Predicting the Evolution of Lung Squamous Cell Carcinoma In Situ Using Computational Pathology
Abstract
1. Introduction
2. Materials and Methods
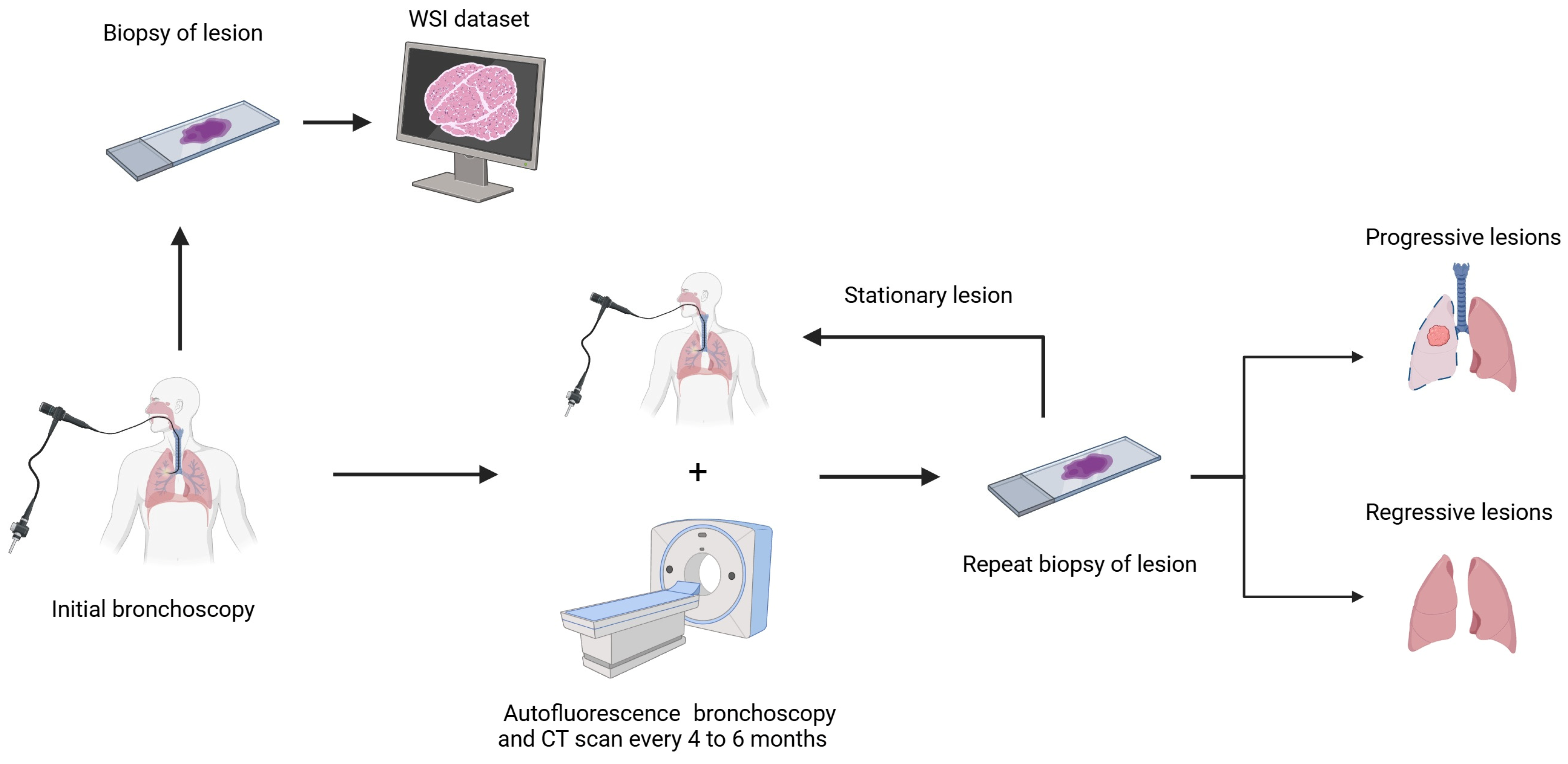
2.1. Dataset
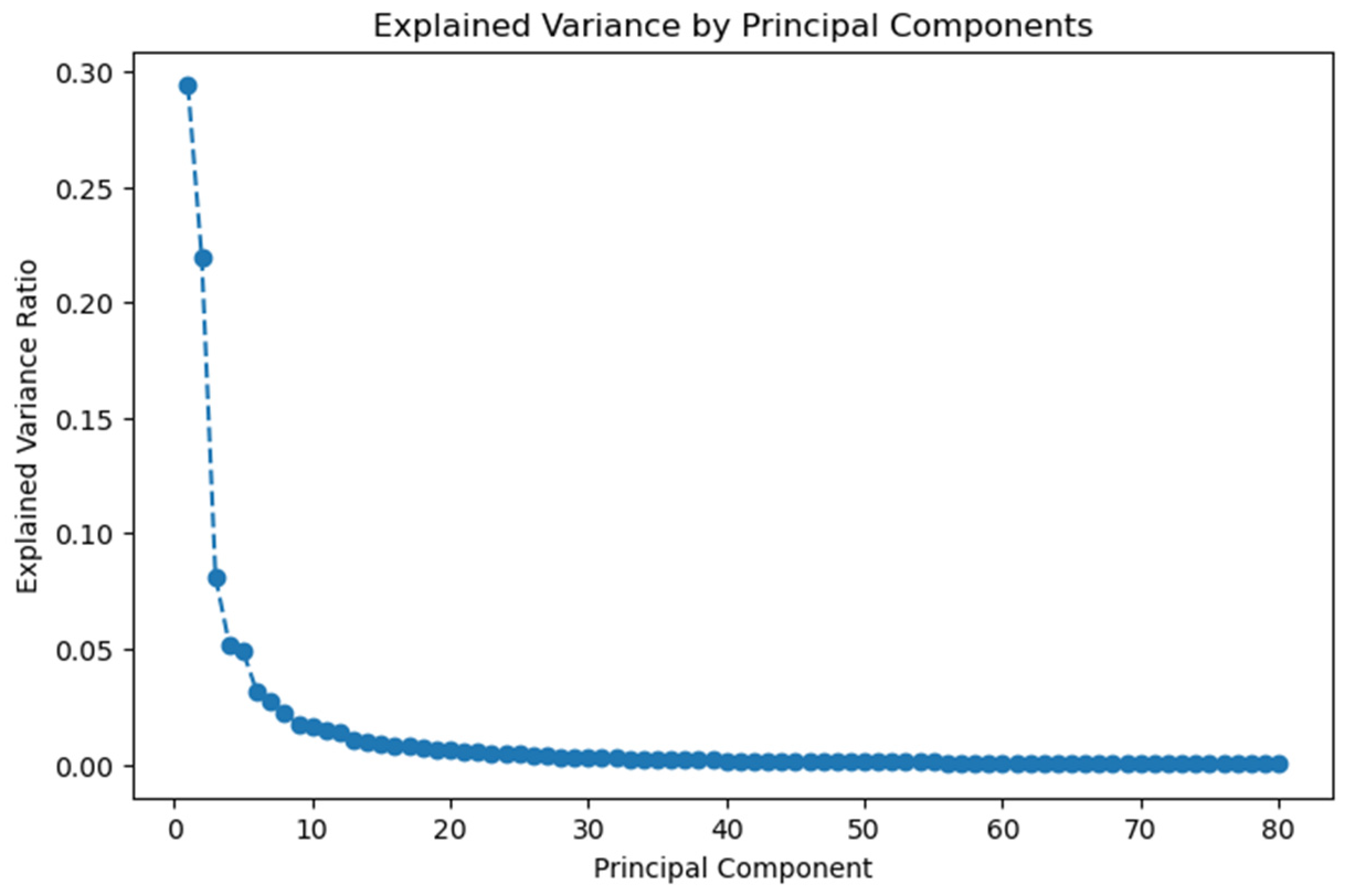
2.2. Feature Extraction and Dimensionality Reduction
2.3. Deep Learning Method
3. Results
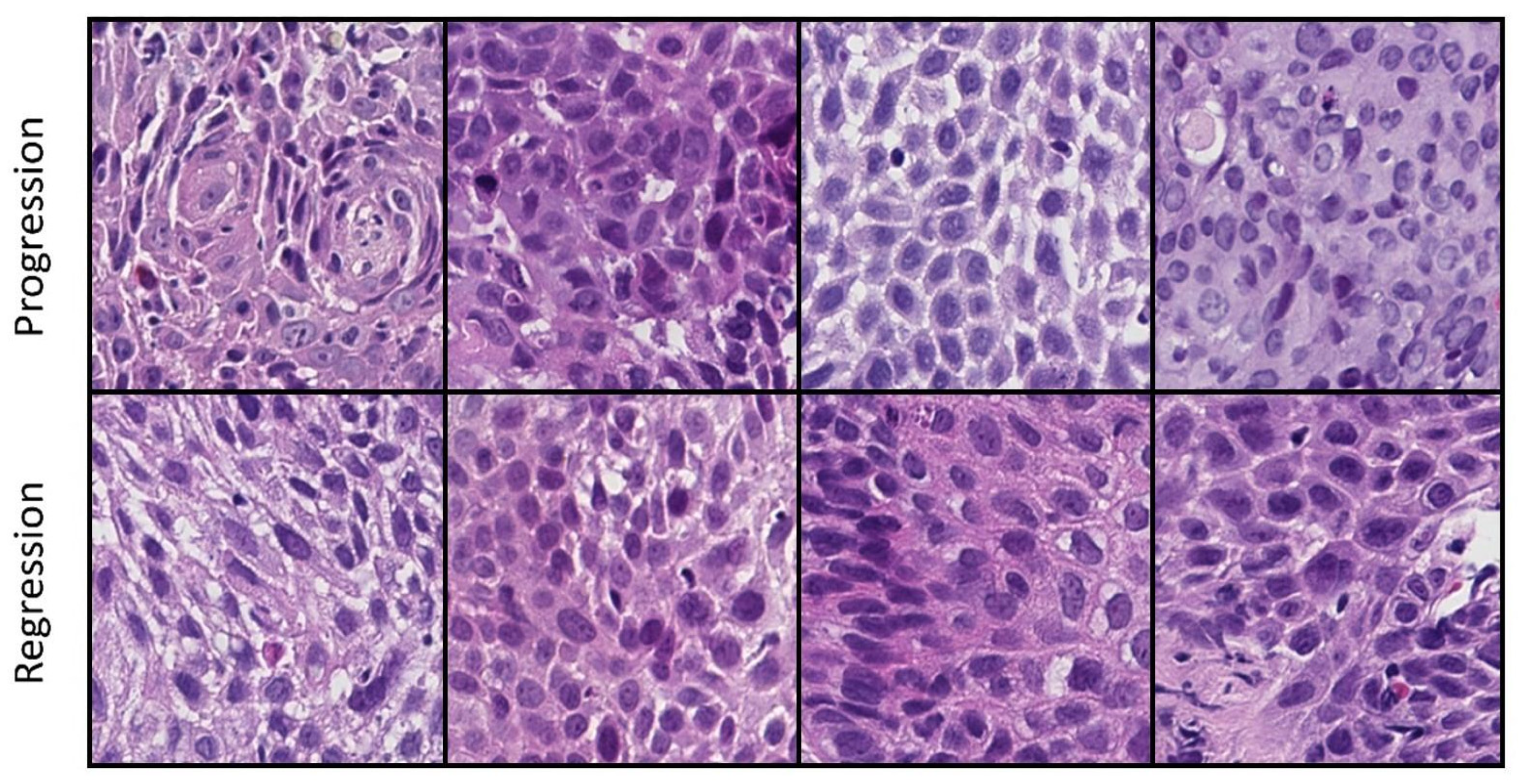
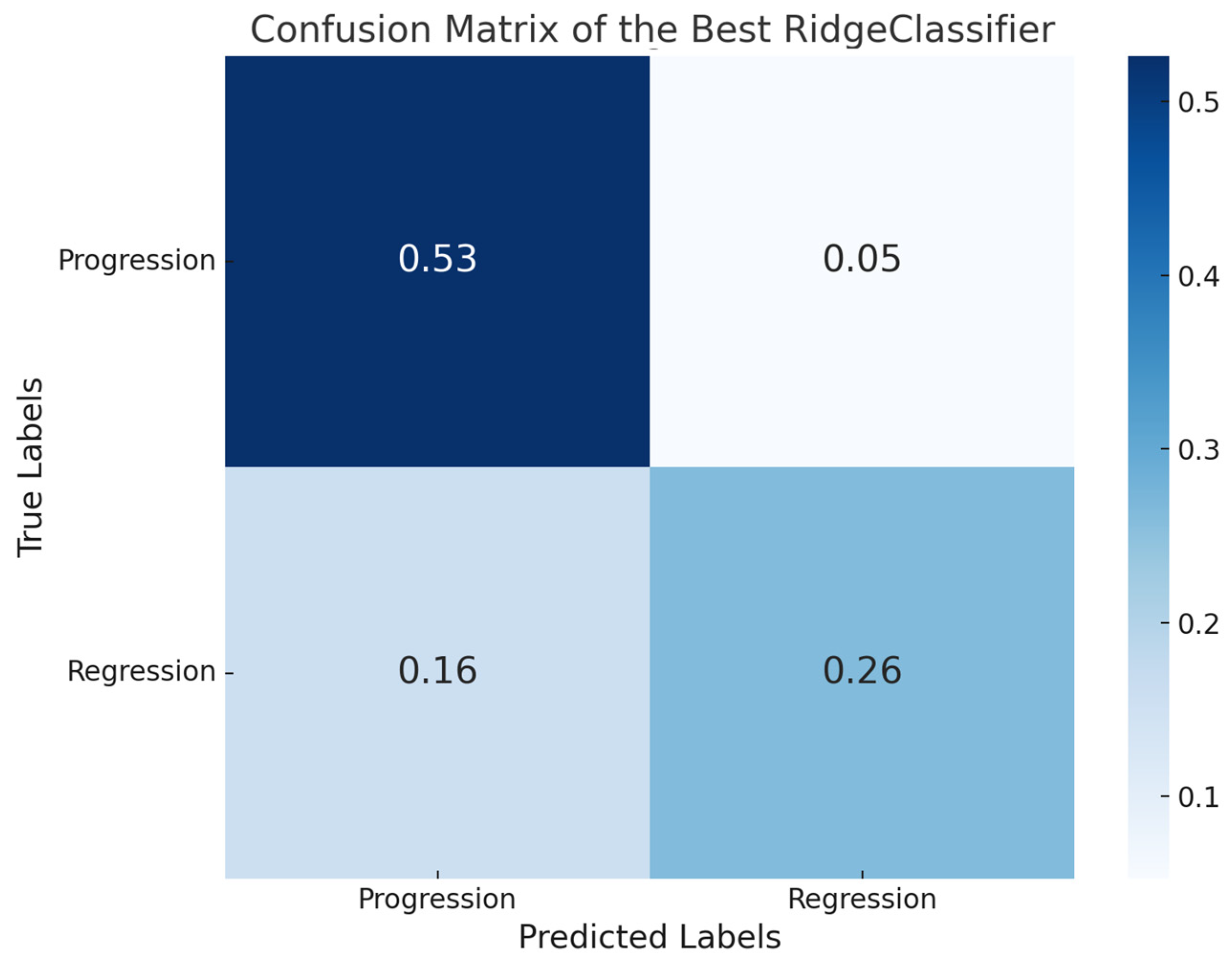
3.1. Pathomics Pipeline
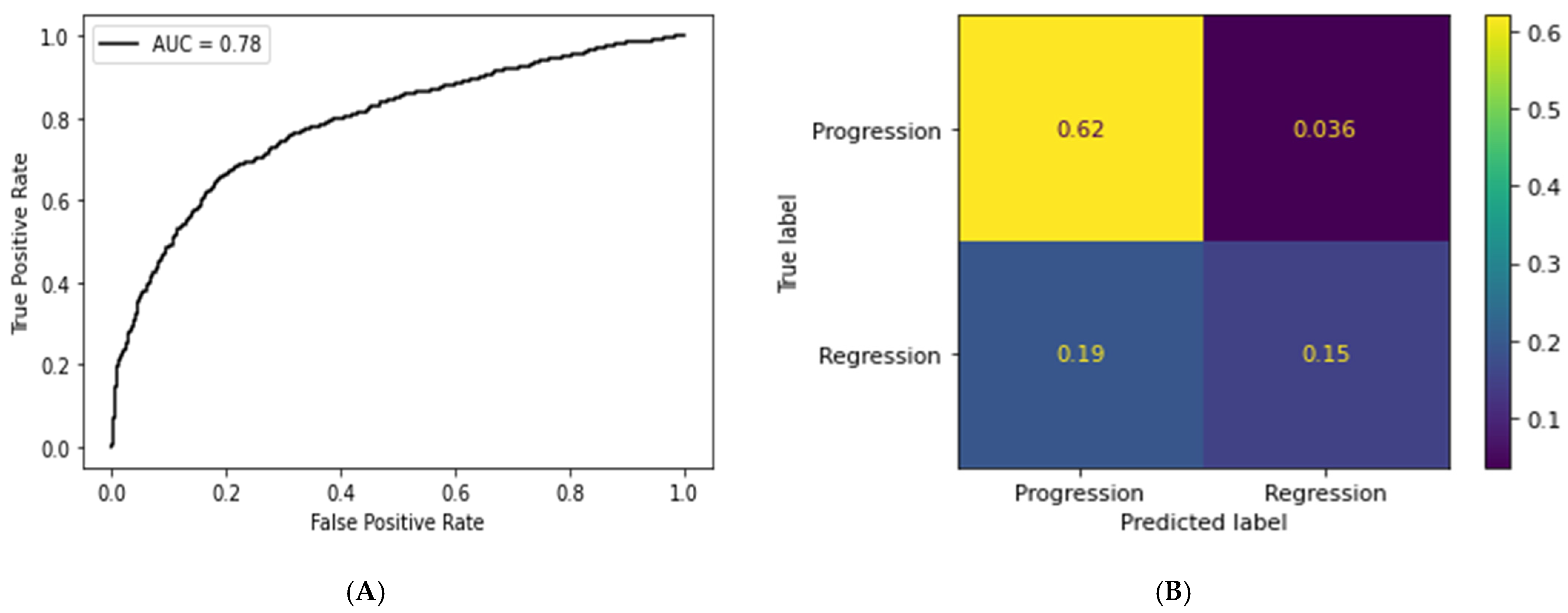
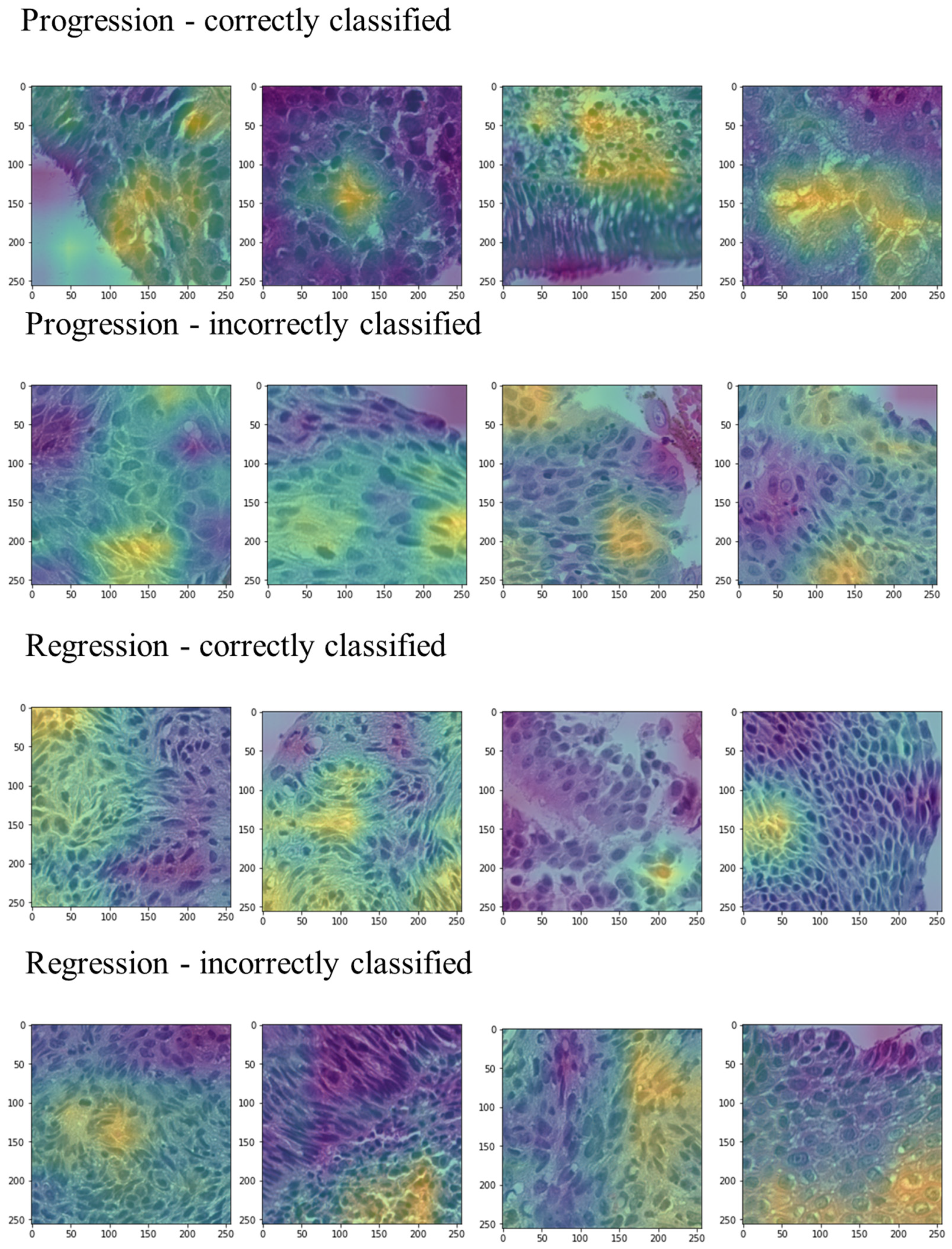
3.2. Deep Learning Pipeline
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Danés, A.; Blanpain, C. Deciphering the Cells of Origin of Squamous Cell Carcinomas. Nat. Rev. Cancer 2018, 18, 549–561. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The Biology and Management of Non-Small Cell Lung Cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Thakrar, R.M.; Pennycuick, A.; Borg, E.; Janes, S.M. Preinvasive Disease of the Airway. Cancer Treat. Rev. 2017, 58, 77–90. [Google Scholar] [CrossRef]
