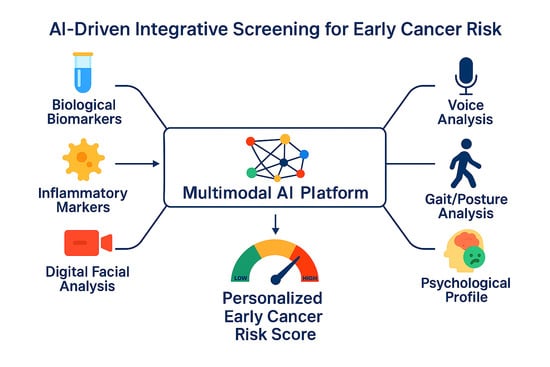
An Integrative Review of Computational Methods Applied to Biomarkers, Psychological Metrics, and Behavioral Signals for Early Cancer Risk Detection
Abstract
1. Introduction
1.1. Motivation for Early AI-Based Cancer Detection
1.2. Conventional Screening Limitations
1.3. Advantages of AI and Multimodal Data Integration
1.4. Novelty of This Review Compared to Existing Surveys
1.5. Roadmap of the Manuscript
1.6. Biological and Psychosocial Context of Cancer Vulnerability
2. Theoretical and Biological Basis of Oncogenesis and Psychological Factors
2.1. Biological Significance of Tumor Markers
2.2. Inflammatory Markers and Oncogenic Pathways
2.3. Psychological Profiling and Stress Vulnerability
2.3.1. Conceptualization of Stress in Oncogenesis
2.3.2. Interrelationship Between Stress, Depression, and Cancer
2.3.3. Psychological Profiling as a Tool for Risk Assessment
- Perceived Stress Scale (PSS)—assessment of perceived stress.
- Hospital Anxiety and Depression Scale (HADS)—screening for anxiety and depression.
- Montgomery–Åsberg Depression Rating Scale (MADRS)—severity of depression.
- Freiburg Personality Inventory (Type C)—evaluation of Type C traits.
- State–Trait Anxiety Inventory (STAI 1 and 2)—situational and dispositional anxiety.
- Holmes and Rahe Stress Scale—quantification of stress based on major life events.
- Self-Efficacy Scale—evaluation of perceived self-efficacy.
2.3.4. Facial Micro-Expression Analysis in Depression Detection
Clinical Analysis of Facial Expressions in Depressed Patients
Pathophysiological Mechanisms Affecting Facial Expressions in Depression
2.3.5. Voice Characteristics as Psychological Biomarkers of Depression
Clinical Analysis of Speech Patterns in Depressed Patients
Pathophysiological Mechanisms Affecting Voice and Speech in Depression
2.3.6. Gait and Posture Analysis in Psychological Assessment
Clinical Analysis of Gait and Posture in Depressed Patients
Pathophysiological Mechanisms Affecting Gait and Posture in Depression
2.4. AI-Assisted Models Used in Cancer Risk Evaluation
2.4.1. AI Models for Cancer Risk Assessment Using Biological Inputs—Tumor and Inflammation Markers
2.4.2. AI Models for Cancer Risk Assessment Using Psychometric Inputs—Psychological Profiling and Stress Vulnerability
2.4.3. AI Models for Cancer Risk Assessment Using Facial Expression Inputs—Micro-Expressions for Depression Detection
2.4.4. AI Models for Cancer Risk Assessment Using Vocal Input—Acoustic Markers for Depression Detection
2.4.5. Gait and Posture Input—Movement Patterns for Psychomotor Evaluation
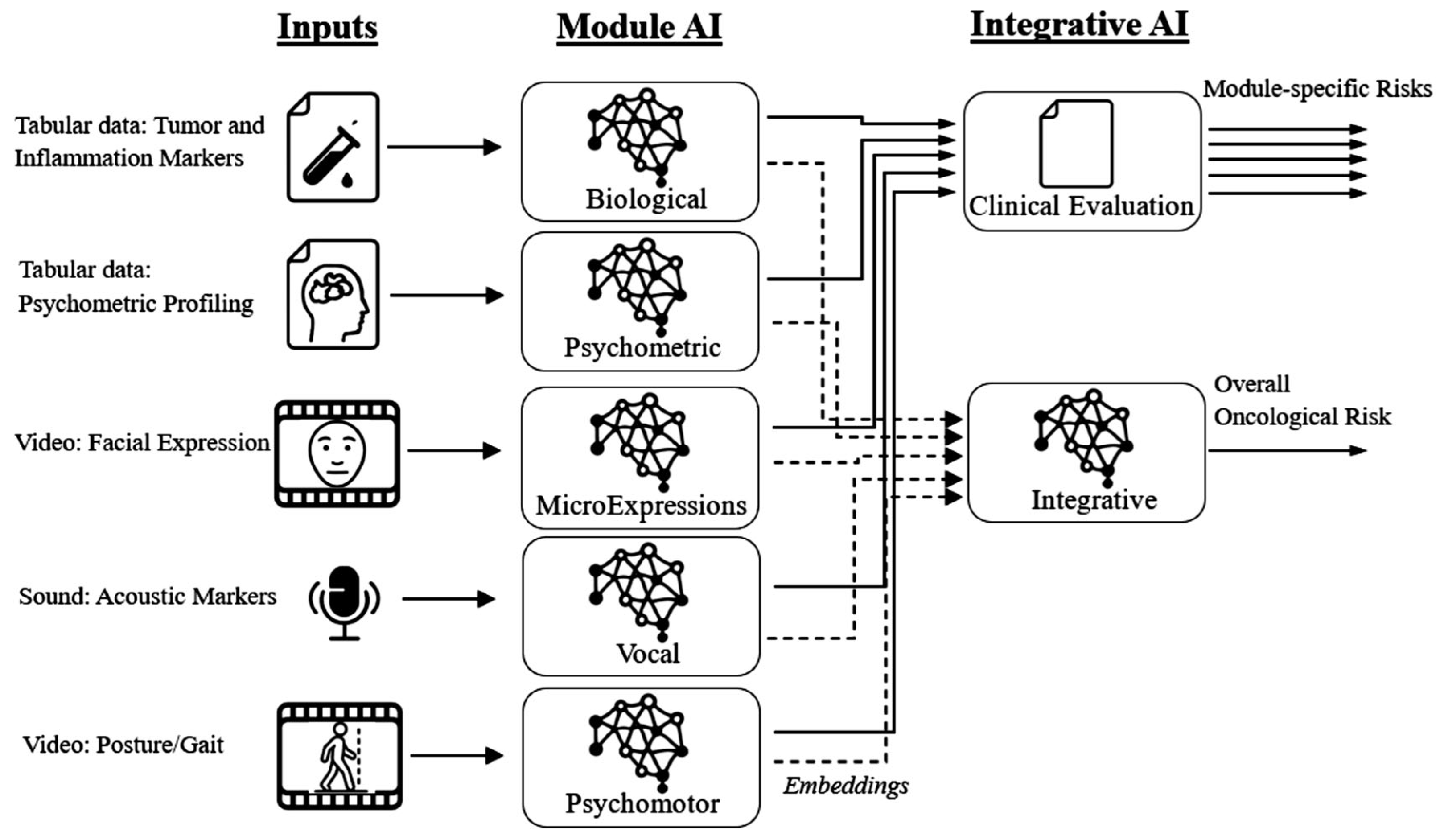
2.4.6. Principles for Modelling an Integrative AI Multimodal Framework
- Pretraining on large-scale image-based datasets such as FER-2013 or AffectNet. Although these datasets consist of single images, pretraining allows the model to learn robust spatial facial representations (e.g., eyes, mouth, eyebrow configurations) and general facial features, providing a strong initialization for downstream tasks.
