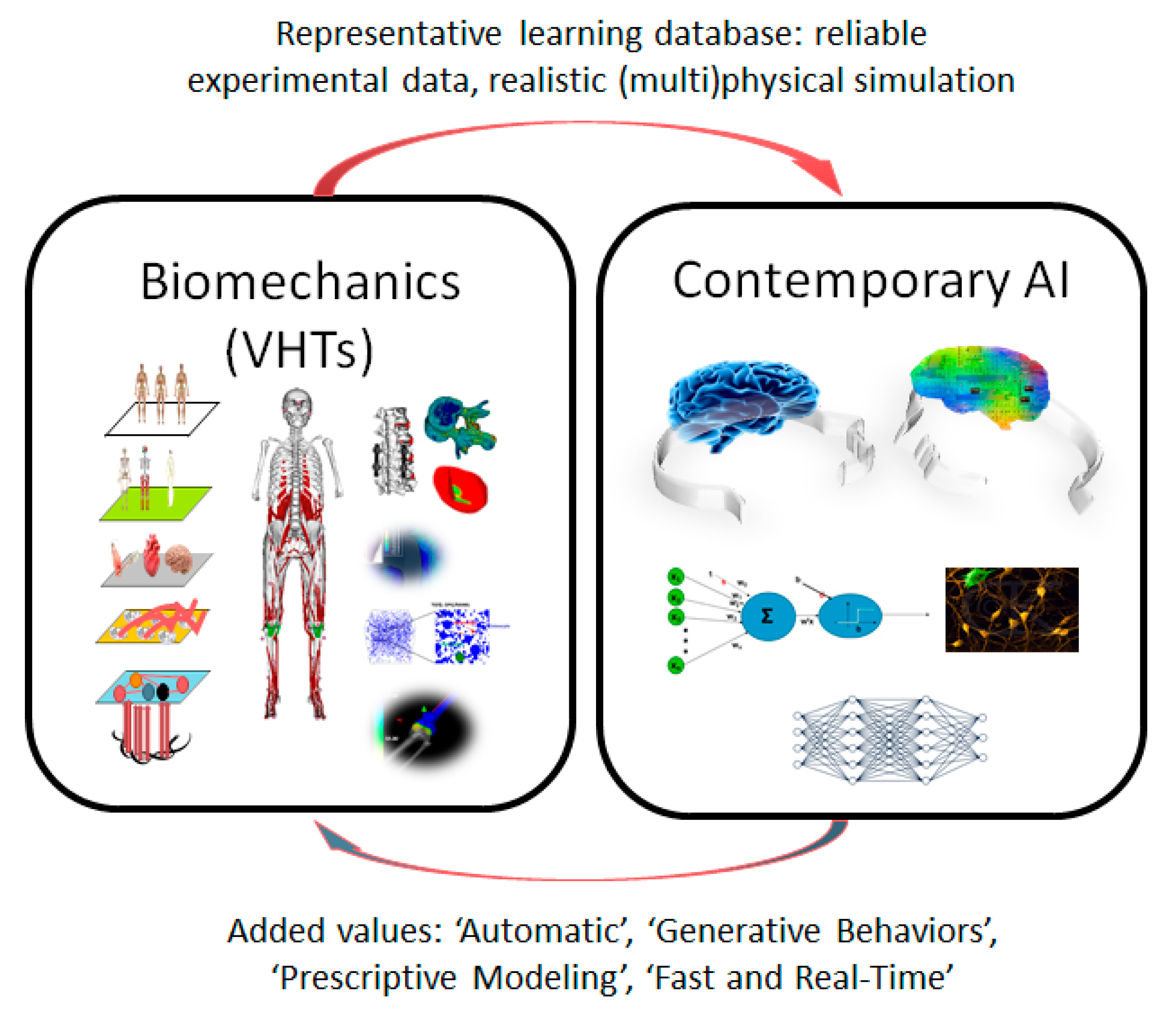
Multiscale Modeling in Computational Biomechanics: A New Era with Virtual Human Twins and Contemporary Artificial Intelligence
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Contributions
- Kang, Y.; Kim, J.; Sim, J.A.; Moon, M.; Park, J.-C.; Cho, S.H.; Lee, B.H. Stress Effect in the Knee Joint Based on the Fibular Osteotomy Level and Varus Deformity: A Finite Element Analysis Study. Bioengineering 2023, 10, 1003. https://doi.org/10.3390/bioengineering10091003.
