Proton Density Fat Fraction Micro-MRI for Non-Invasive Quantification of Bone Marrow Aging and Radiation Effects in Mice
Abstract
:1. Introduction
2. Materials and Methods
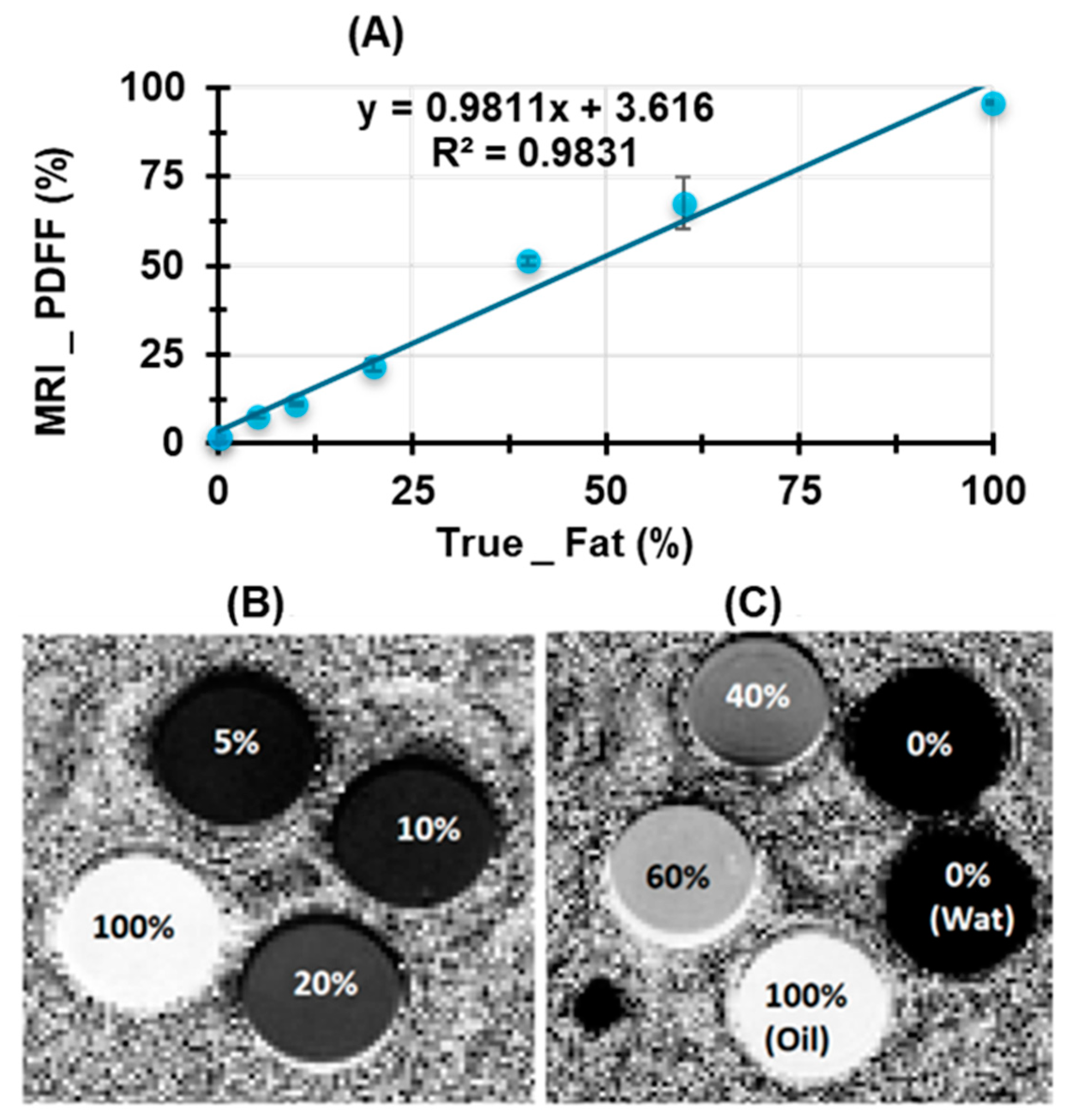
2.1. Phantom Preparation
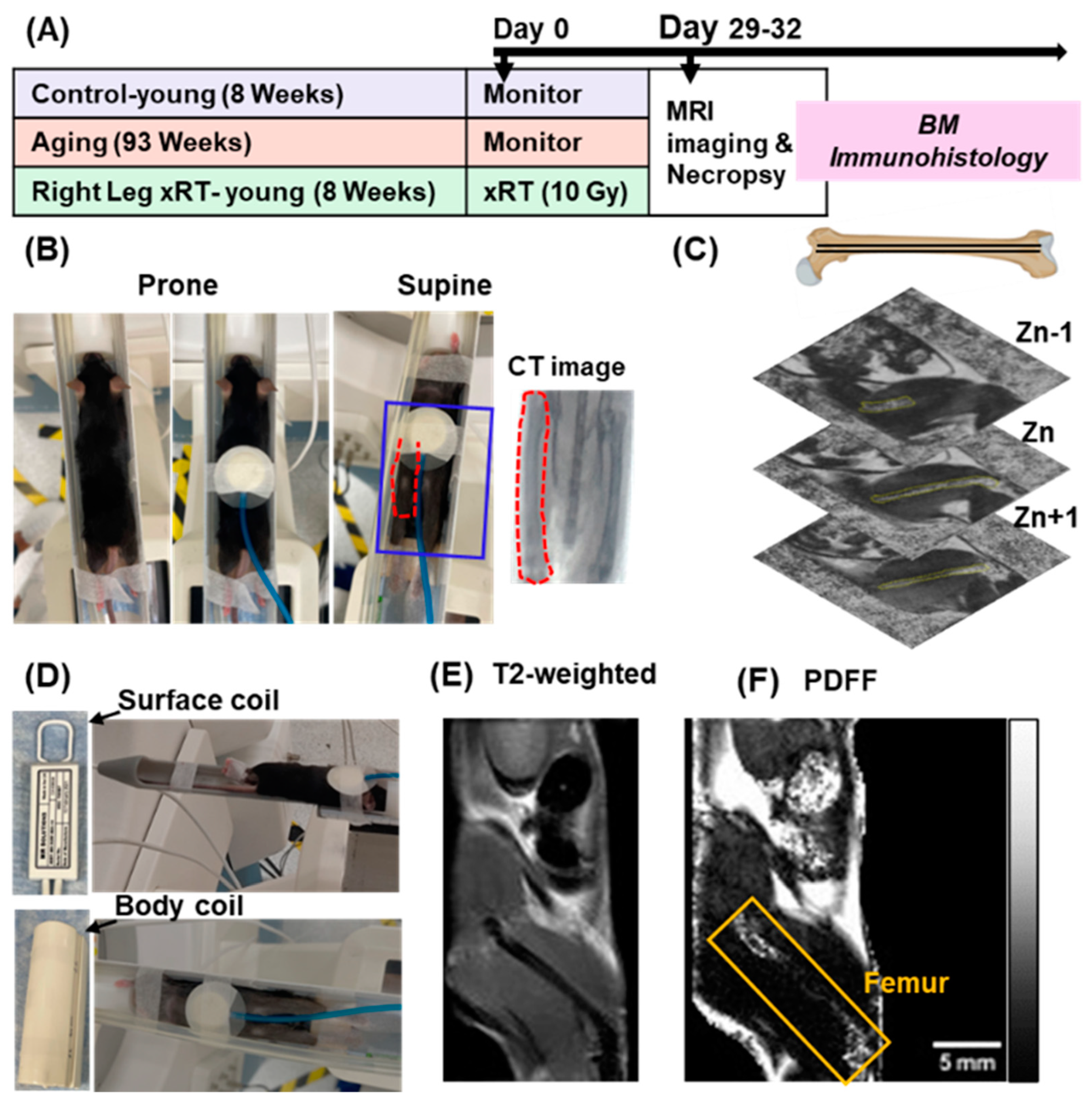
2.2. Mouse Models of Aging and X-Ray Irradiation
2.3. Mouse Preparation and Monitoring for MRI Scanning
2.4. Image Acquisition and Analysis
2.5. Tissue Preparation
2.6. Hematoxylin and Eosin (H&E) Staining and Histological Analysis
2.7. Statistical Analysis
3. Results
3.1. Experimental Design and Positioning
3.2. Optimization of MRI Parameters
3.3. Validation of PDFF Measurements Using Water–Fat Phantom Models
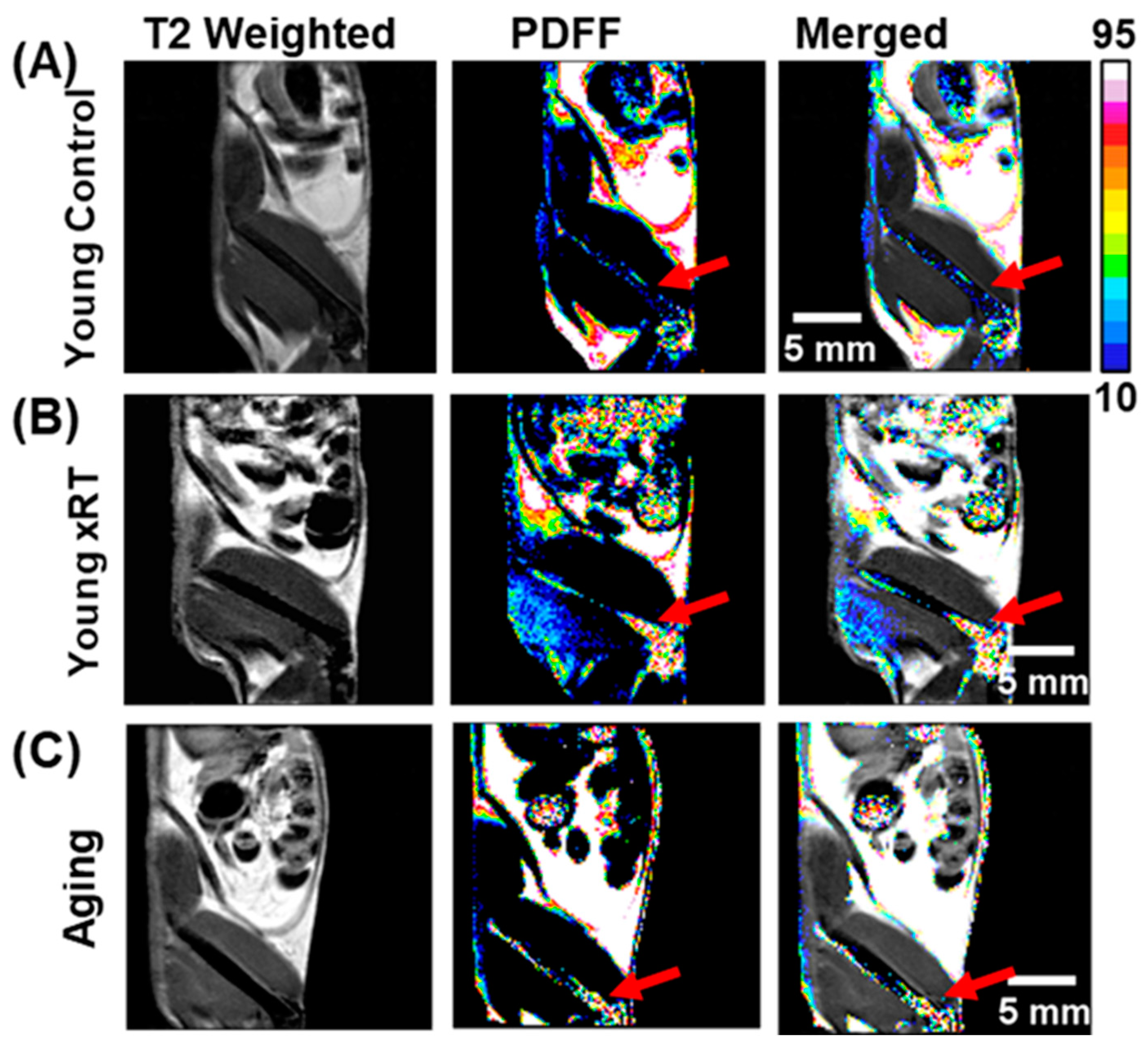
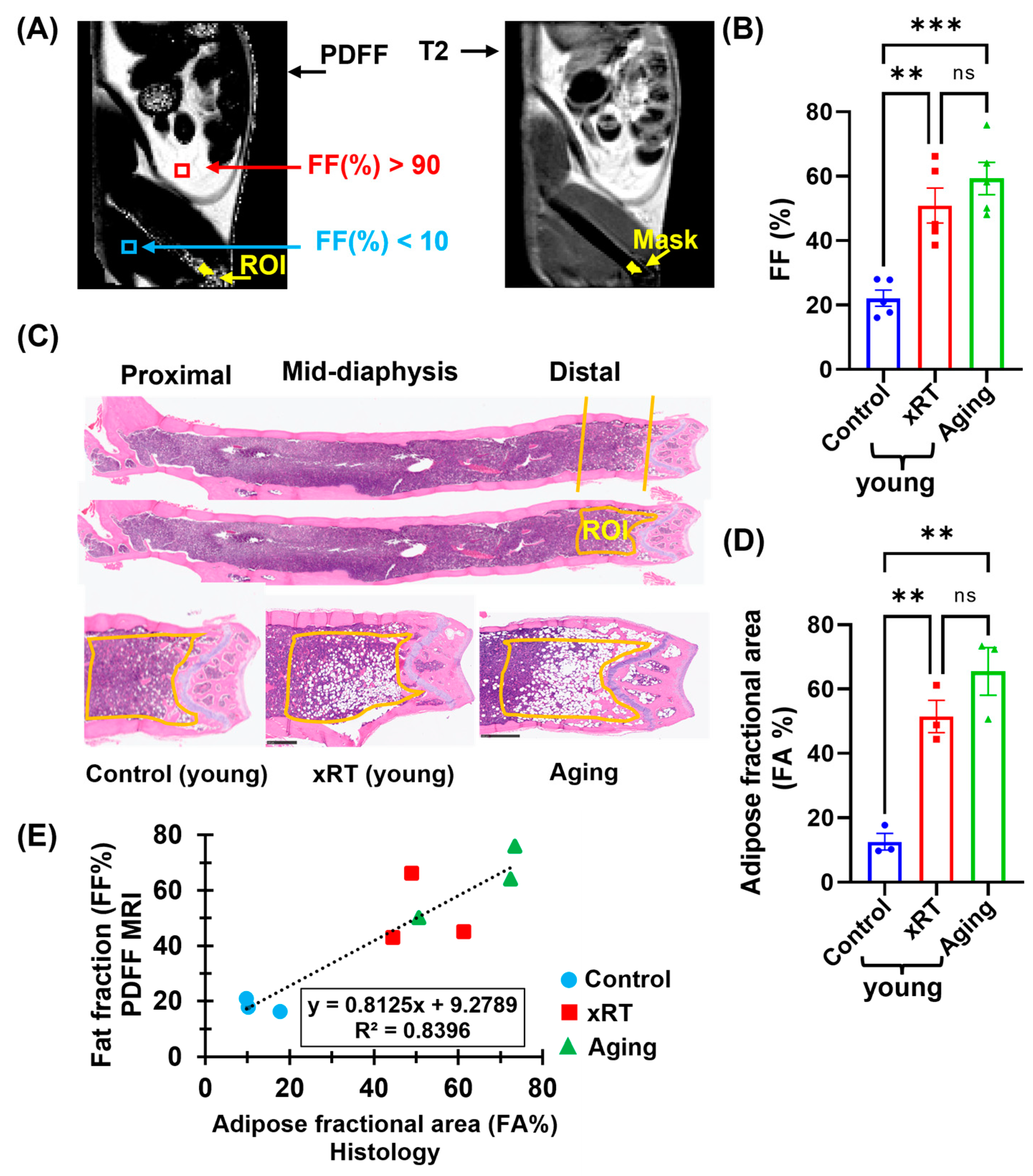
3.4. Effects of Irradiation and Aging on Bone Marrow Fat Fraction
3.5. Correlation of Histological and MRI-Based Bone Marrow Fat Fraction Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salamanna, F.; Contartese, D.; Errani, C.; Sartori, M.; Borsari, V.; Giavaresi, G. Role of bone marrow adipocytes in bone metastasis development and progression: A systematic review. Front. Endocrinol. 2023, 14, 1207416. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Koo, J.S. The role of adipokines and bone marrow adipocytes in breast cancer bone metastasis. Int. J. Mol. Sci. 2020, 21, 4967. [Google Scholar] [CrossRef]
- Hardaway, A.L.; Herroon, M.K.; Rajagurubandara, E.; Podgorski, I. Bone marrow fat: Linking adipocyte-induced inflammation with skeletal metastases. Cancer Metastasis Rev. 2014, 33, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Orellana, M.; Brooks, J.; Madabushi, S.S.; Vishwasrao, P.; Parra, L.E.; Sanchez, J.; Salhotra, A.; Stein, A.; Chen, C.-C.; et al. Exosome-driven lipolysis and bone marrow niche remodeling support leukemia expansion. Haematologica 2021, 106, 1484. [Google Scholar] [CrossRef]
