AI-Assisted Response Surface Methodology for Growth Optimization and Industrial Applicability Evaluation of the Diatom Gedaniella flavovirens GFTA21
Abstract
1. Introduction
2. Materials and Methods
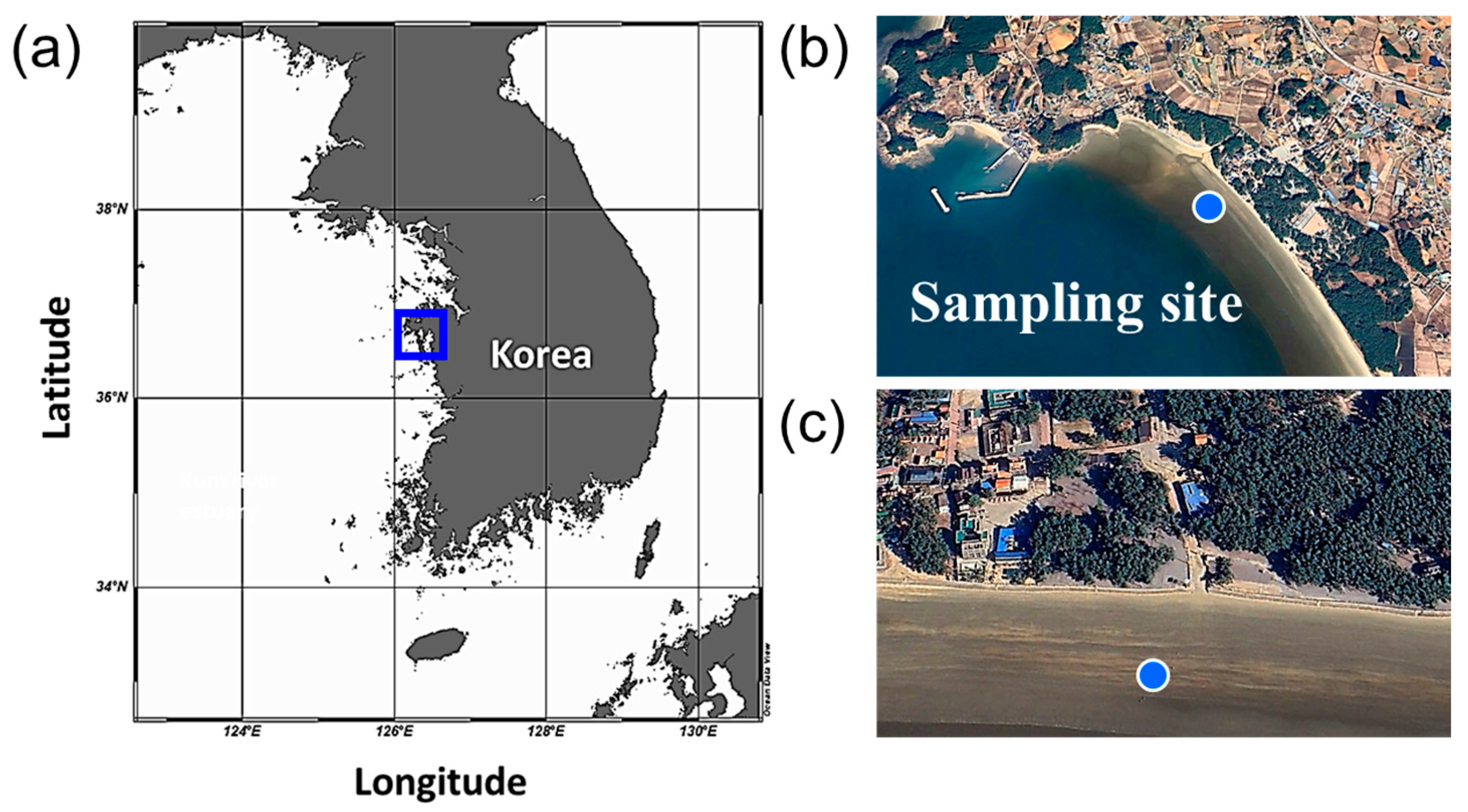
2.1. Axenicity and Cultivation of G. flavovirens
2.2. Design of Experiments (DoE) Through Interaction with ChatGPT-4.0
2.3. Estimation of Optimal Culture Condition Based on RSM
2.4. Photosynthetic Pigment Analysis
2.5. Fatty Acid Composition Analysis
2.6. Statistical Analysis
3. Results and Discussion
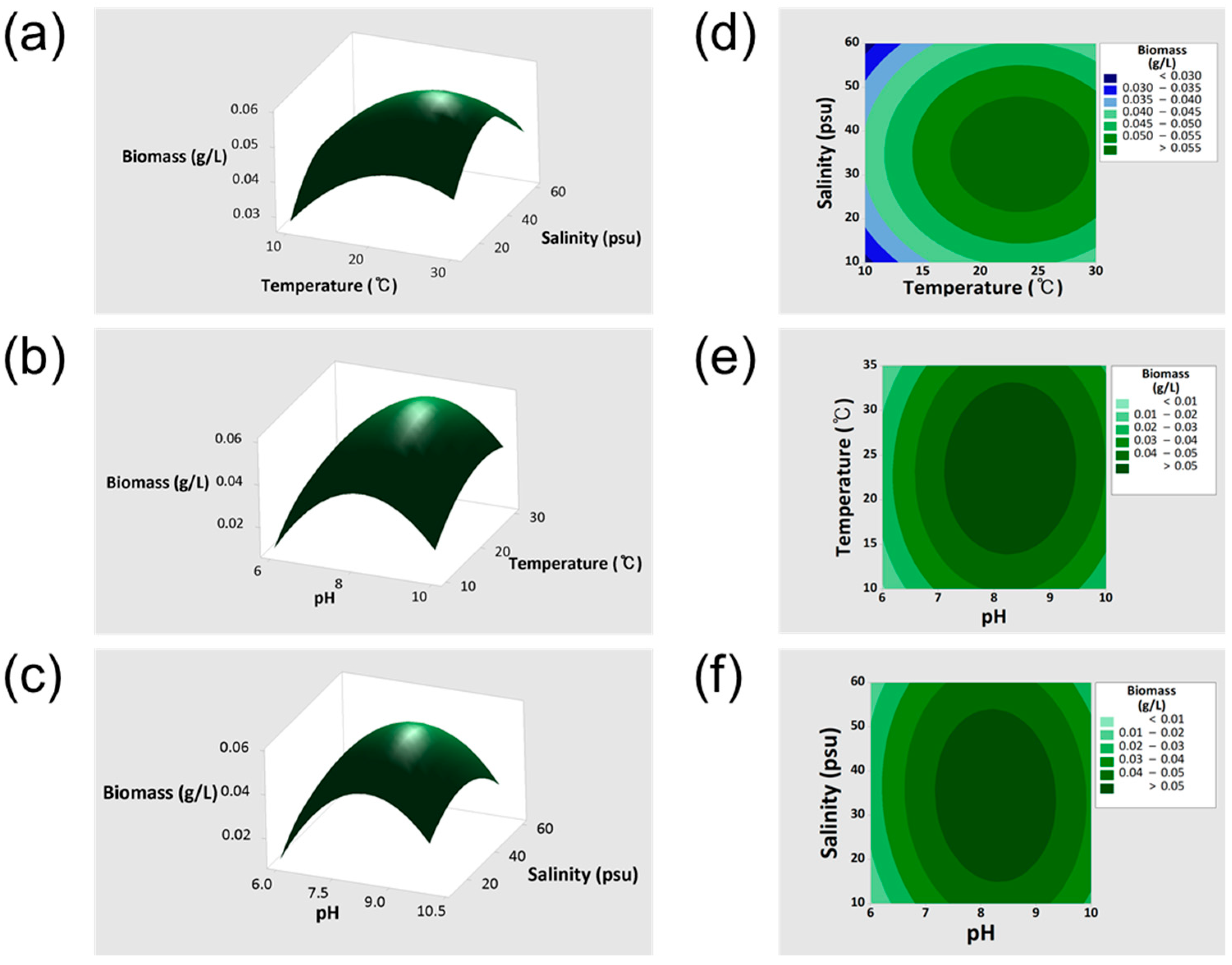
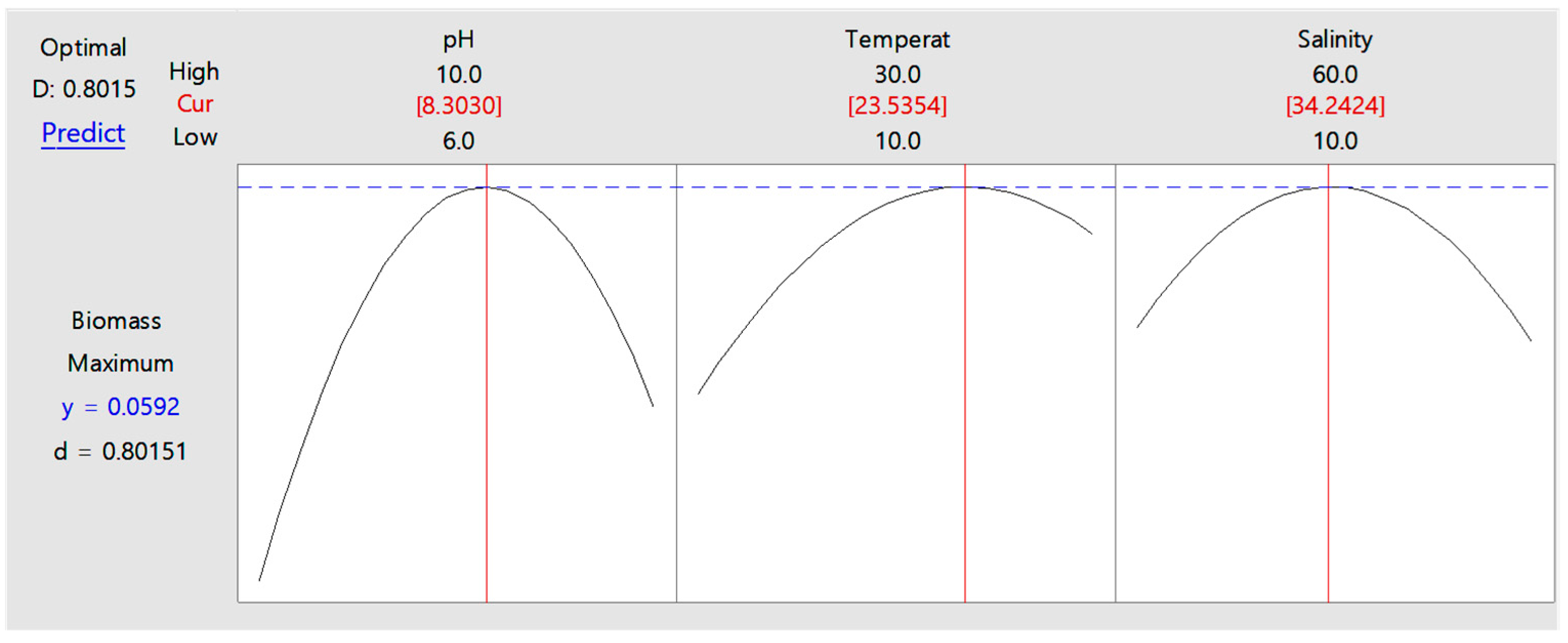
3.1. Optimization Results via ChatGPT-4.0-Assisted RSM Analysis
3.2. Results of Fatty Acid and Photosynthetic Pigment Analysis
3.3. ChatGPT-4.0-Based Evaluation of the Industrial Applicability of G. flavovirens GFTA21
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| DoE | Design of Experiments |
| EPA | Eicosapentaenoic Acid |
| FCCCD | Face-centered central composite design |
| LLM | Large Language Model |
| PSU | Practical Salinity Unit |
| RSM | Response Surface Methodology |
References
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; p. 747. [Google Scholar]
- Smetacek, V. Diatoms and the ocean carbon cycle. Protist 1999, 150, 25–32. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Villanova, V.; Spetea, C. Mixotrophy in diatoms: Molecular mechanism and industrial potential. Physiol. Plant. 2021, 173, 603–611. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Gonçalves, A.; Barbeiro, M.; Bandarra, N.; Nunes, M.L.; Carvalho, M.L.; Silva, J.; Navalho, J.; Dinis, M.T.; Silva, T.; et al. Phaeodactylum tricornutum in finishing diets for gilthead seabream: Effects on skin pigmentation, sensory properties and nutritional value. J. Appl. Phycol. 2017, 29, 1945–1956. [Google Scholar] [CrossRef]
- Uzlaşır, T.; Selli, S.; Kelebek, H. Spirulina platensis and Phaeodactylum tricornutum as sustainable sources of bioactive compounds: Health implications and applications in the food industry. Future Postharvest Food 2024, 1, 34–46. [Google Scholar] [CrossRef]
- Araújo, J.; Candeias-Mendes, A.; Monteiro, I.; Teixeira, D.; Soares, F.; Pousão-Ferreira, P. The use of diatom Skeletonema costatum on aquaculture-produced purple sea urchin (Paracentrotus lividus) larvae and post-larvae diet. Aquac. Res. 2020, 51, 2545–2554. [Google Scholar] [CrossRef]
- Tiwari, A.; Melchor-Martínez, E.M.; Saxena, A.; Kapoor, N.; Singh, K.J.; Saldarriaga-Hernández, S.; Iqbal, H.M. Therapeutic attributes and applied aspects of biological macromolecules (polypeptides, fucoxanthin, sterols, fatty acids, polysaccharides, and polyphenols) from diatoms—A review. Int. J. Biol. Macromol. 2021, 171, 398–413. [Google Scholar] [CrossRef]
- Sharma, N.; Simon, D.P.; Diaz-Garza, A.M.; Fantino, E.; Messaabi, A.; Meddeb-Mouelhi, F.; Desgagné-Penix, I. Diatoms biotechnology: Various industrial applications for a greener tomorrow. Front. Mar. Sci. 2021, 8, 636613. [Google Scholar] [CrossRef]
