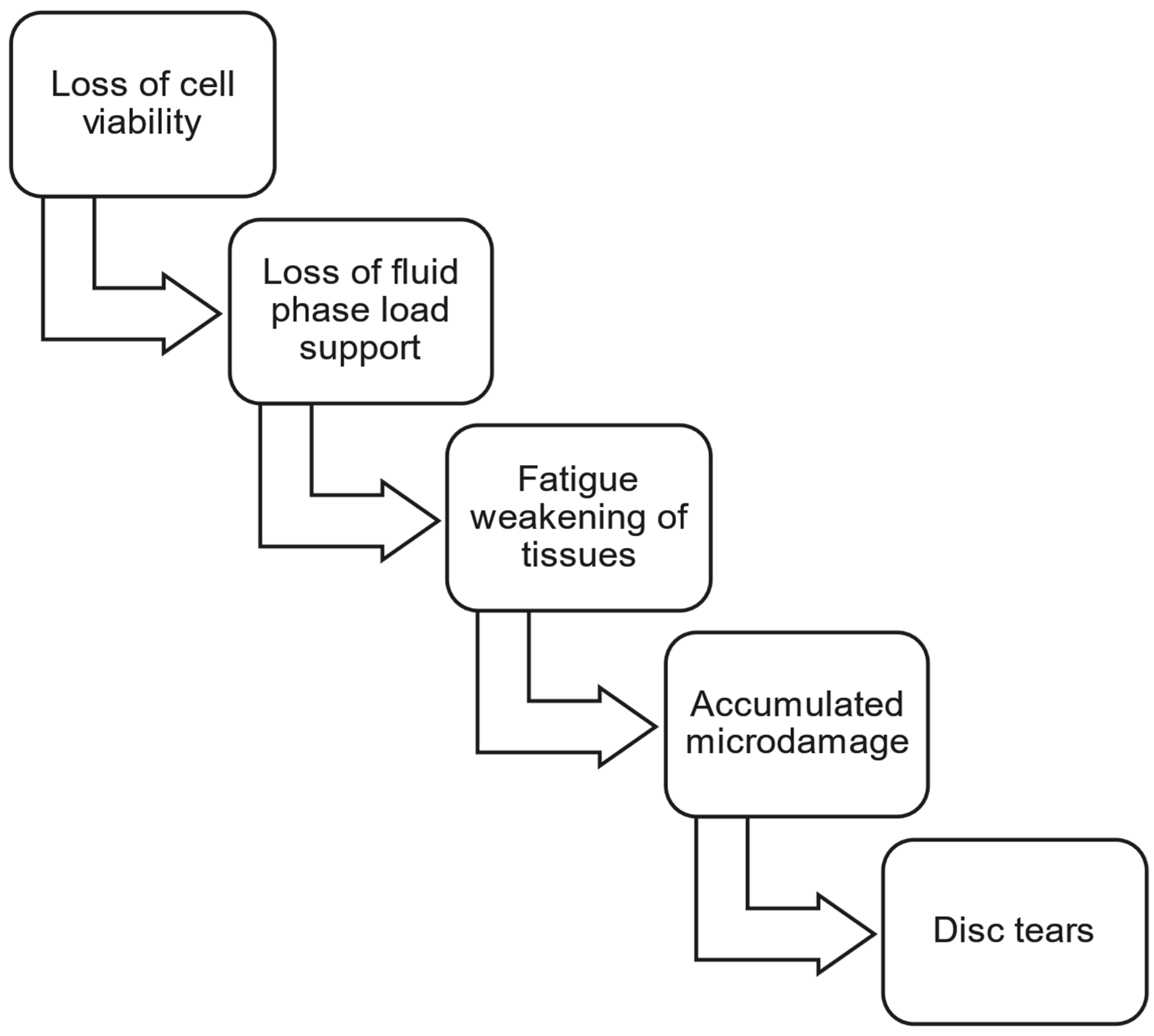
3.2. Fatigue and Accumulated Microdamage
3.3. Disc Delamination and Tears: Early-Stage Prevalence and Clinical Relevance
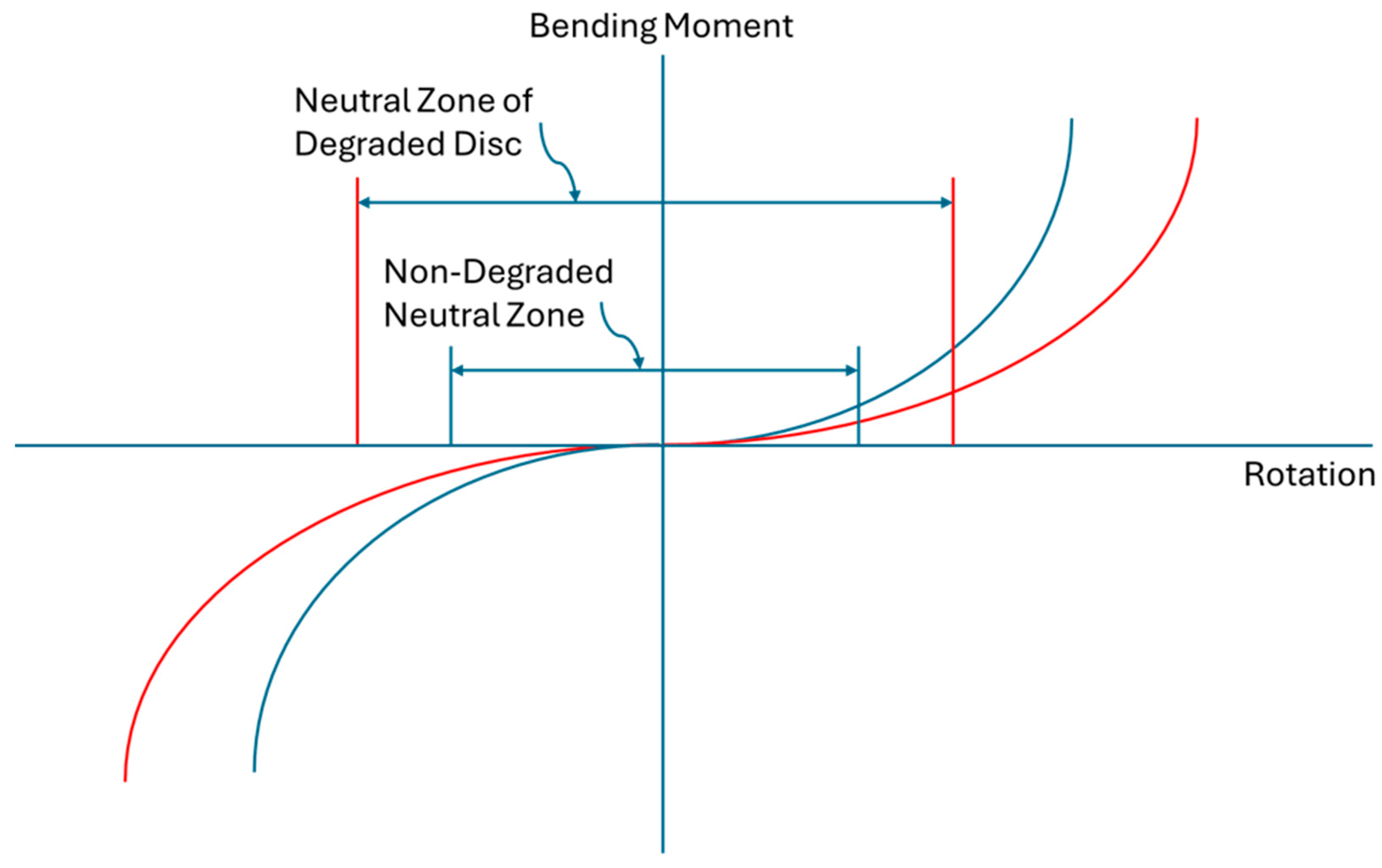
3.4. Segmental Degenerative Instability
3.5. Pathomechanics and Low Back Pain
3.5.1. Discogenic Pain
3.5.2. Peak Age of Onset of Low Back Pain
3.5.3. Prevalence of Low Back Pain
3.5.4. Acute Back Pain
3.6. Preferred Treatment Characteristics for Early-Stage Discogenic Back Pain
3.7. Emerging Intradiscal Therapies for Early-Stage Discogenic Pain
3.7.1. Mechanisms of Load Support in Emerging Intradiscal Therapies
3.7.2. Comparison of Emerging Intradiscal Therapies
4. Discussion and Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stokes, I.A.F.; Iatridis, J.C. Mechanical conditions that accelerate intervertebral disc degeneration: Overload versus immobilization. Spine 2004, 29, 2724–2732. [Google Scholar]
- Molinos, M.; Almeida, C.R.; Caldeira, J.; Cunha, C.; Goncalves, R.M.; Barbosa, M.A. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 2015, 12, 20141191. [Google Scholar] [CrossRef]
- Tsai, T.T.; Cheng, C.M.; Chen, C.F.; Lai, P.L. Mechanotransduction in intervertebral discs. J. Cell. Mol. Med. 2014, 18, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.J.; Veras, M.A.; Kim, M.K.M.; Seguin, C.A. Decoding the intervertebral disc: Unravelling the complexities of cell phenotypes and pathways associated with degeneration and mechanotransduction. Semin. Cell Dev. Biol. 2017, 62, 94–103. [Google Scholar] [PubMed]
- Fearing, B.V.; Hernandez, P.A.; Setton, L.A.; Chahine, N.O. Mechanotransduction and cell biomechanics of the intervertebral disc. JOR Spine 2018, 1, e1026. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Wang, B.; Liao, Z.; Song, Y.; Li, G.; Ma, L.; Wang, K.; Li, S.; Hua, W.; Yang, C. Matrix stiffness induces Drp1-mediated mitochondrial fission through Piezo1 mechanotransduction in human intervertebral disc degeneration. J. Transl. Med. 2023, 21, 711. [Google Scholar]
- Setton, L.A.; Chen, J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J. Bone Joint Surg. 2006, 88-A (Suppl. S2), 52–57. [Google Scholar]
- Battie, M.C.; Videman, T.; Gibbons, L.E.; Fisher, L.D.; Manninen, H.; Gill, K. 1995 Volvo Award in clinical sciences. Determinants of lumbar disc degeneration: A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine 1995, 20, 2601–2612. [Google Scholar] [CrossRef]
- Videman, T.; Leppävuori, J.; Kaprio, J.; Battie, M.C.; Gibbons, L.E.; Peltonen, L.; Koskenvuo, M. Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine 1998, 23, 2477–2485. [Google Scholar] [CrossRef]
- Battié, M.C.; Videman, T.; Parent, E. Lumbar disc degeneration—Epidemiology and genetic influences. Spine 2004, 29, 2679–2690. [Google Scholar] [CrossRef]