- Pipinikas, C.P.; Kiropoulos, T.S.; Teixeira, V.H.; Brown, J.M.; Varanou, A.; Falzon, M.; Capitanio, A.; Bottoms, S.E.; Carroll, B.; Navani, N.; et al. Cell Migration Leads to Spatially Distinct but Clonally Related Airway Cancer Precursors. Thorax 2014, 69, 548–557. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, H.; Jiang, Y.; Chen, Q.; Su, B.; Zhou, X. Treatment Options and Prognosis of Patients with Lung Squamous Cell Cancer in Situ: A Comparative Study of Lung Adenocarcinoma in Situ and Stage IA Lung Squamous Cell Cancer. Transl. Lung Cancer Res. 2023, 12, 1276–1292. [Google Scholar] [CrossRef] [PubMed]
- Wisnivesky, J.P.; Yung, R.C.-W.; Mathur, P.N.; Zulueta, J.J. Diagnosis and Treatment of Bronchial Intraepithelial Neoplasia and Early Lung Cancer of the Central Airways. Chest 2013, 143, e263S–e277S. [Google Scholar] [CrossRef]
- Bertolaccini, L.; Cara, A.; Chiari, M.; Diotti, C.; Glick, N.; Mohamed, S.; Uslenghi, C.; Mazzella, A.; Brambilla, D.; Bertolotti, R.; et al. Real-World Survival Outcomes of Wedge Resection versus Lobectomy for cT1a/b cN0 cM0 Non-Small Cell Lung Cancer: A Single Center Retrospective Analysis. Front. Oncol. 2023, 13, 1226429. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Xiong, X.; Liu, X.; Lin, B.; Xu, B. Recent Advances of Pathomics in Colorectal Cancer Diagnosis and Prognosis. Front. Oncol. 2023, 13, 1094869. [Google Scholar] [CrossRef]