- An, S.M.; Cho, K.; Kim, E.S.; Ki, H.; Choi, G.; Kang, N.S. Description and characterization of the Odontella aurita OAOSH22, a marine diatom rich in eicosapentaenoic acid and fucoxanthin, isolated from Osan Harbor, Korea. Mar. Drugs 2023, 21, 563. [Google Scholar] [CrossRef] [PubMed]
- Marvin, G.; Hellen, N.; Jjingo, D.; Nakatumba-Nabende, J. Prompt engineering in large language models. In Proceedings of the International Conference on Data Intelligence and Cognitive Informatics; Springer: Singapore, 2023; pp. 387–402. [Google Scholar]
- Meskó, B. Prompt engineering as an important emerging skill for medical professionals: Tutorial. J. Med. Internet Res. 2023, 25, e50638. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Q.; Du, J.; Peng, X.; Keloth, V.K.; Zuo, X.; Zhou, Y.; Li, Z.; Jiang, X.; Lu, Z.; et al. Improving large language models for clinical named entity recognition via prompt engineering. J. Am. Med. Inform. Assoc. 2024, 31, 1812–1820. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Pal, S.; Chatterjee, S.; Lee, S.S.; Chakraborty, C. Large language model to multimodal large language model: A journey to shape the biological macromolecules to biological sciences and medicine. Mol. Ther.–Nucleic Acids 2024, 35, 657–661. [Google Scholar] [CrossRef]
- Hatakeyama-Sato, K.; Yamane, N.; Igarashi, Y.; Nabae, Y.; Hayakawa, T. Prompt engineering of GPT-4 for chemical research: What can/cannot be done? Sci. Technol. Adv. Mater. Methods 2023, 3, 2260300. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, W.; Moon, H.; Roell, G.W.; Chen, Y.; Tang, Y.J. Generative artificial intelligence GPT-4 accelerates knowledge mining and machine learning for synthetic biology. ACS Synth. Biol. 2023, 12, 2973–2982. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhang, L. Discovering the next decade’s synthetic biology research trends with ChatGPT. Synth. Syst. Biotechnol. 2023, 8, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Witak, M.; Pędziński, J.; Oliwa, S.; Hetko, D. Biodiversity of benthic diatom flora in the coastal zone of Puck Bay (southern Baltic Sea): A case study of the Hel Peninsula. Oceanol. Hydrobiol. Stud. 2020, 49, 304–318. [Google Scholar] [CrossRef]
- Gao, H. Isolation and evaluation of brackish diatoms for the photobiological treatment of reverse osmosis concentrate. AQUA–Water Infrastruct. Ecosyst. Soc. 2022, 71, 1083–1094. [Google Scholar] [CrossRef]
- Hetko, D.; Witak, M.; Bełdowska, M. The relationship between total mercury, its fractions and species diversity of diatom taphocoenoses deposited in surface sediments (southern Baltic Sea). Water 2023, 15, 3907. [Google Scholar] [CrossRef]
- Gao, H.; Sato, S.; Kodamatani, H.; Fujioka, T.; Ishida, K.P.; Ikehata, K. Optimization of dissolved silica removal from reverse osmosis concentrate by Gedanienella flavovirens for enhanced water recovery. Sustainability 2024, 16, 4052. [Google Scholar] [CrossRef]
