Introduction of a Semi-Quantitative Image-Based Analysis Tool for CBCT-Based Evaluation of Bone Regeneration in Tooth Extraction Sockets
Abstract
1. Introduction
2. Materials and Methods
2.1. Software and Data Format
2.2. Clinical Trial Study Design
2.2.1. Study Design
2.2.2. Inclusion and Exclusion Criteria
2.2.3. Sample Size Calculation
2.2.4. Randomization
2.2.5. Follow-Up and Outcome Measures
2.3. PRF Preparation
2.4. Surgical Procedures
2.4.1. Tooth Extraction and Socket Preservation
2.4.2. Follow-Up and Radiological Evaluation
3. Results
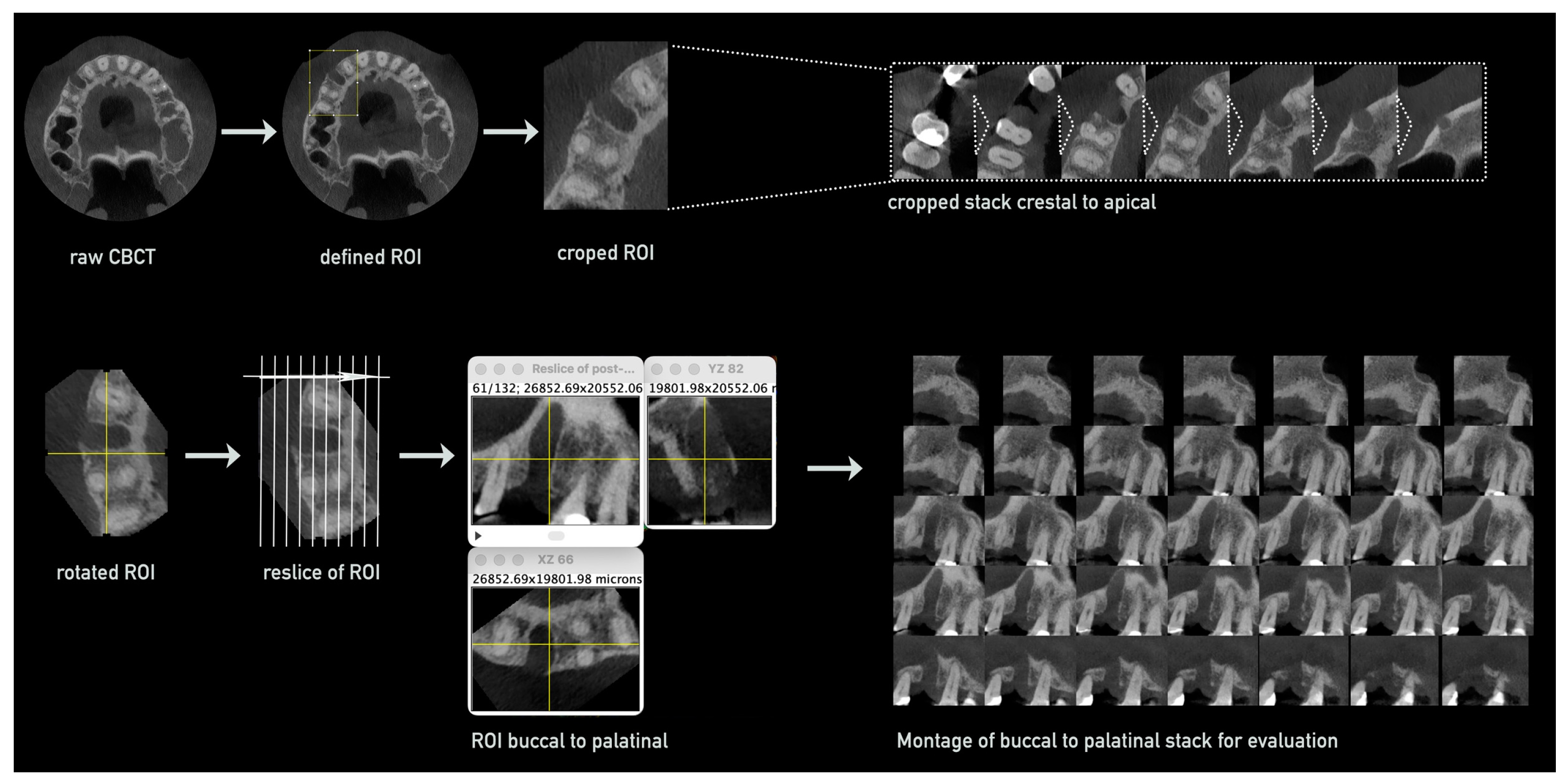
3.1. Method Development
- (i)
- Pre-processing of CBCT images;
- (ii)
- Defining region of interest and measurement;
- (iii)
- Parameter evaluation based on achieved raw data.