- Fine-tuning on high-frame-rate micro-expression datasets such as CASME II [182] or SAMM [183], as well as a curated clinical oncology dataset. During this step, temporal sequences are used to capture subtle facial motions and micro-expression dynamics relevant to stress, depression, or oncological risk. This enables the model to specialize in clinically meaningful patterns while mitigating noise from general-purpose datasets.
2.4.7. Limitations of an Insulated AI Multimodal Platform Model
2.4.8. The Convergent Computerized Platforms Approach
Convergent Transformation of Informational Management for AI-Assisted Multimodal Cancer Screening Platform—The Integrated Specific EHR
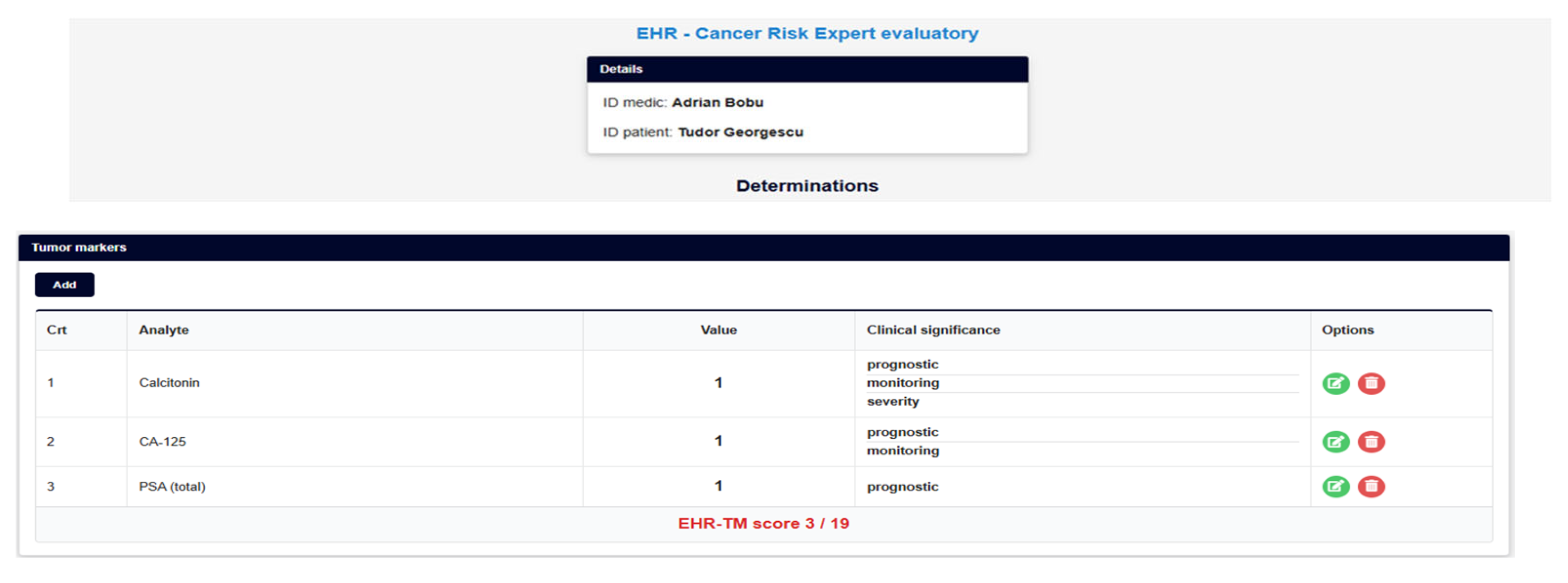
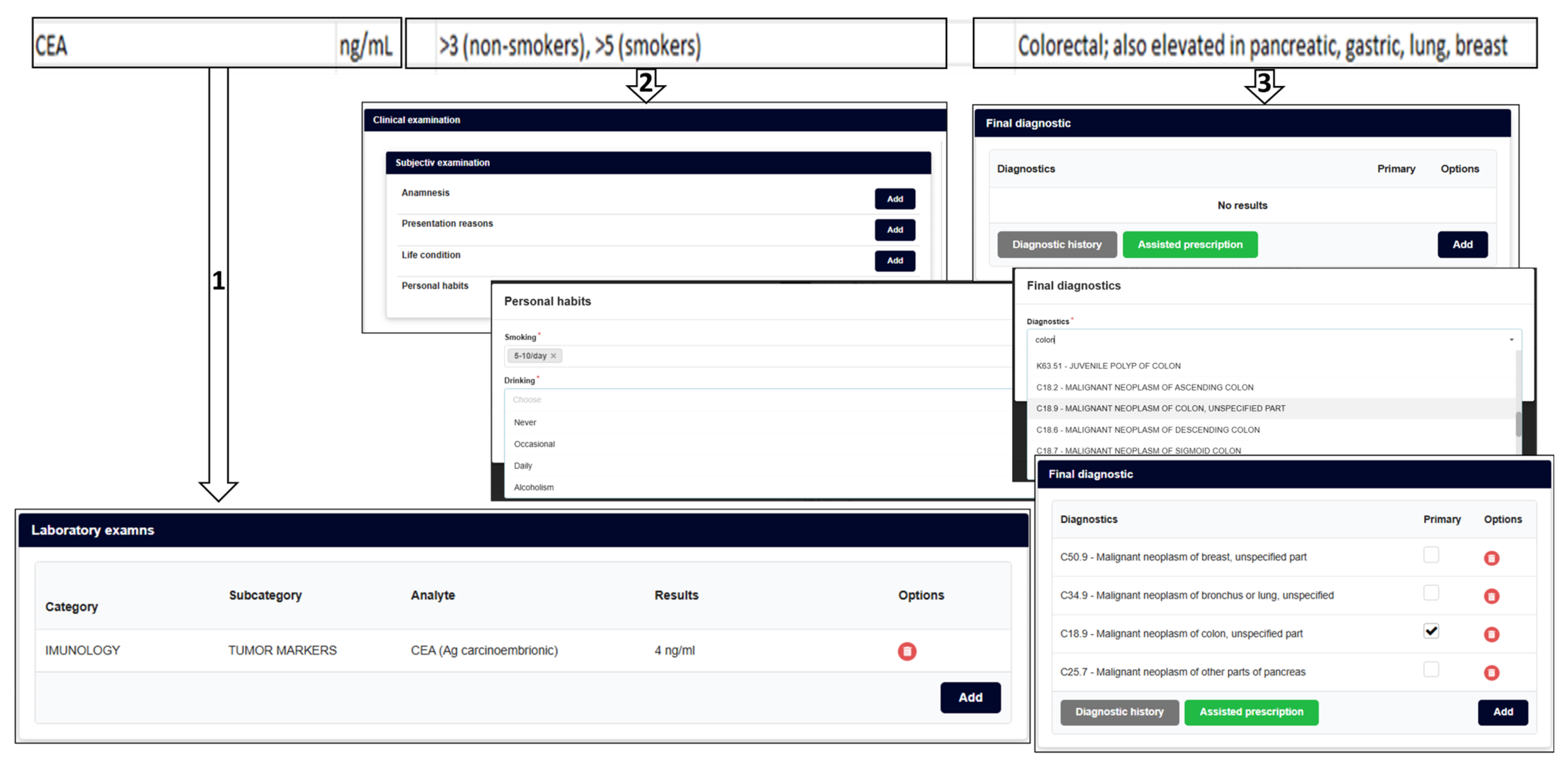
2.5. Illustrative Clinical Scenario (Theoretical Example)
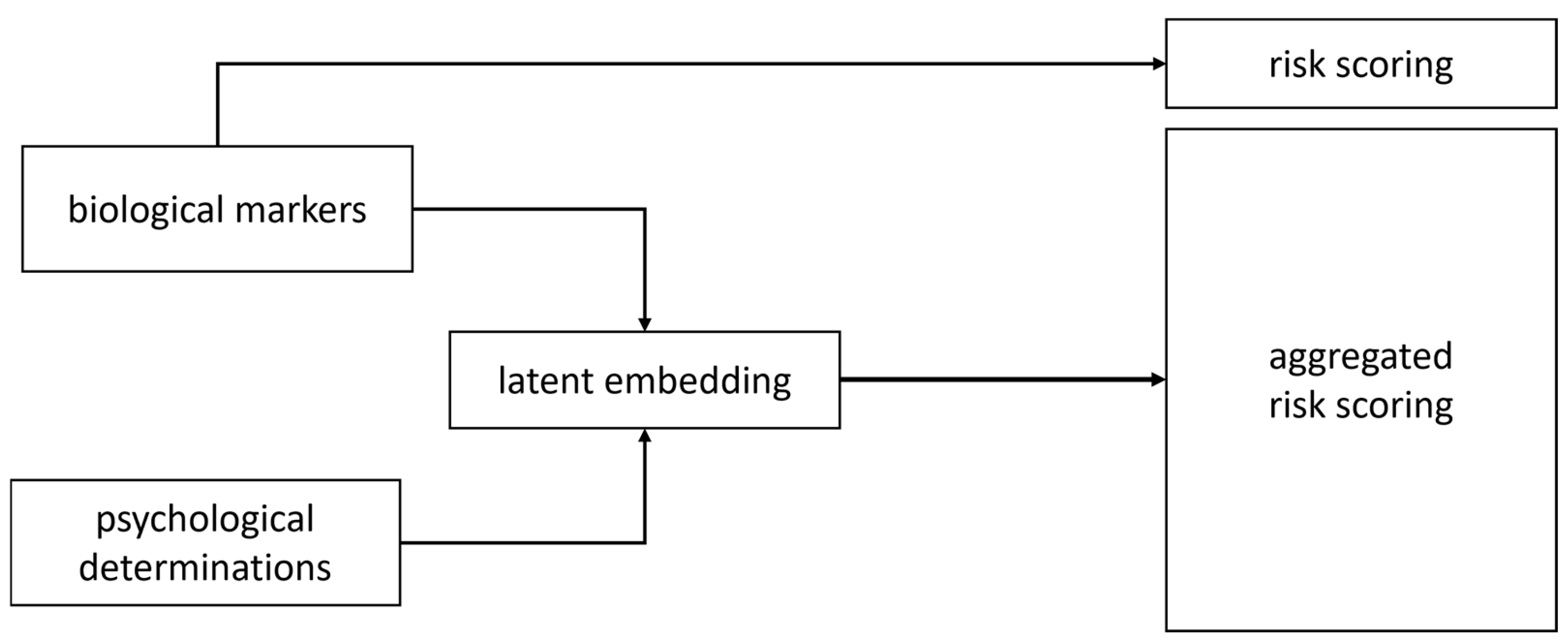
2.5.1. A Mathematical Simple Expert Convergent Model for Further AI-Assisted Multimodal Cancer Screening Platforms
2.5.2. Clarification Note (Methodological Position Statement)
3. Conclusions and Future Directions
- Early risk detection: Identifying latent vulnerabilities before clinical manifestation increases opportunities for preventive intervention.
- Personalized screening: Risk profiles integrating biomarkers and psychosocial indicators support precision medicine strategies.
- Interdisciplinary integration: By bridging oncology, psychology, and biomedical engineering, the platform fosters a holistic view of cancer prevention.
- Non-invasive digital design: Data can be collected remotely using smartphones, webcams, or simple blood tests, facilitating telemedicine and reducing patient burden.
- Longitudinal monitoring: Regular follow-up enables the dynamic tracking of patient trajectories, linking early detection to proactive management.
- Dataset creation and curation: Large-scale, multimodal, clinically annotated datasets are essential for training and validating robust AI models.
- Longitudinal studies: Prospective cohorts integrating biological and psychosocial data will clarify causal relationships between stress, depression, and oncogenesis.
- Explainability and interpretability: Transparent AI models, employing methods such as SHAP values or counterfactual reasoning, are required for clinician trust and regulatory approval.
- Cross-cultural validation: Since psychological and behavioral markers vary across cultures, generalizability must be tested in diverse populations.
- Clinical workflow integration: Implementation science approaches will be needed to align the platform with healthcare infrastructure, policy, and reimbursement models.