- Shafat, M.S.; Oellerich, T.; Mohr, S.; Robinson, S.D.; Edwards, D.R.; Marlein, C.R.; Piddock, R.E.; Fenech, M.; Zaitseva, L.; Abdul-Aziz, A.J.B. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood J. Am. Soc. Hematol. 2017, 129, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Paccou, J.; Hardouin, P.; Cotten, A.; Penel, G.; Cortet, B. The role of bone marrow fat in skeletal health: Usefulness and perspectives for clinicians. J. Clin. Endocrinol. Metab. 2015, 100, 3613–3621. [Google Scholar] [CrossRef]
- Kawai, M.; de Paula, F.J.; Rosen, C.J. New insights into osteoporosis: The bone–fat connection. J. Intern. Med. 2012, 272, 317–329. [Google Scholar] [CrossRef]
- Papadakis, M.A.; McPhee, S.J.; Rabow, M.C. Medical Diagnosis & Treatment; Mc Graw Hill: San Francisco, CA, USA, 2019. [Google Scholar]
- Singhal, V.; Bredella, M.A. Marrow adipose tissue imaging in humans. Bone 2019, 118, 69–76. [Google Scholar] [CrossRef]
- Shen, W.; Chen, J.; Punyanitya, M.; Shapses, S.; Heshka, S.; Heymsfield, S.B. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos. Int. 2007, 18, 641–647. [Google Scholar] [CrossRef]
- Hui, S.K.; Arentsen, L.; Sueblinvong, T.; Brown, K.; Bolan, P.; Ghebre, R.G.; Downs, L.; Shanley, R.; Hansen, K.E.; Minenko, A.G.; et al. A phase I feasibility study of multi-modality imaging assessing rapid expansion of marrow fat and decreased bone mineral density in cancer patients. Bone 2015, 73, 90–97. [Google Scholar] [CrossRef]