- Cheung, K.M.; Chan, D.; Karppinen, J.; Chen, Y.; Jim, J.J.; Yip, S.P.; Ott, J.; Wong, K.K.; Sham, P.; Luk, K.D.; et al. Association of the Taq I Allele in Vitamin D Receptor with Degenerative Disc Disease and Disc Bulge In a Chinese Population. Spine 2006, 31, 1143–1148. [Google Scholar] [PubMed]
- Mayer, J.E.; Iatridis, J.C.; Chan, D.; Qureshi, S.A.; Gottesman, O.; Hecht, A.C. Review: Genetic polymorphisms associated with intervertebral disc degeneration. Spine J. 2013, 13, 299–317. [Google Scholar]
- Li, H.; Tian, L.; Li, J.; Li, Y.; Du, L.; Huo, Z.; Xu, B. The Roles of circRNAs in Intervertebral Disc Degeneration: Inflammation, Extracellular Matrix Metabolism, and Apoptosis. Anal. Cell. Pathol. 2022, 2022, 9550499. [Google Scholar] [CrossRef]
- Roh, E.J.; Darai, A.; Kyung, J.W.; Choi, H.; Kwon, S.Y.; Bhujel, B.; Kim, K.T.; Han, I. Genetic therapy for intervertebral disc degeneration. Int. J. Mol. Sci. 2021, 22, 1579. [Google Scholar] [CrossRef]
- Kirkaldy-Willis, W.H.; Wedge, J.H.; Yong-Hing, K.; Reilly, J. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine 1978, 3, 319–328. [Google Scholar]
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314. [Google Scholar] [PubMed]
- Boos, N.; Weissbach, S.; Rohrbach, H.; Weiler, C.; Spratt, K.F.; Nerlich, A.G. Classification of Age-Related Changes in Lumbar Intervertebral Discs. Spine 2002, 27, 2631–2644. [Google Scholar] [PubMed]
- Urban, J.P.; Smith, S.; Fairbank, J.C. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef]
- Iatridis, J.C.; Liable, J.P.; Krag, M.H. Influence of Fixed Charge Density Magnitude and Distribution on the Intervertebral Disc: Applications of a Poroelastic and Chemical Electric (PEACE) Model. J. Biomech. Eng. 2003, 125, 12–24. [Google Scholar]
- Urban, J.P.G.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef][Green Version]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef]
- Brinckmann, P.; Biggemann, M.; Hilweg, D. Fatigue fracture of human lumbar vertebrae. Clin. Biomech. 1988, 3 (Suppl. S1), 1–22. [Google Scholar]
- Osti, O.L.; Vernon-Roberts, B.; Moore, R.; Fraser, R.D. Annular tears and disc degeneration in the lumbar spine—A post-mortem study of 135 discs. J. Bone Joint Surg. 1992, 74-B, 678–682. [Google Scholar]
- Vernon-Roberts, B.; Moore, R.J.; Fraser, R.D. The Natural History of Age-related Disc Degeneration—The Pathology and Sequelae of Tears. Spine 2007, 32, 2797–2804. [Google Scholar]
- Kirking, B.; Hedman, T.; Criscione, J. Changes in the interfacial shear resistance of disc annulus fibrosus from genipin crosslinking. J. Biomech. 2014, 47, 293–296. [Google Scholar]
- Iatridis, J.C.; MacLean, J.J.; Ryan, D.A. Mechanical damage to the intervertebral disc annulus fibrosus subjected to tensile loading. J. Biomech. 2005, 38, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, A.C.; Aprill, C.N.; Derby, R.; Fortin, J.; Kine, G.; Bogduk, N. The Prevalence and Clinical Features of Internal Disc Disruption in Patients with Chronic Low Back Pain. Spine 1995, 20, 1878–1883. [Google Scholar]