3.1. Ethical and AI Bias Considerations
3.2. Future Outlook
4. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic Hormone |
| AFP | Alpha-Fetoprotein |
| AI | Artificial Intelligence |
| AUC | Area Under the Curve |
| AU | Action Unit (Facial Action Coding System) |
| BRCA1/2 | Breast Cancer Gene 1/2 |
| CEA | Carcinoembryonic Antigen |
| CA-15-3/CA-19-9/CA-125 | Cancer Antigens 15-3, 19-9, 125 |
| CD | Cluster of Differentiation |
| CNN | Convolutional Neural Network |
| CRF | Corticotropin-Releasing Factor |
| CRP | C-Reactive Protein |
| CTL | Cytotoxic T Lymphocyte |
| DL | Deep Learning |
| DNA | Deoxyribonucleic Acid |
| EHR | Electronic Health Record |
| ESR | Erythrocyte Sedimentation Rate |
| FACS | Facial Action Coding System |
| FER | Facial Emotion Recognition (Dataset) |
| GGT | Gamma-Glutamyl Transferase |
| GM | Genetic Marker |
| HADS | Hospital Anxiety and Depression Scale |
| HIPAA | Health Insurance Portability and Accountability Act |
| HPA Axis | Hypothalamic–Pituitary–Adrenal Axis |
| HR | Hazard Ratio |
| HuBERT | Hidden-Unit BERT (Speech Model) |
| ICD-10 | International Classification of Diseases, 10th Revision |
| IL-6 | Interleukin-6 |
| IM | Inflammatory Marker |
| KNN | k-Nearest Neighbors |
| LDH | Lactate Dehydrogenase |
| LMR | Lymphocyte-to-Monocyte Ratio |
| LSTM | Long Short-Term Memory Network |
| MADRS | Montgomery–Åsberg Depression Rating Scale |
| MEN1/MEN2A | Multiple Endocrine Neoplasia Type 1/Type 2A |
| MFCC | Mel-Frequency Cepstral Coefficients |
| ML | Machine Learning |
| MLP | Multilayer Perceptron |
| MRI | Magnetic Resonance Imaging |
| NLP | Natural Language Processing |
| NK Cells | Natural Killer Cells |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| PSS | Perceived Stress Scale |
| PT | Psychological Test |
| PSA | Prostate-Specific Antigen |
| RF | Random Forest |
| RNA | Ribonucleic Acid |
| ROC | Receiver Operating Characteristic |
| ROS | Reactive Oxygen Species |
| RNN | Recurrent Neural Network |
| SVM | Support Vector Machine |
| STAI | State–Trait Anxiety Inventory |
| SIINI | Systemic Immune–Inflammatory–Nutritional Index |
| SN | Substantia Nigra |
| TNF-α | Tumor Necrosis Factor Alpha |
| TAA | Tumor-Associated Antigen |
| TSA | Tumor-Specific Antigen |
| TM | Tumor Marker |
| ViT | Vision Transformer |
| ViViT | Video Vision Transformer |
| VTA | Ventral Tegmental Area |
| WHO | World Health Organization |
| wav2vec 2.0 | Self-supervised Speech Representation Model |
| XGBoost | Extreme Gradient Boosting |
References
- International Agency for Research on Cancer. Romania—Global Cancer Observatory; 2018. Available online: https://gco.iarc.fr/ (accessed on 16 August 2025).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.fr/today/ (accessed on 21 May 2024).
- Marino, P.; Mininni, M.; Deiana, G.; Marino, G.; Divella, R.; Bochicchio, I.; Giuliano, A.; Lapadula, S.; Lettini, A.; Sanseverino, F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients 2024, 16, 800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor Initiation and Early Tumorigenesis: Molecular Mechanisms and Interventional Targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef]
- Li, Y.; Lou, J.; Hong, S.; Hou, D.; Lv, Y.; Guo, Z.; Wang, K.; Xu, Y.; Zhai, Y.; Liu, H. The Role of Heavy Metals in the Development of Colorectal Cancer. BMC Cancer 2023, 23, 616. [Google Scholar] [CrossRef]
- Reiche, E.M.; Morimoto, H.K.; Nunes, S.M. Stress and Depression-Induced Immune Dysfunction: Implications for the Development and Progression of Cancer. Int. Rev. Psychiatry 2005, 17, 515–527. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Yu, K.; Xu, S.; Qiu, P.; Lyu, Z.; Zhang, X.; Xu, Y. Chronic Stress-Induced Immune Dysregulation in Breast Cancer: Implications of Psychosocial Factors. J. Transl. Intern. Med. 2022, 11, 226–233. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Global Cancer Observatory: Cancer Tomorrow (GLOBOCAN 2022 Update). Available online: https://gco.iarc.fr/tomorrow/home (accessed on 9 November 2025).
- European Commission Joint Research Centre (JRC). European Cancer Information System (ECIS): Cancer Incidence and Mortality in Europe, 2020–2022.ECIS Data Release 2023. Available online: https://ecis.jrc.ec.europa.eu (accessed on 9 November 2025).