- Arentsen, L.; Hansen, K.E.; Yagi, M.; Takahashi, Y.; Shanley, R.; McArthur, A.; Bolan, P.; Magome, T.; Yee, D.; Froelich, J.; et al. Use of dual-energy computed tomography to measure skeletal-wide marrow composition and cancellous bone mineral density. J. Bone Miner. Metab. 2017, 35, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Arentsen, L.; Yagi, M.; Takahashi, Y.; Bolan, P.J.; White, M.; Yee, D.; Hui, S. Validation of marrow fat assessment using noninvasive imaging with histologic examination of human bone samples. Bone 2015, 72, 118–122. [Google Scholar] [PubMed]
- Schmeel, F.C.; Luetkens, J.A.; Wagenhäuser, P.J.; Meier-Schroers, M.; Kuetting, D.L.; Feißt, A.; Gieseke, J.; Schmeel, L.C.; Träber, F.; Schild, H.H.; et al. Proton density fat fraction (PDFF) MRI for differentiation of benign and malignant vertebral lesions. Eur. Radiol. 2018, 28, 2397–2405. [Google Scholar]
- Karampinos, D.C.; Ruschke, S.; Dieckmeyer, M.; Diefenbach, M.; Franz, D.; Gersing, A.S.; Krug, R.; Baum, T. Quantitative MRI and spectroscopy of bone marrow. J. Magn. Reson. Imaging 2018, 47, 332–353. [Google Scholar] [PubMed]
- Reeder, S.B.; Hu, H.H.; Sirlin, C.B. Proton density fat-fraction: A standardized MR-based biomarker of tissue fat concentration. J. Magn. Reson. Imaging JMRI 2012, 36, 1011. [Google Scholar]
- Li, Z.; MacDougald, O.A. Preclinical models for investigating how bone marrow adipocytes influence bone and hematopoietic cellularity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101547. [Google Scholar]
- Matsuura, S.; Patterson, S.; Lucero, H.; Leiva, O.; Grant, A.; Herrera, V.; Ravid, K. In vivo magnetic resonance imaging of a mouse model of myelofibrosis. Blood Cancer J. 2016, 6, e497. [Google Scholar]