- Hölscher, D.L.; Bouteldja, N.; Joodaki, M.; Russo, M.L.; Lan, Y.-C.; Sadr, A.V.; Cheng, M.; Tesar, V.; Stillfried, S.V.; Klinkhammer, B.M.; et al. Next-Generation Morphometry for Pathomics-Data Mining in Histopathology. Nat. Commun. 2023, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.H.; Nomikou, S.; Coudray, N.; Jour, G.; Dawood, Z.; Hong, R.; Esteva, E.; Sakellaropoulos, T.; Donnelly, D.; Moran, U.; et al. Deep Learning and Pathomics Analyses Reveal Cell Nuclei as Important Features for Mutation Prediction of BRAF-Mutated Melanomas. J. Investig. Dermatol. 2022, 142, 1650–1658.e6. [Google Scholar] [CrossRef] [PubMed]
- Classe, M.; Lerousseau, M.; Scoazec, J.-Y.; Deutsch, E. Perspectives in Pathomics in Head and Neck Cancer. Curr. Opin. Oncol. 2021, 33, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Peikari, M.; Salama, S.; Nofech-Mozes, S.; Martel, A.L. The Transition Module: A Method for Preventing Overfitting in Convolutional Neural Networks. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2019, 7, 260–265. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Abouzid, M.; Kaczmarek, E. Deep Learning Approaches in Histopathology. Cancers 2022, 14, 5264. [Google Scholar] [CrossRef]
- Alves, N.; Schuurmans, M.; Litjens, G.; Bosma, J.S.; Hermans, J.; Huisman, H. Fully Automatic Deep Learning Framework for Pancreatic Ductal Adenocarcinoma Detection on Computed Tomography. Cancers 2022, 14, 376. [Google Scholar] [CrossRef]
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and Mutation Prediction from Non–Small Cell Lung Cancer Histopathology Images Using Deep Learning. Nat. Med. 2018, 24, 1559–1567. [Google Scholar] [CrossRef]
- Wu, M.; Zhu, C.; Yang, J.; Cheng, S.; Yang, X.; Gu, S.; Xu, S.; Wu, Y.; Shen, W.; Huang, S.; et al. Exploring Prognostic Indicators in the Pathological Images of Ovarian Cancer Based on a Deep Survival Network. Front. Genet. 2022, 13, 1069673. [Google Scholar] [CrossRef]
- Pennycuick, A.; Teixeira, V.H.; AbdulJabbar, K.; Raza, S.E.A.; Lund, T.; Akarca, A.U.; Rosenthal, R.; Kalinke, L.; Chandrasekharan, D.P.; Pipinikas, C.P.; et al. Immune Surveillance in Clinical Regression of Preinvasive Squamous Cell Lung Cancer. Cancer Discov. 2020, 10, 1489–1499. [Google Scholar] [CrossRef]