- Decker, K.M.; Feely, A.; Ratnayake, I.; Bucher, O.; Czaykowski, P.; Galloway, K.; Lambert, P. Measuring the Association between the COVID-19 Pandemic and Cancer Incidence by Sex Using a Quasi-Experimental Study Design. JCO Clin. Cancer Inform. 2025, 9, e2400327. [Google Scholar] [CrossRef]
- Khader, H.; Zeilani, R.; Alloubani, A. Impact of COVID-19 on Anxiety and Depression among Cancer Patients in Jordan. Soc. Sci. Humanit. Open 2025, 12, 101855. [Google Scholar] [CrossRef]
- Kemp, E.; Trigg, J.; Beatty, L.; Dhillon, H.M.; Williams, P.A.H.; Koczwara, B. Priorities for Implementation of Digital Health in Cancer Care: A Qualitative Study of the Perspectives of Australian People Affected by Cancer, Health Professionals, Researchers, Developers, Non-Government Organization and Government Representatives. Cancer Surviv. Res. Care 2024, 2, 1. [Google Scholar] [CrossRef]
- Lazarou, I.; Krooupa, A.-M.; Nikolopoulos, S.; Apostolidis, L.; Sarris, N.; Papadopoulos, S.; Kompatsiaris, I. Cancer Patients’ Perspectives and Requirements of Digital Health Technologies: A Scoping Literature Review. Cancers 2024, 16, 2293. [Google Scholar] [CrossRef] [PubMed]
- Meyskens, F.L., Jr.; Mukhtar, H.; Rock, C.L.; Cuzick, J.; Kensler, T.W.; Yang, C.S.; Ramsey, S.D.; Lippman, S.M.; Alberts, D.S. Cancer Prevention: Obstacles, Challenges and the Road Ahead. J. Natl. Cancer Inst. 2015, 108, djv309. [Google Scholar] [CrossRef] [PubMed]
- Ademola, A.; George, C.; Mapp, G. Addressing the Interoperability of Electronic Health Records: The Technical and Semantic Interoperability, Preserving Privacy and Security Framework. Appl. Syst. Innov. 2024, 7, 116. [Google Scholar] [CrossRef]
- Post, A.R.; Burningham, Z.; Halwani, A.S. Electronic Health Record Data in Cancer Learning Health Systems: Challenges and Opportunities. JCO Clin. Cancer Inform. 2022, 6, e2100158. [Google Scholar] [CrossRef]
- Ge, H.; Li, L.; Zhang, D.; Ma, F. Applications of Digital Medicine in Oncology: Prospects and Challenges. Cancer Innov. 2022, 1, 285–292. [Google Scholar] [CrossRef]
- Casagni, M.; Llewellyn, N.; Kokolus, M.; Chan, M.; Dingwell, R.; Chow, S.; Quina, A. Integrated Electronic Health Record Tools to Access Real-World Data in Oncology Research. JAMIA Open 2024, 7, ooae144. [Google Scholar] [CrossRef]
- Ejezie, C.L.; Sacca, L.; Ayieko, S.; Burgoa, S.; Zerrouki, Y.; Lobaina, D.; Okwaraji, G.; Markham, C. Use of Digital Health Interventions for Cancer Prevention Among People Living With Disabilities in the United States: A Scoping Review. Cancer Med. 2025, 14, e70571. [Google Scholar] [CrossRef]
- Santamato, V.; Tricase, C.; Faccilongo, N.; Iacoviello, M.; Marengo, A. Exploring the Impact of Artificial Intelligence on Healthcare Management: A Combined Systematic Review and Machine-Learning Approach. Appl. Sci. 2024, 14, 10144. [Google Scholar] [CrossRef]
- Almyranti, M.; Sutherland, E.; Ash, D.N.; Eiszele, S. Artificial Intelligence and the Health Workforce: Perspectives from Medical Associations on AI in Health. OECD Artificial Intelligence Papers; OECD Publishing: Paris, France, 2024; Volume 28. [Google Scholar] [CrossRef]
- Wells, B.J.; Nguyen, H.M.; McWilliams, A.; Pallini, M.; Bovi, A.; Kuzma, A.; Kramer, J.; Chou, S.-H.; Hetherington, T.; Corn, P.; et al. A Practical Framework for Appropriate Implementation and Review of Artificial Intelligence (FAIR-AI) in Healthcare. NPJ Digit. Med. 2025, 8, 514. [Google Scholar] [CrossRef]
- Basch, E.; Snyder, C. Overcoming Barriers to Integrating Patient-Reported Outcomes in Clinical Practice and Electronic Health Records. Ann. Oncol. 2017, 28, 2332–2333. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef]
- Antoni, M.H.; Lutgendorf, S.K.; Cole, S.W.; Dhabhar, F.S.; Sephton, S.E.; McDonald, P.G.; Stefanek, M.; Sood, A.K. The Influence of Bio-Behavioural Factors on Tumour Biology: Pathways and Mechanisms. Nat. Rev. Cancer 2006, 6, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.M.; Vaidya, R.; Hershman, D.L.; Minasian, L.M.; Fleury, M.E. Systematic Review and Meta-Analysis of the Magnitude of Structural, Clinical, and Physician and Patient Barriers to Cancer Clinical Trial Participation. J. Natl. Cancer Inst. 2019, 111, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Liu, Z.; Molinari, M. The Role of Social Determinants in Cancer Care. Cancers 2025, 17, 1067. [Google Scholar] [CrossRef] [PubMed]
- Sedani, A.E.; Gomez, S.L.; Lawrence, W.R.; Moore, J.X.; Brandt, H.M.; Rogers, C.R. Social Risks and Nonadherence to Recommended Cancer Screening Among US Adults. JAMA Netw. Open 2025, 8, e2449556. [Google Scholar] [CrossRef]
- Weaver, K.E.; Geiger, A.M.; Lu, L.; Case, L.D. Rural–Urban Disparities in Health Status among U.S. Cancer Survivors. Cancer 2013, 119, 1050–1057. [Google Scholar] [CrossRef]
- El-Kenawi, A.; Ruffell, B. Inflammation, ROS, and mutagenesis. Cancer Cell 2017, 32, 727–729. [Google Scholar] [CrossRef]
- Weinberg, R.A. The Biology of Cancer, 2nd ed.; W.W. Norton & Company: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Butterworth, A.S.; Higgins, J.P.T.; Pharoah, P. Relative and Absolute Risk of Colorectal Cancer for Individuals with a Family History: A Meta-Analysis. Eur. J. Cancer 2006, 42, 216–227. [Google Scholar] [CrossRef]
- Anghel, R. Oncologie Generală; Editura Universității Carol Davila: București, Romania, 2019; pp. 22–23. [Google Scholar]
- Anghel, R.; Blidaru, A. Oncologie Generală; Editura Universității Carol Davila: București, Romania, 2007; pp. 69–78. [Google Scholar]
- Bubulac, L. Causal Links Between Psychological Imbalances and the Appearance of Diseases; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2025. [Google Scholar]
- Harsanyi, S.; Kupcova, I.; Danisovic, L.; Klein, M. Selected biomarkers of depression: What are the effects of cytokines and inflammation? Int. J. Mol. Sci. 2023, 24, 578. [Google Scholar] [CrossRef]