- Gomes, A.L.; Gribben, J.; Siow, B.; Passaro, D.; Bonnet, D. Dynamic contrast-enhanced magnetic resonance imaging quantification of leukemia-induced changes in bone marrow vascular function. Haematologica 2021, 106, 2281. [Google Scholar]
- Robison, T.H.; Solipuram, M.; Heist, K.; Amouzandeh, G.; Lee, W.Y.; Humphries, B.A.; Buschhaus, J.M.; Bevoor, A.; Zhang, A.; Luker, K.E.; et al. Multiparametric MRI to quantify disease and treatment response in mice with myeloproliferative neoplasms. JCI Insight 2022, 7, e161457. [Google Scholar]
- Robison, T.H.; Solipuram, M.; Lee, W.Y.; Luker, K.; Pettit, K.M.; Talpaz, M.; Malyarenko, D.; Chenevert, T.; Ross, B.; Luker, G. Quantitative MRI Identifies Heterogeneous Bone Marrow Treatment Responses in a Mouse Model of Myelofibrosis. Blood 2022, 140 (Suppl. S1), 3860–3861. [Google Scholar]
- Justesen, J.; Stenderup, K.; Ebbesen, E.; Mosekilde, L.; Steiniche, T.; Kassem, M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001, 2, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hines, C.D.; Agni, R.; Roen, C.; Rowland, I.; Hernando, D.; Bultman, E.; Horng, D.; Yu, H.; Shimakawa, A.; Brittain, J.H.; et al. Validation of MRI biomarkers of hepatic steatosis in the presence of iron overload in the ob/ob mouse. J. Magn. Reson. Imaging 2012, 35, 844–851. [Google Scholar] [PubMed]
- Bray, T.J.; Chouhan, M.D.; Punwani, S.; Bainbridge, A.; Hall-Craggs, M.A. Fat fraction mapping using magnetic resonance imaging: Insight into pathophysiology. Br. J. Radiol. 2017, 91, 20170344. [Google Scholar]