- Roychoudhury, P.; Golubeva, A.; Dąbek, P.; Gloc, M.; Dobrucka, R.; Kurzydłowski, K.; Witkowski, A. Diatom mediated production of fluorescent flower shaped silver–silica nanohybrid. Materials 2021, 14, 7284. [Google Scholar] [CrossRef]
- De Lillo, A.; Ashley, F.P.; Palmer, R.M.; Munson, M.A.; Kyriacou, L.; Weightman, A.J.; Wade, W.G. Novel subgingival bacterial phylotypes detected using multiple universal polymerase chain reaction primer sets. Oral Microbiol. Immunol. 2006, 21, 61–68. [Google Scholar] [CrossRef]
- Baek, K.; Yu, J.; Jeong, J.; Sim, S.J.; Bae, S.; Jin, E. Photoautotrophic production of macular pigment in a Chlamydomonas reinhardtii strain generated by using DNA-free CRISPR-Cas9 RNP-mediated mutagenesis. Biotechnol. Bioeng. 2018, 115, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef]
- Cho, K.; Kim, E.S.; Ki, H.; Kim, K.Y.; Pan, C.H.; Hwang, H.J.; An, S.M. Estimation of optimal culture conditions for Gedanienella panicellus GPYS21 (Fragilariaceae) as a high-yield bioresource for palmitoleic acid and fucoxanthin production. Biochem. Biophys. Res. Commun. 2025, 756, 151579. [Google Scholar] [CrossRef]
- Rijstenbil, J.W.; Mur, L.R.; Wijnholds, J.J.; Sinke, J.J. Impact of a temporal salinity decrease on growth and nitrogen metabolism of the marine diatom Skeletonema costatum in continuous cultures. Mar. Biol. 1989, 101, 121–129. [Google Scholar] [CrossRef]
- Berges, J.A.; Varela, D.E.; Harrison, P.J. Effects of temperature on growth rate, cell composition and nitrogen metabolism in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). Mar. Ecol. Prog. Ser. 2002, 225, 139–146. [Google Scholar] [CrossRef]
- Grouneva, I.; Jakob, T.; Wilhelm, C.; Goss, R. Influence of ascorbate and pH on the activity of the diatom xanthophyll cycle-enzyme diadinoxanthin de-epoxidase. Physiol. Plant. 2006, 126, 205–211. [Google Scholar] [CrossRef]
- Goldman, J.A.; Bender, M.L.; Morel, F.M. The effects of pH and pCO2 on photosynthesis and respiration in the diatom Thalassiosira weissflogii. Photosynth. Res. 2017, 132, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Koester, J.A.; Liefer, J.D.; Irwin, A.J.; Finkel, Z.V. Molecular mechanisms of temperature acclimation and adaptation in marine diatoms. ISME J. 2019, 13, 2415–2425. [Google Scholar] [CrossRef]
- Rousch, J.M.; Bingham, S.E.; Sommerfeld, M.R. Changes in fatty acid profiles of thermo-intolerant and thermo-tolerant marine diatoms during temperature stress. J. Exp. Mar. Biol. Ecol. 2003, 295, 145–156. [Google Scholar] [CrossRef]
- Bose, A.; Tiwari, B.S.; Chattopadhyay, M.K.; Gupta, S.; Ghosh, B. Thermal stress induces differential degradation of Rubisco in heat-sensitive and heat-tolerant rice. Physiol. Plant. 1999, 105, 89–94. [Google Scholar] [CrossRef]
- Souffreau, C.; Vanormelingen, P.; Verleyen, E.; Sabbe, K.; Vyverman, W. Tolerance of benthic diatoms from temperate aquatic and terrestrial habitats to experimental desiccation and temperature stress. Phycologia 2010, 49, 309–324. [Google Scholar] [CrossRef]