- Harris, T.; Ferrucci, L.; Tracy, R.; Corti, M.C.; Wacholder, S.; Ettinger, W.J.; Heimovitz, H.; Cohen, H.J.; Wallace, R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999, 106, 506–512. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Esterling, B.A.; Kiecolt-Glaser, J.K.; Glaser, R. Psychosocial Modulation of Cytokine-Induced Natural Killer Cell Activity in Older Adults. Psychosom. Med. 1996, 58, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Moulton, C.D.; Malys, M.; Hopkins, C.W.P.; Rokakis, A.S.; Young, A.H.; Powell, N. Activation of the interleukin-23/Th17 axis in major depression: A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 275, 1653–1673. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Glaser, R. Stress and immunity: Age enhances the risks. Curr. Dir. Psychol. Sci. 2001, 10, 18–21. [Google Scholar] [CrossRef]
- Polsky, L.R.; Rentscher, K.E.; Carroll, J.E. Stress-induced biological aging: A review and guide for research priorities. Brain Behav. Immun. 2022, 104, 97–109. [Google Scholar] [CrossRef]
- Hawkley, L.C.; Cacioppo, J.T. Stress and the aging immune system. Brain Behav. Immun. 2004, 18, 114–119. [Google Scholar] [CrossRef]
- Parham, P. The Immune System, 2nd ed.; Garland Science: New York, NY, USA, 2005. [Google Scholar]
- Liang, Z.; Dong, X.; Zhang, Z.; Zhang, Q.; Zhao, Y. Age-related thymic involution: Mechanisms and functional impact. Aging Cell 2022, 21, e13671. [Google Scholar] [CrossRef]
- Lord, J.M.; Butcher, S.; Killampali, V.; Lascelles, D.; Salmon, M. Neutrophil ageing and immunosenescence. Mech. Ageing Dev. 2001, 122, 1521–1535. [Google Scholar] [CrossRef]
- Miller, R.A. The aging immune system: Primer and prospectus. Science 1996, 273, 70–74. [Google Scholar] [CrossRef]
- Quiros-Roldan, E.; Sottini, A.; Natali, P.G.; Imberti, L. The impact of immune system aging on infectious diseases. Microorganisms 2024, 12, 775. [Google Scholar] [CrossRef]
- Heffner, K.L.; Loving, T.J.; Robles, T.F.; Kiecolt-Glaser, J.K. Examining psychosocial factors related to cancer incidence and progression: In search of the silver lining. Brain Behav. Immun. 2003, 17, S109–S111. [Google Scholar] [CrossRef]
- Keller, S.E.; Schleifer, S.J.; Bartlett, J.A.; Shiflett, S.C.; Rameshwar, P. Stress, depression, immunity, and health. In Psychoneuroimmunology: Stress, Mental Disorders, and Health; Goodkin, K., Visser, A.P., Eds.; American Psychiatric Press: Washington, DC, USA, 2000; pp. 1–25. [Google Scholar]
- Andersen, B.L.; Kiecolt-Glaser, J.K.; Glaser, R. A biobehavioral model of cancer stress and disease course. Am. Psychol. 1994, 49, 389–404. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, Z.; Zhang, C.; Zhang, Q.; Liu, Z.; Cheng, Q.; Zhang, J.; Lin, A.; Luo, P. NK cell senescence in cancer: From molecular mechanisms to therapeutic opportunities. Aging Dis. 2025. [Google Scholar] [CrossRef]
- Bondar, T.; Medzhitov, R. The origins of tumor-promoting inflammation. Cancer Cell 2013, 24, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Niraula, A.; Wang, Y.; Godbout, J.P.; Sheridan, J.F. Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance, and neurovascular adhesion molecule expression. J. Neurosci. 2018, 38, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Ader, R.; Felten, D.L.; Cohen, N. Psychoneuroimmunology: Interactions between the nervous system and the immune system. Lancet 1995, 345, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Ader, R.; Cohen, N.; Felten, D.L. Psychoneuroimmunology: Conditioning and stress. Annu. Rev. Psychol. 1993, 44, 53–85. [Google Scholar] [CrossRef]
- Lempesis, I.G.; Georgakopoulou, V.E.; Papalexis, P.; Chrousos, G.P.; Spandidos, D.A. Role of Stress in the Pathogenesis of Cancer (Review). Int. J. Oncol. 2023, 63, 124. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Li, Q. Mechanisms Underlying the Effects of Stress on Tumorigenesis and Metastasis. Int. J. Oncol. 2018, 53, 2332–2342. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, S.; Ning, B.; Huang, T.; Li, Y.; Wei, Y. Stress and Cancer: The Mechanisms of Immune Dysregulation and Management. Front. Immunol. 2022, 13, 1032294. [Google Scholar] [CrossRef]
- Kanter, N.G.; Cohen-Woods, S.; Balfour, D.A.; Burt, M.G.; Waterman, A.L.; Koczwara, B. Hypothalamic–pituitary–adrenal axis dysfunction in people with cancer: A systematic review. Cancer Med. 2024, 13, e70366. [Google Scholar] [CrossRef]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic stress promotes cancer development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef] [PubMed]
- Nihira, N.T.; Kudo, R.; Ohta, T. Inflammation and tumor immune escape in response to DNA damage. Semin. Cancer Biol. 2025, 110, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pan, J.; Chen, W.; Jiang, J.; Huang, J. Chronic stress-induced immune dysregulation in cancer: Implications for initiation, progression, metastasis, and treatment. Am. J. Cancer Res. 2020, 10, 1294–1307. [Google Scholar] [PubMed]
- Krizanova, O.; Babula, P.; Pacak, K. Stress, the catecholaminergic system, and cancer. Stress 2016, 19, 419–428. [Google Scholar] [CrossRef]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interaction between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Stephens, R.E.; Lipetz, P.D.; Speicher, C.E.; Glaser, R. Distress and DNA repair in human lymphocytes. J. Behav. Med. 1985, 8, 311–320. [Google Scholar] [CrossRef]
- Lei, Y.; Liao, F.; Tian, Y.; Wang, Y.; Xia, F.; Wang, J. Investigating the crosstalk between chronic stress and immune cells: Implications for enhanced cancer therapy. Front. Neurosci. 2023, 17, 1321176. [Google Scholar] [CrossRef]
- Khan, A.; Song, M.; Dong, Z. Chronic stress: A fourth etiology in tumorigenesis? Mol. Cancer 2025, 24, 196. [Google Scholar] [CrossRef]
- Curtin, N.M.; Boyle, N.T.; Mills, K.H.; Connor, T.J. Psychological stress suppresses innate IFN-γ production via glucocorticoid receptor activation: Reversal by the anxiolytic chlordiazepoxide. Brain Behav. Immun. 2009, 23, 535–547. [Google Scholar] [CrossRef]
- Baum, A.; Cohen, L.; Hall, M. Control and intrusive memories as possible determinants of chronic stress. Psychosom. Med. 1993, 55, 274–286. [Google Scholar] [CrossRef]
- Frankenhauser, M. Experimental approaches to the study of catecholamines. In Emotions—Their Parameters and Measurement; Levi, L., Ed.; Raven Press: New York, NY, USA, 1975; pp. 209–234. [Google Scholar]