- Yu, H.; Shimakawa, A.; McKenzie, C.A.; Brodsky, E.; Brittain, J.H.; Reeder, S.B. Multiecho water-fat separation and simultaneous R estimation with multifrequency fat spectrum modeling. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2008, 60, 1122–1134. [Google Scholar]
- Gee, C.S.; Nguyen, J.T.; Marquez, C.J.; Heunis, J.; Lai, A.; Wyatt, C.; Han, M.; Kazakia, G.; Burghardt, A.J.; Karampinos, D.C.; et al. Validation of bone marrow fat quantification in the presence of trabecular bone using MRI. J. Magn. Reson. Imaging 2015, 42, 539–544. [Google Scholar]
- Mobini, N.; Malekzadeh, M.; Haghighatkhah, H.; Saligheh Rad, H. A hybrid (iron–fat–water) phantom for liver iron overload quantification in the presence of contaminating fat using magnetic resonance imaging. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 385–392. [Google Scholar]
- Cui, C.; Wu, X.; Newell, J.D.; Jacob, M. Fat water decomposition using globally optimal surface estimation (GOOSE) algorithm. Magn. Reson. Med. 2015, 73, 1289–1299. [Google Scholar] [PubMed]
- Chenevert, T.; Malyarenko, D. Proton Density Fat Fraction (PDFF) Measurement of Myelofibrosis in Mouse Tibia. Protocol, University of Michigan. 2023. Available online: https://deepblue.lib.umich.edu/bitstream/handle/2027.42/175838/UMich_PDFF_SOP-Profile_20230214.pdf?sequence=2&isAllowed=y (accessed on 24 March 2025).
- Bolan, P.J.; Arentsen, L.; Sueblinvong, T.; Zhang, Y.; Moeller, S.; Carter, J.S.; Downs, L.S.; Ghebre, R.; Yee, D.; Froelich, J.; et al. Water–fat MRI for assessing changes in bone marrow composition due to radiation and chemotherapy in gynecologic cancer patients. J. Magn. Reson. Imaging 2013, 38, 1578–1584. [Google Scholar]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114. [Google Scholar]