- (i)
- Preparation and Processing of CBCTs for Measurements
- (ii)
- Defining Region of Interest and Measurement within Montages
- (iii)
- Parameter Evaluation Based on Achieved Raw Data
3.2. Radiological Bone Density Evaluation
3.3. Application of the Novel Method in the Clinical Trial
3.3.1. Quality Assessment of Datasets for Evaluation
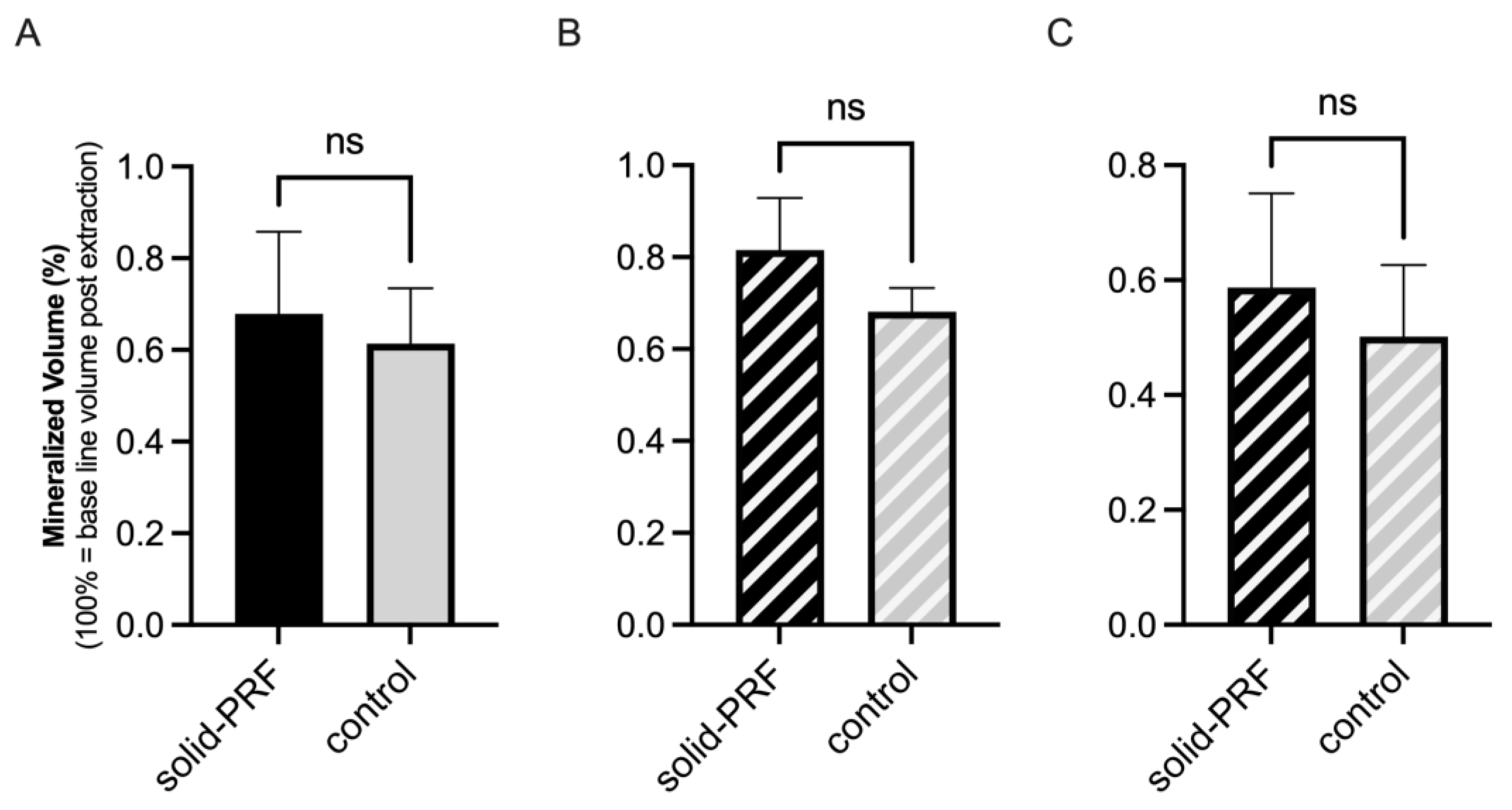
3.3.2. Evaluation of Mineralized Bone Volume
Mineralized Bone Volume in Molars
Mineralized Bone Volume in Premolars
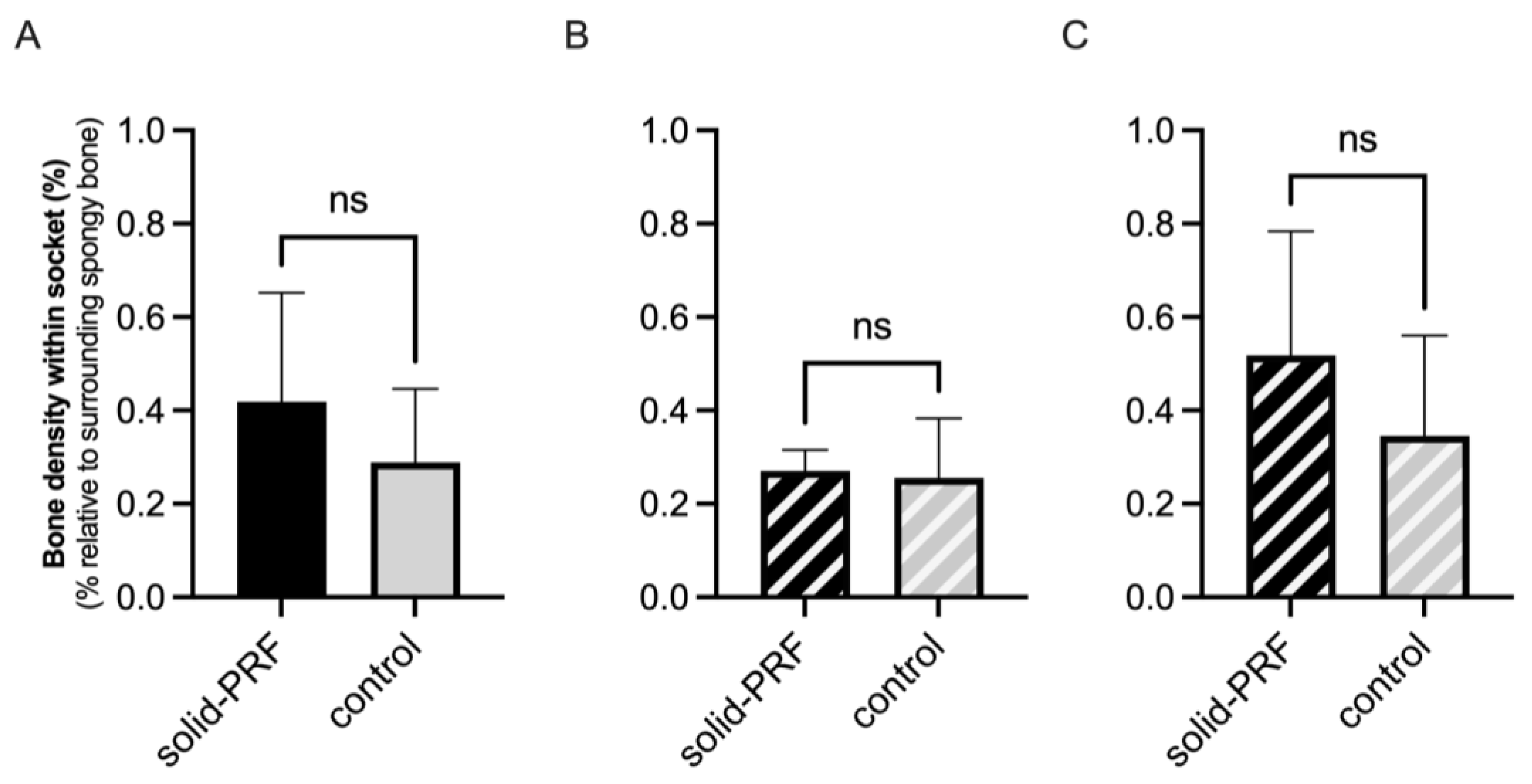
3.3.3. Evaluation of Radiological Bone Density Within Former Extraction Sockets
Radiological Bone Density in Molars
Radiological Bone Density in Premolars
4. Discussion
4.1. Development of a Novel Image Analysis-Based Evaluation Method
4.2. Efficacy of Solid PRF for Bone Regeneration—Mineralized Tissue Volume
4.3. Efficacy of Solid PRF for Bone Regeneration—Radiological Bone Density
4.4. Strength and Limitation of Radiological Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRF | Platelet-rich fibrin |
| CBCT | Cone beam computer tomography |
References
- Amler, M.H. The time sequence of tissue regeneration in human extraction wounds. Oral Surg. Oral Med. Oral Pathol. 1969, 27, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Udeabor, S.E.; Heselich, A.; Al-Maawi, S.; Alqahtani, A.F.; Sader, R.; Ghanaati, S. Current Knowledge on the Healing of the Extraction Socket: A Narrative Review. Bioengineering 2023, 10, 1145. [Google Scholar] [CrossRef]
- Araujo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontology 2000 2015, 68, 122–134. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Marzola, A.; Bozzi, L.; Liljenberg, B.; Lindhe, J. Modeling and remodeling of human extraction sockets. J. Clin. Periodontol. 2008, 35, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Smieszek-Wilczewska, J.; Al-Maawi, S.; Heselich, A.; Sader, R. After Extraction, Upper Premolars Undergo Programmed Socket Collapse with Development of Cavitations Rather than Complete Socket Healing: A Radiological Study. Bioengineering 2025, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Fickl, S.; Zuhr, O.; Wachtel, H.; Stappert, C.F.; Stein, J.M.; Hurzeler, M.B. Dimensional changes of the alveolar ridge contour after different socket preservation techniques. J. Clin. Periodontol. 2008, 35, 906–913. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Araujo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Cardaropoli, G.; Araujo, M.; Lindhe, J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef]