- Gunzburg, R.; Parkinson, R.; Moore, R.; Cantraine, F.; Hutton, W.; Vernon-Roberts, B.; Fraser, R. A cadaveric study comparing discography, magnetic resonance imaging, histology, and mechanical behavior of the human lumbar disc. Spine 1992, 17, 417–426. [Google Scholar] [CrossRef]
- Sehgal, N.; Fortin, J.D. Internal disc disruption and low back pain. Pain. Physician 2000, 3, 143–157. [Google Scholar]
- Fujiwara, A.; Tamai, K.; An, H.S.; Kurihashi, A.; Lim, T.H.; Yoshida, H.; Saotome, K. The Relationship Between Disc Degeneration, Facet Joint Osteoarthritis, and Stability of the Degenerative Lumbar Spine. J. Spinal Disord. 2000, 13, 444–450. [Google Scholar]
- Oxland, T.R.; Panjabi, M.M. The onset and progression of spinal injury: A demonstration of neutral zone sensitivity. J. Biomech. 1992, 25, 1165–1172. [Google Scholar] [CrossRef]
- Panjabi, M.M. Clinical spinal instability and low back pain. J. Electromyogr. Kinesiol. 2003, 13, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Stokes, I.A. Bulging of lumbar intervertebral discs: Non-contacting measurements of anatomical specimens. J. Spinal Disord. 1988, 1, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Khalsa, P.S. Encoding of compressive stress during indentation by group III and IV muscle mechano-nociceptors in rat gracilis muscle. J. Neurophysiol. 2003, 89, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Coppes, M.H.; Marani, E.; Thomeer, R.T.; Groen, G.J. Innervation of “painful” lumbar discs. Spine 1997, 22, 2342–2349. [Google Scholar] [CrossRef]
- Freemont, A.J.; Peacock, T.E.; Goupille, P.; Hoyland, J.A.; O’brien, J.; Jayson, M.I.V. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997, 350, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.M. Neural Mechanisms of Lumbar Pain. Spine 1995, 20, 1804–1809. [Google Scholar] [CrossRef]
- Adams, M.A.; Hutton, W.C.; Stott, J.R.R. The resistance to flexion of the lumbar intervertebral joint. Spine 1980, 5, 245–253. [Google Scholar] [CrossRef]
- Bartynski, W.S.; Dejohn, L.M.; Rothfus, W.E.; Gerszten, P.C. ‘Progressive-Onset’ versus injury-associated discogenic low back pain: Features of disc internal derangement in patients studied with provocation lumbar discography. Interv. Neuroradiol. 2013, 19, 110–120. [Google Scholar] [CrossRef]
- Von Korff, M. Studying the natural history of back pain. Spine 1994, 19, 2041S–2046S. [Google Scholar] [CrossRef]
- Adams, M.A.; Freeman, B.J.C.; Morrison, H.P.; Nelson, I.W.; Dolan, P. Mechanical initiation of intervertebral disc degeneration. Spine 2000, 25, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Kuslich, S.D.; Ulstrom, C.L.; Michael, C.J. The tissue origin of low back pain and sciatica: A report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop. Clin. North. Am. 1991, 22, 181–187. [Google Scholar] [CrossRef]