- Burkhardt, S.; Amoroso, G.; Riebesell, U.; Sültemeyer, D. CO2 and HCO3− uptake in marine diatoms acclimated to different CO2 concentrations. Limnol. Oceanogr. 2001, 46, 1378–1391. [Google Scholar] [CrossRef]
- Kettles, N.L.; Kopriva, S.; Malin, G. Insights into the regulation of DMSP synthesis in the diatom Thalassiosira pseudonana through APR activity, proteomics and gene expression analyses on cells acclimating to changes in salinity, light and nitrogen. PLoS ONE 2014, 9, e94795. [Google Scholar] [CrossRef] [PubMed]
- Pinseel, E.; Nakov, T.; Van den Berge, K.; Downey, K.M.; Judy, K.J.; Kourtchenko, O.; Alverson, A.J. Strain-specific transcriptional responses overshadow salinity effects in a marine diatom sampled along the Baltic Sea salinity cline. ISME J. 2022, 16, 1776–1787. [Google Scholar] [CrossRef]
- Uzlasir, O.T.; Isik, O.; Uslu, L.H.; Selli, S.; Kelebek, H. Impact of different salt concentrations on growth, biochemical composition and nutrition quality of Phaeodactylum tricornutum and Spirulina platensis. Food Chem. 2023, 429, 136843. [Google Scholar] [CrossRef]
- Baek, S.H.; Jung, S.W.; Shin, K. Effects of temperature and salinity on growth of Thalassiosira pseudonana (Bacillariophyceae) isolated from ballast water. J. Freshw. Ecol. 2011, 26, 547–552. [Google Scholar] [CrossRef]
- Kirrolia, A.; Bishnoi, N.R.; Singh, R. Response surface methodology as a decision-making tool for optimization of culture conditions of green microalgae Chlorella spp. for biodiesel production. Ann. Microbiol. 2014, 64, 1133–1147. [Google Scholar] [CrossRef]
- Akış, S.; Özçimen, D. Optimization of pH-induced flocculation of marine and freshwater microalgae via central composite design. Biotechnol. Prog. 2019, 35, e2801. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Alharthi, S. Use of response surface methodology in optimization of biomass, lipid productivity and fatty acid profiles of marine microalga Dunaliella parva for biodiesel production. Environ. Technol. Innov. 2021, 22, 101485. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Saeid, A. Plant growth biostimulants, dietary feed supplements and cosmetics formulated with supercritical CO2 algal extracts. Molecules 2017, 22, 66. [Google Scholar] [CrossRef]
- Los, D.A. Fatty Acid Desaturases; Nauchnyj Mir: Moscow, Russia, 2014. (In Russian) [Google Scholar]
- Maltsev, Y.; Maltseva, K. Fatty acids of microalgae: Diversity and applications. Rev. Environ. Sci. Biotechnol. 2021, 20, 515–547. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.V.; Lambrinidis, G.; Parry, D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Sukenik, A. Production of eicosapentaenoic acid by the marine eustigmatophyte Nannochloropsis. In Chemicals from Microalgae; Cohen, Z., Ed.; Taylor & Francis: London, UK, 1999; pp. 41–56. [Google Scholar]