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef]
- Chappuis, V.; Engel, O.; Reyes, M.; Shahim, K.; Nolte, L.P.; Buser, D. Ridge alterations post-extraction in the esthetic zone: A 3D analysis with CBCT. J. Dent. Res. 2013, 92, 195S–201S. [Google Scholar] [CrossRef]
- Hammerle, C.H.; Araujo, M.G.; Simion, M.; Osteology Consensus, G. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin. Oral Implant. Res. 2012, 23 (Suppl. 5), 80–82. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.V. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef]
- Darby, I.; Chen, S.T.; Buser, D. Ridge preservation techniques for implant therapy. Int. J. Oral Maxillofac. Implant. 2009, 24, 260–271. [Google Scholar]
- Ten Heggeler, J.M.; Slot, D.E.; Van der Weijden, G.A. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: A systematic review. Clin. Oral Implant. Res. 2011, 22, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.A.; Murriky, A.; Shafik, S. Influence of platelet rich fibrin on post-extraction socket healing: A clinical and radiographic study. Saudi Dent. J. 2017, 29, 149–155. [Google Scholar] [CrossRef]
- Canellas, J.; da Costa, R.C.; Breves, R.C.; de Oliveira, G.P.; Figueredo, C.; Fischer, R.G.; Thole, A.A.; Medeiros, P.J.D.; Ritto, F.G. Tomographic and histomorphometric evaluation of socket healing after tooth extraction using leukocyte- and platelet-rich fibrin: A randomized, single-blind, controlled clinical trial. J. Craniomaxillofac. Surg. 2020, 48, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.B.; Van Dessel, J.; Temmerman, A.; Jacobs, R.; Quirynen, M. Effect of different platelet-rich fibrin matrices for ridge preservation in multiple tooth extractions: A split-mouth randomized controlled clinical trial. J. Clin. Periodontol. 2021, 48, 984–995. [Google Scholar] [CrossRef]
- Girish Kumar, N.; Chaudhary, R.; Kumar, I.; Arora, S.S.; Kumar, N.; Singh, H. To assess the efficacy of socket plug technique using platelet rich fibrin with or without the use of bone substitute in alveolar ridge preservation: A prospective randomised controlled study. Oral Maxillofac. Surg. 2018, 22, 135–142. [Google Scholar] [CrossRef]
- Hauser, F.; Gaydarov, N.; Badoud, I.; Vazquez, L.; Bernard, J.P.; Ammann, P. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: A prospective randomized controlled study. Implant. Dent. 2013, 22, 295–303. [Google Scholar] [CrossRef]