- Williams, E.; Moore, J.; Li, S.W.; Rustici, G.; Tarkowska, A.; Chessel, A.; Leo, S.; Antal, B.; Ferguson, R.K.; Sarkans, U.; et al. Image Data Resource: A Bioimage Data Integration and Publication Platform. Nat. Methods 2017, 14, 775–781. [Google Scholar] [CrossRef]
- Teixeira, V.H.; Pipinikas, C.P.; Pennycuick, A.; Lee-Six, H.; Chandrasekharan, D.; Beane, J.; Morris, T.J.; Karpathakis, A.; Feber, A.; Breeze, C.E.; et al. Deciphering the Genomic, Epigenomic, and Transcriptomic Landscapes of Pre-Invasive Lung Cancer Lesions. Nat. Med. 2019, 25, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in Speed, Utility and Usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Müller, A.; Nothman, J.; Louppe, G.; et al. Scikit-Learn: Machine Learning in Python. arXiv 2012, arXiv:1201.0490. [Google Scholar] [CrossRef]
- Deng, C.; Han, D.; Feng, M.; Lv, Z.; Li, D. Differential Diagnostic Value of the ResNet50, Random Forest, and DS Ensemble Models for Papillary Thyroid Carcinoma and Other Thyroid Nodules. J. Int. Med. Res. 2022, 50, 3000605221094276. [Google Scholar] [CrossRef] [PubMed]