- McNally, D.S.; Shackleford, I.M.; Goodship, A.E.; Mulholland, R.C. In-vivo stress measurements can predict pain on discography. Spine 1996, 21, 2580–2587. [Google Scholar] [CrossRef]
- Yoshizawa, H.; O’Brien, J.P.; Smith, W.T.; Trumper, M. The neuropathology of intervertebral discs removed for low-back pain. J. Pathol. 1980, 132, 95–104. [Google Scholar] [CrossRef]
- Rowe, M.L. Low back pain in industry—A position paper. J. Occup. Med. 1969, 11, 161–169. [Google Scholar] [CrossRef]
- Deyo, R.A.; Tsui-Wu, Y.J. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine 1987, 12, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Laslett, M.; Crothers, C.; Beattie, P.; Cregten, L.; Moses, A. The frequency and incidence of low back pain/sciatica in an urban population. New Zealand Med. J. 1991, 104, 424–426. [Google Scholar] [PubMed]
- Biering-Sorensen, F. A prospective study of low back pain in a general population. I. Occurrence, recurrence and aetiology. Scand. J. Rehabil. Med. 1983, 15, 71–79. [Google Scholar] [CrossRef]
- van Tulder, M.; Koes, B.; Bombardier, C. Low back pain. Best. Pract. Res. Clin. Rheumatol. 2002, 16, 761–775. [Google Scholar] [CrossRef]
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef]
- Rubin, D.I. Epidemiology and risk factors for spine pain. Neurol. Clin. 2007, 25, 353–371. [Google Scholar] [CrossRef]
- Haldemann, S.; Dagenais, S. A supermarket approach to the evidence-informed management of chronic low back pain. Spine J. 2008, 8, 1–7. [Google Scholar]
- Von Korff, M.; Deyo, R.A.; Cherkin, D.; Barlow, W. Back pain in primary care—Outcomes at 1 year. Spine 1993, 18, 855–862. [Google Scholar]
- Meucci, R.D.; Fassa, A.G.; Faria, N.M. Prevalence of chronic low back pain: Systematic review. Rev. Saude Publica 2015, 49, 73. [Google Scholar]
- Balagué, F.; Mannion, A.F.; Pellisé, F.; Cedraschi, C. Non-specific low back pain. Lancet 2012, 379, 482–491. [Google Scholar]
- Druss, B.G.; Marcus, S.C.; Olfson, M.; Pincus, H.A. The most expensive medical conditions in America. Health Aff. 2002, 21, 105–111. [Google Scholar]
- Schlenk, R.P.; Stewart, T.; Benzel, E.C. The biomechanics of iatrogenic spinal destabilization and implant failure. Neurosurg. Focus. 2003, 15, E2. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J. Bone Joint Surg. Am. 2006, 88 (Suppl. S2), 21–24. [Google Scholar] [PubMed]
- Lorio, M.P.; Tate, J.L.; Myers, T.J.; Block, J.E.; Beall, D.P. Perspective on Intradiscal Therapies for Lumbar Discogenic Pain: State of the Science, Knowledge Gaps, and Imperatives for Clinical Adoption. J. Pain Res. 2024, 17, 1171–1182. [Google Scholar]
- Airiksinen, O.; Borx, J.I.; Cedraschi, C. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006, 15 (Suppl. S2), S192–S300. [Google Scholar]