- Suttapreyasri, S.; Leepong, N. Influence of platelet-rich fibrin on alveolar ridge preservation. J. Craniofacial Surg. 2013, 24, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Yewale, M.; Bhat, S.; Kamath, A.; Tamrakar, A.; Patil, V.; Algal, A.S. Advanced platelet-rich fibrin plus and osseous bone graft for socket preservation and ridge augmentation—A randomized control clinical trial. J. Oral Biol. Craniofacial Res. 2021, 11, 225–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, Z.; Shen, M.; Tan, L.; Huang, W.; Wang, L.; Huang, Y. Clinical effect of platelet-rich fibrin on the preservation of the alveolar ridge following tooth extraction. Exp. Ther. Med. 2018, 15, 2277–2286. [Google Scholar] [CrossRef]
- Clark, D.; Rajendran, Y.; Paydar, S.; Ho, S.; Cox, D.; Ryder, M.; Dollard, J.; Kao, R.T. Advanced platelet-rich fibrin and freeze-dried bone allograft for ridge preservation: A randomized controlled clinical trial. J. Periodontol. 2018, 89, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, B.; Das, P.; Rana, M.M.; Qureshi, A.Q.; Vaidya, K.C.; Ahmed Raziuddin, S.J. Wound Healing and Bone Regeneration in Postextraction Sockets with and without Platelet-rich Fibrin. Ann. Maxillofac. Surg. 2018, 8, 28–34. [Google Scholar] [CrossRef]
- Temmerman, A.; Vandessel, J.; Castro, A.; Jacobs, R.; Teughels, W.; Pinto, N.; Quirynen, M. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: A split-mouth, randomized, controlled clinical trial. J. Clin. Periodontol. 2016, 43, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, D.J.; Deshpande, N.C.; Dave, D.H.; Narayankar, S.D. A comparative evaluation of extraction socket preservation with demineralized freeze-dried bone allograft alone and along with platelet-rich fibrin: A clinical and radiographic study. Contemp. Clin. Dent. 2016, 7, 371–376. [Google Scholar] [CrossRef]
- Sharma, A.; Ingole, S.; Deshpande, M.; Ranadive, P.; Sharma, S.; Kazi, N.; Rajurkar, S. Influence of platelet-rich fibrin on wound healing and bone regeneration after tooth extraction: A clinical and radiographic study. J. Oral Biol. Craniofacial Res. 2020, 10, 385–390. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ghanaati, S.; Smieszek-Wilczewska, J.; Al-Maawi, S.; Neff, P.; Zadeh, H.H.; Sader, R.; Heselich, A.; Rutkowski, J.L. Solid PRF Serves as Basis for Guided Open Wound Healing of the Ridge after Tooth Extraction by Accelerating the Wound Healing Time Course-A Prospective Parallel Arm Randomized Controlled Single Blind Trial. Bioengineering 2022, 9, 661. [Google Scholar] [CrossRef]