- Yoon, W.J.; Kim, M.J.; Moon, J.Y.; Kang, H.J.; Kim, G.O.; Lee, N.H.; Hyun, C.G. Effect of palmitoleic acid on melanogenic protein expression in murine B16 melanoma. J. Oleo Sci. 2010, 59, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yano, S.; Kawai, T.; Jinbo, Y.; Nonomura, Y. Selective antibacterial activity of palmitoleic acid in emulsions and other formulations. J. Surfactants Deterg. 2021, 24, 973–979. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The role of the novel lipokine palmitoleic acid in health and disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef]
- Narayan, B.; Miyashita, K.; Hosakawa, M. Physiological effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—A review. Food Rev. Int. 2006, 22, 291–307. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Salem, N., Jr. Dietary polyunsaturated fatty acids and depression: When cholesterol does not satisfy. Am. J. Clin. Nutr. 1995, 62, 1–9. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic pigments in diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef]
- Gelzinis, A.; Butkus, V.; Songaila, E.; Augulis, R.; Gall, A.; Büchel, C.; Robert, B.; Abramavicius, D.; Zigmantas, D.; Valkunas, L. Mapping energy transfer channels in fucoxanthin–chlorophyll protein complex. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 241–247. [Google Scholar] [CrossRef]
- Erdogan, A.; Demirel, Z.; Dalay, M.C.; Eroglu, A.E. Fucoxanthin content of Cylindrotheca closterium and its oxidative stress mediated enhancement. Turk. J. Fish. Aquat. Sci. 2016, 16, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Chen, S.H.Y.; Lu, X.; Yu, J.; Liu, B. High silicate concentration facilitates fucoxanthin and eicosapentaenoic acid (EPA) production under heterotrophic condition in the marine diatom Nitzschia laevis. Algal Res. 2020, 52, 102086. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; García-Oliveira, P.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Sun, H.; Yang, S.; Zhao, W.; Kong, Q.; Zhu, C.; Fu, X.; He, Y. Fucoxanthin from marine microalgae: A promising bioactive compound for industrial production and food application. Crit. Rev. Food Sci. Nutr. 2023, 63, 7996–8012. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Hu, Q. Microalgae as feed sources and feed additives for sustainable aquaculture: Prospects and challenges. Rev. Aquac. 2024, 16, 818–835. [Google Scholar] [CrossRef]
- Budiarso, F.S.; Leong, Y.K.; Chang, J.J.; Chen, C.Y.; Chen, J.H.; Yen, H.W.; Chang, J.S. Current advances in microalgae-based fucoxanthin production and downstream processes. Bioresour. Technol. 2025, 428, 132455. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, H.; Ding, Y.; Liu, S.; Ding, Y.; Lu, B.; Xiao, J.; Zhou, X. Dietary protective potential of fucoxanthin as an active food component on neurological disorders. J. Agric. Food Chem. 2023, 71, 3599–3619. [Google Scholar] [CrossRef]
- Sørensen, M.; Berge, G.M.; Reitan, K.I.; Ruyter, B. Microalga Phaeodactylum tricornutum in feed for Atlantic salmon (Salmo salar)—Effect on nutrient digestibility, growth and utilization of feed. Aquaculture 2016, 460, 116–123. [Google Scholar] [CrossRef]