- Kanan, M.; Eby, O.; Kelkar, A.; Serhan, H.; Zodak, Y.; Aldoohan, S.; Elsamaloty, H.; Goel, V.; Yildirim-Ayan, E. Electrical Stimulation-Mediated Tissue Healing in Porcine Intervertebral Disc Under Mechanically Dynamic Organ Culture Conditions. Spine 2022, 47, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Beall, D.P.; Amirdelfan, K.; Nunley, P.D.; Phillips, T.R.; Navarro, L.C.I.; Spath, A. Hydrogel Augmentation of the Lumbar Intervertebral Disc: An Early Feasibility Study of a Treatment for Discogenic Low Back Pain. J. Vasc. Interv. Radiol. 2024, 35, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Don, A.S.; Hurwitz, E.L.; Cuellar, J.M.; Carrino, J.; Herzog, R. Does discography cause accelerated progression of degeneration changes in the lumbar disc: A ten-year matched cohort study. Spine 2009, 34, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Michalek, A.J.; Buckley, M.R.; Bonassar, L.J.; Cohen, I.; Iatridis, J.C. The effects of needle puncture injury on microscale shear strain in the intervertebral disc annulus fibrosus. Spine J. 2010, 10, 1098–1105. [Google Scholar] [CrossRef]
- Hedman, T.; Rogers, A.; Beall, D. A Self-Polymerizing Mesh of Nano-Tethers for the Mechanical Constraint of Degraded Intervertebral Discs—A Review of 25 Years of Pre-Clinical and Early Clinical Research. Bioengineering 2024, 11, 535. [Google Scholar] [CrossRef]
- Hedman, T.; Yu, J.; Singh, H.; Deer, T. Early clinical results of intervertebral joint stabilization by injectable load-sharing polymers. J. Pain Res. 2023, 16, 2777–2789. [Google Scholar] [CrossRef]
| Criteria | Rationale |
|---|---|
| Effective in reducing pain and disability | Primary reason patient sought medical attention |
| Provide load support and motion constraint, joint re-stabilization | To address cause of pain and to resist progression of disorder |
| Immediate effectiveness | Preferred by patients |
| Lasting effect, durability | To reduce repeat visits to healthcare providers, reduce cumulative healthcare costs |
| Single procedure | To avoid patient non-compliance with multiple procedure visits |
| Demonstrated mechanical benefits in the lab and in the clinic | To aid adoption of new therapy by demonstrating key benefit over palliative care |
| No adverse effects to disc or adjacent tissues | To promote long-term health benefits |
| Procedure simplicity, not requiring specialized equipment or training | To promote availability of treatment, remove potential hindrances to physicians |
| Healthcare and societal cost reductions | To encourage adoption by all the medical care stakeholders |
| Accessibility to marginalized and economically challenged groups, countries | Reducing treatment cost, time away from work, and need for repeated treatments helps the inclusion of these groups |
| Emerging Therapy | Load Support Mechanism (s) | Comments |
|---|---|---|