- de Almeida Barros Mourao, C.F.; de Mello-Machado, R.C.; Javid, K.; Moraschini, V. The use of leukocyte- and platelet-rich fibrin in the management of soft tissue healing and pain in post-extraction sockets: A randomized clinical trial. J. Craniomaxillofac. Surg. 2020, 48, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Ghanaati, S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. Eur. J. Trauma Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- Anitua, E.; Murias-Freijo, A.; Alkhraisat, M.H.; Orive, G. Clinical, radiographical, and histological outcomes of plasma rich in growth factors in extraction socket: A randomized controlled clinical trial. Clin. Oral Investig. 2015, 19, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Celio-Mariano, R.; de Melo, W.M.; Carneiro-Avelino, C. Comparative radiographic evaluation of alveolar bone healing associated with autologous platelet-rich plasma after impacted mandibular third molar surgery. J. Oral Maxillofac. Surg. 2012, 70, 19–24. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heselich, A.; Neff, P.; Śmieszek-Wilczewska, J.; Sader, R.; Ghanaati, S. Introduction of a Semi-Quantitative Image-Based Analysis Tool for CBCT-Based Evaluation of Bone Regeneration in Tooth Extraction Sockets. Bioengineering 2025, 12, 301. https://doi.org/10.3390/bioengineering12030301
Heselich A, Neff P, Śmieszek-Wilczewska J, Sader R, Ghanaati S. Introduction of a Semi-Quantitative Image-Based Analysis Tool for CBCT-Based Evaluation of Bone Regeneration in Tooth Extraction Sockets. Bioengineering. 2025; 12(3):301. https://doi.org/10.3390/bioengineering12030301
Chicago/Turabian StyleHeselich, Anja, Pauline Neff, Joanna Śmieszek-Wilczewska, Robert Sader, and Shahram Ghanaati. 2025. "Introduction of a Semi-Quantitative Image-Based Analysis Tool for CBCT-Based Evaluation of Bone Regeneration in Tooth Extraction Sockets" Bioengineering 12, no. 3: 301. https://doi.org/10.3390/bioengineering12030301
APA StyleHeselich, A., Neff, P., Śmieszek-Wilczewska, J., Sader, R., & Ghanaati, S. (2025). Introduction of a Semi-Quantitative Image-Based Analysis Tool for CBCT-Based Evaluation of Bone Regeneration in Tooth Extraction Sockets. Bioengineering, 12(3), 301. https://doi.org/10.3390/bioengineering12030301