- Paszke, A.; Gross, S.; Massa, F.; Lerer, A.; Bradbury, J.; Chanan, G.; Killeen, T.; Lin, Z.; Gimelshein, N.; Antiga, L.; et al. PyTorch: An Imperative Style, High-Performance Deep Learning Library. arXiv 2019, arXiv:1912.01703. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Sun, J.; Garfield, D.H.; Lam, B.; Yan, J.; Gu, A.; Shen, J.; Han, B. The Value of Autofluorescence Bronchoscopy Combined with White Light Bronchoscopy Compared with White Light Alone in the Diagnosis of Intraepithelial Neoplasia and Invasive Lung Cancer: A Meta-Analysis. J. Thorac. Oncol. 2011, 6, 1336–1344. [Google Scholar] [CrossRef]
- McWilliams, A.; Shaipanich, T.; Lam, S. Fluorescence and Navigational Bronchoscopy. Thorac. Surg. Clin. 2013, 23, 153–161. [Google Scholar] [CrossRef]
- Lam, S.; Standish, B.; Baldwin, C.; McWilliams, A.; leRiche, J.; Gazdar, A.; Vitkin, A.I.; Yang, V.; Ikeda, N.; MacAulay, C. In Vivo Optical Coherence Tomography Imaging of Preinvasive Bronchial Lesions. Clin. Cancer Res. 2008, 14, 2006–2011. [Google Scholar] [CrossRef]