- Scheller, E.L.; Doucette, C.R.; Learman, B.S.; Cawthorn, W.P.; Khandaker, S.; Schell, B.; Wu, B.; Ding, S.Y.; Bredella, M.A.; Fazeli, P.K.; et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat. Commun. 2015, 6, 7808. [Google Scholar]
- Wang, L.; Zhang, H.; Wang, S.; Chen, X.; Su, J. Bone marrow adipocytes: A critical player in the bone marrow microenvironment. Front. Cell Dev. Biol. 2021, 9, 770705. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.K.; Kapatoes, J.; Fowler, J.; Henderson, D.; Olivera, G.; Manon, R.R.; Gerbi, B.; Mackie, T.R.; Welsh, J.S. Feasibility study of helical tomotherapy for total body or total marrow irradiation a. Med. Phys. 2005, 32, 3214–3224. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Filippi, A.R.; Scorsetti, M.; Hui, S.; Muren, L.P.; Mancosu, P. Total marrow and total lymphoid irradiation in bone marrow transplantation for acute leukaemia. Lancet Oncol. 2020, 21, e477–e487. [Google Scholar] [CrossRef]
- Zuro, D.; Madabushi, S.S.; Brooks, J.; Chen, B.T.; Goud, J.; Salhotra, A.; Song, J.Y.; Parra, L.E.; Pierini, A.; Sanchez, J.F.; et al. First multimodal, three-dimensional, image-guided total marrow irradiation model for preclinical bone marrow transplantation studies. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.K.; Sharkey, L.; Kidder, L.S.; Zhang, Y.; Fairchild, G.; Coghill, K.; Xian, C.J.; Yee, D. The influence of therapeutic radiation on the patterns of bone marrow in ovary-intact and ovariectomized mice. PLoS ONE 2012, 7, e42668. [Google Scholar] [CrossRef]