- Lillberg, K.; Verkasalo, P.K.; Kaprio, J.; Teppo, L.; Helenius, H.; Koskenvuo, M. Stressful life events and risk of breast cancer in 10,808 women: A cohort study. Am. J. Epidemiol. 2003, 157, 415–423. [Google Scholar] [CrossRef]
- Sridhar, A.; Sekhon, V.K.; Nguyen, C.; Abushalha, K.; Tahanan, A.; Rahbar, M.H.; Jafri, S.H. Major stressful life events and the risk of pancreatic, head and neck cancers: A case–control study. Cancers 2024, 16, 451. [Google Scholar] [CrossRef]
- Bergelt, C.; Prescott, E.; Gronbak, M.; Koch, U.; Johansen, C. Stressful life events and cancer risk. Br. J. Cancer 2006, 95, 1579–1581. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Pfaltz, M.C.; Schnyder, U. Allostatic load and allostatic overload: Preventive and clinical implications. Psychother. Psychosom. 2023, 92, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Gupta, S.; Soerjomataram, I.; Ferlay, J.; Steliarova-Foucher, E.; Bray, F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: A population-based study. Lancet Oncol. 2017, 18, 1579–1589. [Google Scholar] [CrossRef]
- Heller, E.A.; Hamilton, P.J.; Burek, D.D.; Lombroso, S.I.; Pena, C.J.; Neve, R.L.; Nestler, E.J. Targeted epigenetic remodeling of the Cdk5 gene in nucleus accumbens regulates cocaine- and stress-evoked behavior. Nat. Neurosci. 2016, 19, 4690–4697. [Google Scholar] [CrossRef]
- Patel, V.; Saxena, S.; Lund, C.; Thornicroft, G.; Baingana, F.; Bolton, P.; Chisholm, D.; Collins, P.Y.; Cooper, J.L.; Eaton, J.; et al. The Lancet Commission on global mental health and sustainable development. Lancet 2018, 392, 1553–1598. [Google Scholar] [CrossRef]
- Martinez, M.E.; Schmeler, K.M.; Lajous, M.; Newman, L.A. Cancer screening in low- and middle-income countries. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e431272. [Google Scholar] [CrossRef]
- Paquet, A.; Lacroix, A.; Calvet, B.; Girard, M. Psychomotor Semiology in Depression: A Standardized Clinical Psychomotor Approach. BMC Psychiatry 2022, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Buyukdura, J.S.; McClintock, S.M.; Croarkin, P.E. Psychomotor Retardation in Depression: Biological Underpinnings, Measurement, and Treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K. Depression May Increase Cancer Risk. Lancet Oncol. 2003, 4, 390. [Google Scholar] [CrossRef]
- Huang, T.; Poole, E.M.; Okereke, O.I.; Kubzansky, L.D.; Eliassen, A.H.; Sood, A.K.; Wang, M.; Tworoger, S.S. Depression and risk of epithelial ovarian cancer: Results from two large prospective cohort studies. Gynecol. Oncol. 2015, 139, 481–486. [Google Scholar] [CrossRef]
- Hu, J. Stress-induced metastasis: The NET effect. Cancer Cell 2024, 42, 335–337. [Google Scholar] [CrossRef]
- Spiegel, D.; Giese-Davis, J. Depression and cancer: Mechanisms and disease progression. Biol. Psychiatry 2003, 54, 269–282. [Google Scholar] [CrossRef]
- Jia, Y.; Li, F.; Liu, Y.F.; Zhao, J.P.; Leng, M.M.; Chen, L. Depression and cancer risk: A systematic review and meta-analysis. Public Health 2017, 149, 138–148. [Google Scholar] [CrossRef]
- Roy, V.; Ruel, S.; Ivers, H.; Savard, M.H.; Gouin, J.P.; Caplette-Gingras, A.; Lemieux, J.; Couture, F.; Savard, J. Stress-buffering effect of social support on immunity and infectious risk during chemotherapy for breast cancer. Brain Behav. Immun. Health 2020, 10, 100186. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy mechanism in human agency. Am. Psychol. 1982, 37, 122–147. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy. In Encyclopedia of Human Behavior; Ramachaudran, V.S., Ed.; Academic Press: New York, NY, USA, 1994; Volume 4, pp. 71–81, reprinted in Encyclopedia of Mental Health; Friedman, H., Ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Carver, C.S.; Scheier, M.F.; Segerstrom, S.C. Optimism. Clin. Psychol. Rev. 2010, 30, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Kobasa, S.C. Stressful life events, personality, and health: An inquiry into hardiness. J. Pers. Soc. Psychol. 1979, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Temoshok, L.R.; Wald, R.L. Change Is Complex: Rethinking Research on Psychosocial Interventions and Cancer. Integr. Cancer Ther. 2002, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Nemiah, J.C.; Sifneos, P.E. Affects and Fantasy in Patients with Psychosomatic Disorders. In Modern Trends in Psychosomatic Medicine; Hill, O., Ed.; Butterworths: London, UK, 1970. [Google Scholar]
- Contrada, R.J.; Czarnecki, E.M.; Pan, R.L.-C. Health-Damaging Personality Traits and Verbal–Autonomic Dissociation: The Role of Self-Control and Environmental Control. Health Psychol. 1997, 16, 451–457. [Google Scholar] [CrossRef]
- Madani, H.; Pourmemari, M.; Moghimi, M.; Rashvand, F. Hopelessness, perceived social support and their relationship in Iranian patients with cancer. Asia-Pac. J. Oncol. Nurs. 2018, 5, 314–319. [Google Scholar] [CrossRef]
- Penton-Voak, I.S.; Wezowski, K. Relationship between low mood and micro-expression processing: Evidence of negative bias in interpreting fleeting facial expressions. J. Affect. Disord. 2025, 300, 131–138. [Google Scholar] [CrossRef]
- Shah, J.; Lin, M.; Schmuter, G.; Kovacs, K.D.; Godfrey, K.J. Association of Ptosis with Mental Health Conditions in Adults from a Large United States Research Database. Complex Psychiatry 2025, 11, 94–98. [Google Scholar] [CrossRef]
- Lee, Y.T.; Chang, Y.H.; Barquero, C.; Wu, C.S.; Chao, S.P.; Chen, D.Y.; Chen, J.T.; Cherng, Y.G.; Wang, C.A. Pupil and eye blink response abnormalities during emotional conflict processing in late-life depression. J. Geriatr. Psychiatry Neurol. 2025, 38, 378–393. [Google Scholar] [CrossRef]
- Ekman, P.; Friesen, W.V. Facial Action Coding System: A Technique for the Measurement of Facial Movement; Consulting Psychologists Press: Palo Alto, CA, USA, 1978. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.H.; Liang, J.; Yan, W.J. The dynamic features of lip corners in genuine and posed smiles. Front. Psychol. 2018, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Ekman, P.; Rosenberg, E.L. What the Face Reveals: Basic and Applied Studies of Spontaneous Expression Using the Facial Action Coding System; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Hidalgo Julia, N.; Lewis, R.; Ferguson, C.; Goldberg, S.; Lau, W.; Swords, C.; Valdivia, G.; Wilson-Mendenhall, C.; Tartar, R.; Picard, R.; et al. Identifying Vocal and Facial Biomarkers of Depression in Large-Scale Remote Recordings: A Multimodal Study Using Mixed-Effects Modeling. In Proceedings of the Interspeech, Rotterdam, The Netherlands, 17–21 August 2025; Volume 2025, pp. 5263–5267. [Google Scholar] [CrossRef]