- Alom, M.Z.; Taha, T.M.; Yakopcic, C.; Westberg, S.; Sidike, P.; Nasrin, M.S.; Hasan, M.; Van Essen, B.C.; Awwal, A.A.S.; Asari, V.K. A State-of-the-Art Survey on Deep Learning Theory and Architectures. Electronics 2019, 8, 292. [Google Scholar] [CrossRef]
| Feature Category | Feature Examples |
|---|---|
| Area and Shape | Mean cytoplasmic area Median nuclear compactness Mean cytoplasmic form factor |
| Perimeter and Boundary | Mean cytoplasmic perimeter area Mean nuclear bounding box area Mean nuclear ferret diameter |
| Texture | Mean cytoplasm eosin contrast Mean nuclear angular second moment Median cytoplasm hematoxylin texture correlation |
| Granularity | Eosin granularity Mean nuclear hematoxylin granularity Median eosin cytoplasm granularity |
| Intensity | Eosin intensity Mean nuclear hematoxylin intensity Median cytoplasm hematoxylin intensity |
| Location | Mean cytoplasm center location Mean hematoxylin center location Median cytoplasm mass center |
| Object Counts | Cytoplasmic object count Nuclear count |
| Accuracy | F1-Score | Sensitivity | Precision | |
|---|---|---|---|---|
| Tile | 0.77 | 0.84 | 0.94 | 0.76 |