- Medina-Félix, D.; López-Elías, J.A.; Martínez-Córdova, L.R.; López-Torres, M.A.; Hernández-López, J.; Rivas-Vega, M.E.; Mendoza-Cano, F. Evaluation of the productive and physiological responses of Litopenaeus vannamei infected with WSSV and fed diets enriched with Dunaliella sp. J. Invertebr. Pathol. 2014, 117, 9–12. [Google Scholar] [CrossRef]
- Patil, V.; Reitan, K.I.; Knutsen, G.; Mortensen, L.M.; Källqvist, T.; Olsen, E.; Gislerød, H.R. Microalgae as source of polyunsaturated fatty acids for aquaculture. Plant Biol. 2005, 6, 57–65. [Google Scholar]
- Nath, P.R.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S.; Zilberg, D. Dietary supplementation with the microalgae Parietochloris incisa increases survival and stress resistance in guppy (Poecilia reticulata) fry. Aquac. Nutr. 2012, 18, 167–180. [Google Scholar] [CrossRef]
- Marques, A.E.M.L.; Balen, R.E.; da Silva Pereira Fernandes, L.; Motta, C.M.; de Assis, H.C.S.; Taher, D.M.; Cestari, M.M. Diets containing residual microalgae biomass protect fishes against oxidative stress and DNA damage. J. Appl. Phycol. 2019, 31, 2933–2940. [Google Scholar] [CrossRef]
- Zhu, K.; Huang, M.; Wang, Y.; Gu, Y.; Li, W.; Liu, G.; Tang, Y. MetaPredictor: In silico prediction of drug metabolites based on deep language models with prompt engineering. Brief. Bioinform. 2024, 25, bbae374. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, J.; Garikipati, A.; Singh, N.P.; Cyrus, L.; Sharma, M.; Ciobanu, M.; Barnes, G.; Thapa, R.; Mao, Q.; Das, R. OpenMedLM: Prompt engineering can out-perform fine-tuning in medical question-answering with open-source large language models. Sci. Rep. 2024, 14, 14156. [Google Scholar] [CrossRef] [PubMed]
- Cahan, P.; Treutlein, B. A conversation with ChatGPT on the role of computational systems biology in stem cell research. Stem Cell Rep. 2023, 18, 1–2. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| pH | 6 | 8 | 10 |
| Temperature (°C) | 10 | 20 | 30 |
| Salinity (psu) | 10 | 35 | 60 |
| No. | pH | Temperature (°C) | Salinity (psu) | Biomass (g/L) |
|---|---|---|---|---|
| 1 | −1 | 1 | 1 | 0.01 |
| 2 | 1 | −1 | 1 | 0.01 |
| 3 | 1 | 1 | −1 | 0.04 |
| 4 | −1 | −1 | −1 | 0.00 |
| 5 | −1 | −1 | −1 | 0.01 |
| 6 | −1 | 1 | 1 | 0.01 |
| 7 | 0 | 0 | 0 | 0.07 |
| 8 | 1 | −1 | 1 | 0.01 |
| 9 | 0 | 0 | 0 | 0.07 |
| 10 | 1 | 1 | −1 | 0.02 |
| 11 | 0 | 0 | 0 | 0.04 |
| 12 | 0 | 0 | 0 | 0.06 |
| 13 | −1 | −1 | 1 | 0.00 |
| 14 | 1 | −1 | −1 | 0.02 |
| 15 | 1 | 1 | 1 | 0.02 |
| 16 | 1 | 1 | 1 | 0.02 |
| 17 | 0 | 0 | 0 | 0.06 |
| 18 | 0 | 0 | 0 | 0.07 |
| 19 | −1 | −1 | 1 | 0.00 |
| 20 | −1 | 1 | −1 | 0.01 |
| 21 | 1 | −1 | −1 | 0.02 |
| 22 | 0 | 0 | 0 | 0.05 |
| 23 | −1 | 1 | −1 | 0.01 |
| 24 | 0 | 0 | 0 | 0.06 |
| 25 | 0 | 0 | 0 | 0.05 |
| 26 | 0 | −1 | 0 | 0.02 |
| 27 | 0 | 0 | 0 | 0.06 |
| 28 | 0 | 0 | 1 | 0.03 |
| 29 | −1 | 0 | 0 | 0.01 |
| 30 | 0 | 0 | 1 | 0.04 |