- Carmona, R.; Pritz, J.; Bydder, M.; Gulaya, S.; Zhu, H.; Williamson, C.W.; Welch, C.S.; Vaida, F.; Bydder, G.; Mell, L.K. Fat composition changes in bone marrow during chemotherapy and radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 155–163. [Google Scholar]
- Ghimire, H.; Madabushi, S.S.; Vercellino, J.; Brooks, J.; Zuro, D.; Lim, J.E.; Vishwasrao, P.; Abdelhamid, A.M.H.; Strome, G.; Eichenbaum, G.; et al. Thrombopoietin mimetic therapy alleviates radiation-induced bone marrow vascular injury in a bone marrow transplant mouse model. Front. Oncol. 2024, 14, 1414488. [Google Scholar]
| Clinical Area | Potential Implication of PDFF-MRI |
|---|---|
| SCD and Marrow Failure Syndromes | To characterize BM microenvironment remodeling in SCD and marrow failure syndromes, supporting individualized treatment strategies and improving BM response monitoring to allogeneic BMT [33]. |
| Hematological and Skeletal Metastases | To enable non-invasive monitoring of BM metabolic remodeling in leukemia and bone metastases, providing insights into disease progression and therapeutic response [4]. |
| Pelvic Radiation and Chemotherapy for Gynecological Cancers | To evaluate BM damage from pelvic irradiation and chemotherapy, facilitating the development of BM-sparing radiation strategies to reduce hematologic toxicity [11,30]. |
| Metabolic Disorders | To investigate BM fat as a potential biomarker for obesity and diabetes and its influence on hematopoiesis and immune function, potentially leading to novel therapeutic interventions [4]. |
| BMT Conditioning Regimens | To assess the impact of advanced BM transplant conditioning strategies, such as TMI and TMLI, on local marrow damage, marrow regeneration, and graft failure, aiding in transplant optimization [34,35,36]. |