- Mehr, J.A.; Hatami-Marbini, H. Finite Deformation of Scleral Tissue under Electrical Stimulation: An Arbitrary Lagrangian-Eulerian Finite Element Method. Bioengineering 2023, 10, 920. https://doi.org/10.3390/bioengineering10080920.
- Wang, Z.; Ge, W.; Zhang, Y.; Liu, B.; Liu, B.; Jin, S.; Li, Y. Optimization Design and Performance Analysis of a Bionic Knee Joint Based on the Geared Five-Bar Mechanism. Bioengineering 2023, 10, 582. https://doi.org/10.3390/bioengineering10050582.
- Song, Y.; Cen, X.; Zhang, Y.; Bíró, I.; Ji, Y.; Gu, Y. Development and Validation of a Subject-Specific Coupled Model for Foot and Sports Shoe Complex: A Pilot Computational Study. Bioengineering 2022, 9, 553. https://doi.org/10.3390/bioengineering9100553.
- Zhu, C.; Song, Y.; Xu, Y.; Zhu, A.; Baker, J.S.; Liu, W.; Gu, Y. Toe Box Shape of Running Shoes Affects In-Shoe Foot Displacement and Deformation: A Randomized Crossover Study. Bioengineering 2024, 11, 57. https://doi.org/10.3390/bioengineering11050457.
- Nguyen, D.-P.; Nguyen, T.-N.; Dakpé, S.; Ho Ba Tho, M.-C.; Dao, T.-T. Fast 3D Face Reconstruction from a Single Image Using Different Deep Learning Approaches for Facial Palsy Patients. Bioengineering 2022, 9, 619. https://doi.org/10.3390/bioengineering9110619.
- Tran, V.-D.; Nguyen, T.-N.; Ballit, A.; Dao, T.-T. Novel Baseline Facial Muscle Database Using Statistical Shape Modeling and In Silico Trials toward Decision Support for Facial Rehabilitation. Bioengineering 2023, 10, 737. https://doi.org/10.3390/bioengineering10060737.
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Curiel-Regueros, A.; Rubio-Zarapuz, A.; Tornero-Aguilera, J.F. Neuro-Nutrition and Exercise Synergy: Exploring the Bioengineering of Cognitive Enhancement and Mental Health Optimization. Bioengineering 2025, 12, 208. https://doi.org/10.3390/bioengineering12020208.
References
- Viceconti, M.; De Vos, M.; Mellone, S.; Geris, L. Position paper from the digital twins in healthcare to the Virtual Human Twin: A moon-shot project for digital health research. IEEE J. Biomed. Health Inform. 2023, 28, 491–501. [Google Scholar] [CrossRef]
- Nguyen, D.P.; Tho, M.C.H.B.; Dao, T.T. Reinforcement Learning coupled with Finite Element Modeling for Facial Motion Learning. Comput. Methods Programs Biomed. 2022, 221, 106904. [Google Scholar]
- Nowakowski, K.; El Kirat, K.; Dao, T.T. Deep reinforcement learning coupled with musculoskeletal modeling for a better understanding of elderly falls. Med. Biol. Eng. Comput. 2022, 60, 1745–1761. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dao, T.-T. Multiscale Modeling in Computational Biomechanics: A New Era with Virtual Human Twins and Contemporary Artificial Intelligence. Bioengineering 2025, 12, 320. https://doi.org/10.3390/bioengineering12030320
Dao T-T. Multiscale Modeling in Computational Biomechanics: A New Era with Virtual Human Twins and Contemporary Artificial Intelligence. Bioengineering. 2025; 12(3):320. https://doi.org/10.3390/bioengineering12030320
Chicago/Turabian StyleDao, Tien-Tuan. 2025. "Multiscale Modeling in Computational Biomechanics: A New Era with Virtual Human Twins and Contemporary Artificial Intelligence" Bioengineering 12, no. 3: 320. https://doi.org/10.3390/bioengineering12030320
APA StyleDao, T.-T. (2025). Multiscale Modeling in Computational Biomechanics: A New Era with Virtual Human Twins and Contemporary Artificial Intelligence. Bioengineering, 12(3), 320. https://doi.org/10.3390/bioengineering12030320