| Biological therapies—MSCs, PRP, allograft, tissue engineered materials, etc. | (1) New or repaired extracellular matrix to restore mechanics; (2) Restored disc hydration, liquid-phase load support | Regenerated or repaired tissue is a worthy goal, and biologic therapies work in other load-supporting tissues; however, the disc has deficient nutritional supply to added cells, challenges to durability of effect due to harsh biological conditions that led to pre-existing degradation, patient specific outcomes, and delayed effect |
| Nucleus allograft | Increased nucleus hydration, liquid-phase load support | Nucleus augmentation without repair of degraded annulus may increase stress in annulus and not resist progression of mechanical degradation |
| Allogeneic fibrin sealant | None without Factor XIII-A crosslinking component | Fibrin is unstable and breaks down without the crosslinking component |
| Multi-electrode implanted catheter that provides intradiscal electrical stimulation | Increase nucleus hydration, liquid-phase load support | Nucleus augmentation without repair of degraded annulus may increase stress in annulus and not resist progression of mechanical degradation |
| Polymer composite hydrophilic hydrogel augmentation material | Compressive load-supporting bulk filler material, increase fluid-phase load support | Disc augmentation without repair of degraded annulus may increase stress in annulus and not resist progression of mechanical degradation, space for filler may not be available in early-stage discs, potential tissue damage from hot injectate, possible implant material expulsion |
| Injectable, intra-annular polymer mesh | Mesh of tensile load-carrying polymers attached to collagen matrix | Polymeric mesh augments collagen tensile load-carrying to provide durable motion constraint and load support, requires second treatment for high mechanical demand patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hedman, T.; Rogers, A. Pathomechanics of Early-Stage Lumbar Intervertebral Disc Degradation Leading to Discogenic Pain—A Narrative Review. Bioengineering 2025, 12, 389. https://doi.org/10.3390/bioengineering12040389
Hedman T, Rogers A. Pathomechanics of Early-Stage Lumbar Intervertebral Disc Degradation Leading to Discogenic Pain—A Narrative Review. Bioengineering. 2025; 12(4):389. https://doi.org/10.3390/bioengineering12040389
Chicago/Turabian StyleHedman, Thomas, and Adam Rogers. 2025. "Pathomechanics of Early-Stage Lumbar Intervertebral Disc Degradation Leading to Discogenic Pain—A Narrative Review" Bioengineering 12, no. 4: 389. https://doi.org/10.3390/bioengineering12040389
APA StyleHedman, T., & Rogers, A. (2025). Pathomechanics of Early-Stage Lumbar Intervertebral Disc Degradation Leading to Discogenic Pain—A Narrative Review. Bioengineering, 12(4), 389. https://doi.org/10.3390/bioengineering12040389