| 31 | 0 | 1 | 0 | 0.06 |
| 32 | 0 | −1 | 0 | 0.02 |
| 33 | 1 | 0 | 0 | 0.02 |
| 34 | 1 | 0 | 0 | 0.03 |
| 35 | 0 | 0 | −1 | 0.02 |
| 36 | −1 | 0 | 0 | 0.01 |
| 37 | 0 | 1 | 0 | 0.05 |
| 38 | 0 | 0 | −1 | 0.03 |
| 39 | 0 | 0 | 0 | 0.06 |
| 40 | 0 | 0 | 0 | 0.06 |
| Source | DF | Adj. SS | Adj. MS | F | p |
|---|---|---|---|---|---|
| Model | 11 | 0.016667 | 0.001515 | 19.31 | <0.001 |
| Linear | 3 | 0.001904 | 0.000635 | 8.09 | <0.001 |
| pH (A) | 1 | 0.000989 | 0.000989 | 12.61 | 0.001 |
| Temperature (B) | 1 | 0.000912 | 0.000912 | 11.63 | 0.002 |
| Salinity (C) | 1 | 0.000002 | 0.000002 | 0.02 | 0.889 |
| Square | 3 | 0.014352 | 0.004784 | 60.97 | <0.001 |
| A·A | 1 | 0.003733 | 0.003733 | 47.57 | <0.001 |
| B·B | 1 | 0.000526 | 0.000526 | 6.71 | 0.015 |
| C·C | 1 | 0.000903 | 0.000903 | 11.51 | 0.002 |
| Interaction | 3 | 0.000096 | 0.000032 | 0.41 | 0.748 |
| A·B | 1 | 0.000012 | 0.000012 | 0.42 | 0.524 |
| A·C | 1 | 0.000063 | 0.000063 | 0.81 | 0.377 |
| B·C | 1 | <0.001 | <0.001 | 0.00 | 0.988 |
| Error | 28 | 0.002197 | 0.000078 | ||
| Lack-of-Fit | 5 | 0.001226 | 0.000245 | 5.81 | 0.001 |
| Pure Error | 23 | 0.000971 | 0.000042 | ||
| Total | 39 | 0.018864 |
| Fatty Acid | Category | Amount (mg/g) | Weight (w/w. %) | |
|---|---|---|---|---|
| Myristic acid | C14:0 | 3.40 ± 0.00 | 1.70 | |
| Palmitic acid | C16:0 | 37.82 ± 0.02 | 18.90 | |
| Palmitoleic acid | C16:1 | ω-7 | 116.61 ± 0.04 | 58.28 |
| Stearic acid | C18:0 | 0.42 ± 0.00 | 0.21 | |
| Oleic acid | C18:1 | ω-9 | 1.64 ± 0.00 | 0.82 |
| Linoleic acid | C18:2 | ω-6 | 3.04 ± 0.00 | 1.52 |
| Gamma-linolenic acid | C18:3 | ω-6 | 1.60 ± 0.00 | 0.80 |
| Dihomo-gamma-linolenic acid | C20:3 | ω-6 | 0.53 ± 0.00 | 0.27 |
| Arachidonic acid | C20:4 | ω-6 | 12.43 ± 0.03 | 6.21 |
| Eicosapentaenoic acid | C20:5 | ω-3 | 22.60 ± 0.04 | 11.29 |
| Total fatty acids | 200.10 | 100.00 | ||
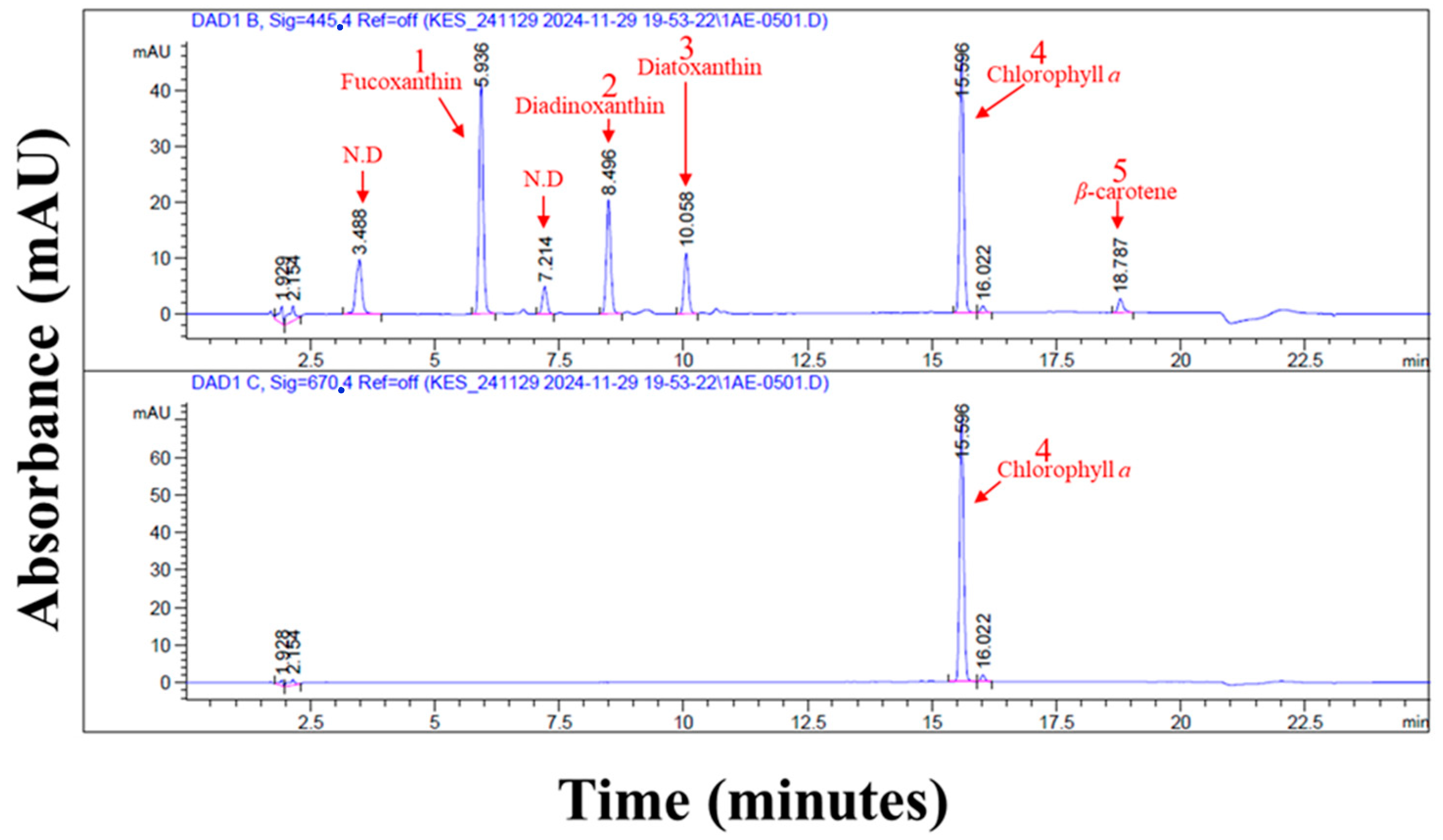
| Pigments | Retention Time (min) | Peak Area | Amount (mg/g) |
|---|---|---|---|
| Fucoxanthin | 5.94 ± 0.00 | 249.27 ± 11.55 | 8.67 ± 0.20 |
| Diadinoxanthin | 8.50 ± 0.00 | 129.37 ± 5.67 | 3.47 ± 0.07 |
| Diatoxanthin | 10.06 ± 0.00 | 69.41 ± 3.09 | 2.16 ± 0.05 |
| Chlorophyll a | 15.59 ± 0.01 | 412.99 ± 1.52 | 56.56 ± 1.62 |
| β-carotene | 18.78 ± 0.01 | 18.05 ± 0.85 | 0.46 ± 0.01 |
| Rank | Industry | Reason for Potential |
|---|---|---|
| 1 | Aquafeeds | Fatty acids and pigments support fish growth and improve pigmentation in aquafeeds, enhancing product quality for the aquaculture industry. |
| 2 | Nutraceuticals | High content of Eicosapentaenoic acid (EPA) and fucoxanthin, offering cardiovascular, anti-inflammatory, and anti-obesity benefits. These compounds are highly valued in health supplements. |
| 3 | Functional Foods | Abundance of EPA, Arachidonic acid (ARA), and carotenoids, which enhance the nutritional profile of food products, supporting health-conscious consumer trends. |