| Osteoporosis and Aging | To quantify BM fat fraction to assess its relationship with bone density and fracture risk, supporting early diagnosis and intervention strategies [11,12,13]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, H.; Malekzadeh, M.; Lim, J.E.; Madabushi, S.S.; Zampini, M.A.; Camacho, A.; Hu, W.; Baran, N.; Storme, G.; Al Malki, M.M.; et al. Proton Density Fat Fraction Micro-MRI for Non-Invasive Quantification of Bone Marrow Aging and Radiation Effects in Mice. Bioengineering 2025, 12, 349. https://doi.org/10.3390/bioengineering12040349
Ghimire H, Malekzadeh M, Lim JE, Madabushi SS, Zampini MA, Camacho A, Hu W, Baran N, Storme G, Al Malki MM, et al. Proton Density Fat Fraction Micro-MRI for Non-Invasive Quantification of Bone Marrow Aging and Radiation Effects in Mice. Bioengineering. 2025; 12(4):349. https://doi.org/10.3390/bioengineering12040349
Chicago/Turabian StyleGhimire, Hemendra, Malakeh Malekzadeh, Ji Eun Lim, Srideshikan Sargur Madabushi, Marco Andrea Zampini, Angela Camacho, Weidong Hu, Natalia Baran, Guy Storme, Monzr M. Al Malki, and et al. 2025. "Proton Density Fat Fraction Micro-MRI for Non-Invasive Quantification of Bone Marrow Aging and Radiation Effects in Mice" Bioengineering 12, no. 4: 349. https://doi.org/10.3390/bioengineering12040349
APA StyleGhimire, H., Malekzadeh, M., Lim, J. E., Madabushi, S. S., Zampini, M. A., Camacho, A., Hu, W., Baran, N., Storme, G., Al Malki, M. M., & Hui, S. K. (2025). Proton Density Fat Fraction Micro-MRI for Non-Invasive Quantification of Bone Marrow Aging and Radiation Effects in Mice. Bioengineering, 12(4), 349. https://doi.org/10.3390/bioengineering12040349