| WSI | 0.75 | 0.80 | 0.90 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigdorovits, A.; Olteanu, G.-E.; Tica, O.; Pascalau, A.; Boros, M.; Pop, O. Predicting the Evolution of Lung Squamous Cell Carcinoma In Situ Using Computational Pathology. Bioengineering 2025, 12, 377. https://doi.org/10.3390/bioengineering12040377
Vigdorovits A, Olteanu G-E, Tica O, Pascalau A, Boros M, Pop O. Predicting the Evolution of Lung Squamous Cell Carcinoma In Situ Using Computational Pathology. Bioengineering. 2025; 12(4):377. https://doi.org/10.3390/bioengineering12040377
Chicago/Turabian StyleVigdorovits, Alon, Gheorghe-Emilian Olteanu, Ovidiu Tica, Andrei Pascalau, Monica Boros, and Ovidiu Pop. 2025. "Predicting the Evolution of Lung Squamous Cell Carcinoma In Situ Using Computational Pathology" Bioengineering 12, no. 4: 377. https://doi.org/10.3390/bioengineering12040377
APA StyleVigdorovits, A., Olteanu, G.-E., Tica, O., Pascalau, A., Boros, M., & Pop, O. (2025). Predicting the Evolution of Lung Squamous Cell Carcinoma In Situ Using Computational Pathology. Bioengineering, 12(4), 377. https://doi.org/10.3390/bioengineering12040377