| 4 | Cosmetics | Rich in fucoxanthin, β-carotene, and chlorophyll a, which provide antioxidant, anti-aging, and skin-brightening effects. Ideal for premium skincare products. |
| 5 | Pharmaceuticals | EPA and ARA have therapeutic properties for managing inflammation, metabolic disorders, and cardiovascular diseases, making them essential in advanced medicine. |
| 6 | Food Colorants | Natural pigments such as β-carotene and fucoxanthin meet the demand for clean-label, health-promoting food colorants in the food industry. |
| Compounds (Class) | Aquaculture Benefit(s) | Microalgal Source/ Feeding Trial | References |
|---|---|---|---|
| Fucoxanthin (xanthophyll) | Skin and fillet pigmentation enhancement; supports growth and nutrient retention | Phaeodactylum tricornutum/whole biomass, 2.5–6% of diet (gilthead seabream, Atlantic salmon) | [6,63] |
| β-Carotene (carotene) | Growth promotion; immune enhancement | Dunaliella sp./1–2% algal meal in diet (Pacific white shrimp & black-tiger prawn) | [64] |
| EPA (20:5 n-3 PUFA) | Improved growth rate, feed efficiency, and muscle lipid composition | Various marine microalgae/commonly used in aquaculture feeds and live feed production for fish larvae and shellfish | [65] |
| ARA (20:4 n-6 PUFA) | Improved larval survival and resilience to stress | Parietochloris incisa/diet supplement during first-month fry stage (guppy) | [66] |
| Chlorophyll a (tetrapyrrole) | Enhanced antioxidant status and DNA protection | Acutodesmus obliquus/1–3% residual algal biomass in diet (Rhamdia quelen) | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.S.; Lee, S.J.; Lee, J.A.; An, S.M.; Hwang, H.-J.; Park, B.S.; Lee, H.-W.; Pan, C.-H.; Kim, D.; Cho, K. AI-Assisted Response Surface Methodology for Growth Optimization and Industrial Applicability Evaluation of the Diatom Gedaniella flavovirens GFTA21. Bioengineering 2025, 12, 1277. https://doi.org/10.3390/bioengineering12111277
Kim ES, Lee SJ, Lee JA, An SM, Hwang H-J, Park BS, Lee H-W, Pan C-H, Kim D, Cho K. AI-Assisted Response Surface Methodology for Growth Optimization and Industrial Applicability Evaluation of the Diatom Gedaniella flavovirens GFTA21. Bioengineering. 2025; 12(11):1277. https://doi.org/10.3390/bioengineering12111277
Chicago/Turabian StyleKim, Eun Song, Soo Jeong Lee, Jung A Lee, Sung Min An, Hyun-Ju Hwang, Bum Soo Park, Hae-Won Lee, Cheol-Ho Pan, Daekyung Kim, and Kichul Cho. 2025. "AI-Assisted Response Surface Methodology for Growth Optimization and Industrial Applicability Evaluation of the Diatom Gedaniella flavovirens GFTA21" Bioengineering 12, no. 11: 1277. https://doi.org/10.3390/bioengineering12111277
APA StyleKim, E. S., Lee, S. J., Lee, J. A., An, S. M., Hwang, H.-J., Park, B. S., Lee, H.-W., Pan, C.-H., Kim, D., & Cho, K. (2025). AI-Assisted Response Surface Methodology for Growth Optimization and Industrial Applicability Evaluation of the Diatom Gedaniella flavovirens GFTA21. Bioengineering, 12(11), 1277. https://doi.org/10.3390/bioengineering12111277