- Penton-Voak, I.S.; Wezowski, K. Relationship between low mood and micro-expression processing: Evidence of negative bias in interpreting fleeting facial expressions. R. Soc. Open Sci. 2024, 11, 231944. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kwak, S.; Yoo, S.S.; Lee, E.C.; Park, S.; Ko, H.; Bae, M.; Seo, M.; Nam, G.; Lee, J.-Y. Facial Expressions Track Depressive Symptoms in Old Age. Sensors 2023, 23, 7080. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Tao, Y.; Martinez, A.M. Compound Facial Expressions of Emotion. Proc. Natl. Acad. Sci. USA 2014, 111, E1454–E1462. [Google Scholar] [CrossRef]
- Bomfim, A.J.L.; Ribeiro, R.A.D.S.; Chagas, M.H.N. Recognition of Dynamic and Static Facial Expressions of Emotion among Older Adults with Major Depression. Trends Psychiatry Psychother. 2019, 41, 159–166. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Xu, R.; Wei, X.; Zhang, J. Supporting the Development of Contactless Mental Health Assessment: An Explorations of the Relationships Between Gaze Patterns, Depression, Anxiety, and Insomnia. In Human Aspects of IT for the Aged Population. HCII 2025. Lecture Notes in Computer Science; Gao, Q., Zhou, J., Eds.; Springer: Cham, Switzerland, 2025; Volume 15809, p. 27. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, L.N. Are the dorsal and ventral hippocampus functionally distinct? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Edwards, L.S.; Ganesan, S.; Tay, J.; Elliott, E.S.; Misaki, M.; White, E.J.; Paulus, M.P.; Guinjoan, S.M.; Tsuchiyagaito, A. Increased Insular Functional Connectivity during Repetitive Negative Thinking in Major Depression and Healthy Volunteers. Psychol. Med. 2025, 55, e268. [Google Scholar] [CrossRef]
- Parent, A.; Hazrati, L.N. Functional Anatomy of the Basal Ganglia. I. The Cortico-Basal Ganglia-Thalamo-Cortical Loop. Brain Res. Brain Res. Rev. 1995, 20, 91–127. [Google Scholar] [CrossRef]
- Schrijvers, D.; Hulstijn, W.; Sabbe, B.G. Psychomotor Symptoms in Depression: A Diagnostic, Pathophysiological and Therapeutic Tool. J. Affect. Disord. 2008, 109, 1–20. [Google Scholar] [CrossRef]
- Goldman-Rakic, P.S. Regional and cellular fractionation of working memory in the primate prefrontal cortex. Proc. Natl. Acad. Sci. USA 1996, 93, 13473–13480. [Google Scholar] [CrossRef]
- Malhi, G.S.; Berk, M. Does dopamine dysfunction drive depression? ActaPsychiatr. Scand. 2007, 115, 116–124. [Google Scholar] [CrossRef]
- Little, B.; Alshabrawy, O.; Stow, D.; Ferrier, I.N.; McNaney, R.; Jackson, D.G.; Ladha, K.; Ladha, C.; Ploetz, T.; Bacardit, J.; et al. Deep Learning-Based Automated Speech Detection as a Marker of Social Functioning in Late-Life Depression. Psychol. Med. 2021, 51, 1441–1450. [Google Scholar] [CrossRef]
- Sanchez, M.H.; Vergyri, D.; Ferrer, L.; Richey, C.; Garcia, P.; Knoth, B.; Jarrold, W. Using Prosodic and Spectral Features in Detecting Depression in Elderly Males. In Proceedings of the Interspeech, Florence, Italy, 27–31 August 2011; pp. 3001–3004. [Google Scholar]
- Mundt, J.C.; Snyder, P.J.; Cannizzaro, M.S.; Chappie, K.; Geralts, D.S. Voice acoustic measures of depression severity and treatment response collected via interactive voice response (IVR) technology. J. Neurolinguistics 2007, 20, 50–64. [Google Scholar] [CrossRef]
- Cummins, N.; Scherer, S.; Krajewski, J.; Schnieder, S.; Epps, J.; Quatieri, T.F. A review of depression and suicide risk assessment using speech analysis. Speech Commun. 2015, 71, 10–49. [Google Scholar] [CrossRef]
- Shen, P.; Changjun, Z.; Chen, X. Automatic Speech Emotion Recognition Using Support Vector Machine. In Proceedings of the 2011 International Conference on Electronic & Mechanical Engineering and Information Technology, Harbin, China, 12–14 August 2011; pp. 621–625. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Steinholtz, L.; Persson, J.; Thörnblom, E.; Wall, A.; Lubberink, M.; Fällmar, D.; Bodén, R. Inhibitory Neurotransmission in Frontostriatal Circuitry: Implications for Psychomotor and Vegetative Symptoms in Depressive Episodes. arXiv 2025. [Google Scholar] [CrossRef]
- Miller, A.H. Advancing an Inflammatory Subtype of Major Depression. Am. J. Psychiatry 2025, 182, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Topa, K.; Haluska-Vass, E.; Simon, L. Movement Analysis in the Diagnosis of Depression and Schizophrenia: A Systematic Review. Acta Psychol. 2025, 259, 105161. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.; Schreiber, S.; Pick, C.G.; Been, E. Gait, Balance, Mobility and Muscle Strength in People with Anxiety Compared to Healthy Individuals. Hum. Mov. Sci. 2019, 67, 102513. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, B.; Wen, Z.; Zhang, Y. From Healing and Martial Roots to Global Health Practice: Reimagining Tai Chi (Taijiquan) in the Modern Public Fitness Movement. Front. Public Health 2025, 13, 1677470. [Google Scholar] [CrossRef]
- Parida, S.; Kumar, A.; Verma, A.; Krishna, A.; Singh, V.K.; Pathak, A.; Joshi, D. White Matter Correlates of Gait and Balance Dysfunction in Essential Tremor Patients. J. Clin. Neurosci. 2024, 130, 110920. [Google Scholar] [CrossRef] [PubMed]
- BelvederiMurri, M.; Triolo, F.; Coni, A.; Tacconi, C.; Nerozzi, E.; Escelsior, A.; Zanetidou, S.; Amore, M. Instrumental Assessment of Balance and Gait in Depression: A Systematic Review. Psychiatry Res. 2020, 284, 112687. [Google Scholar] [CrossRef] [PubMed]
- Pranzatelli, M.R.; Mott, S.H.; Pavlakis, S.G.; Conry, J.A. Clinical spectrum of secondary parkinsonism in childhood: A reversible disorder. Pediatr. Neurol. 1994, 10, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Lemke, M.R.; Wendorff, T.; Mieth, B.; Buhl, K.; Linnemann, M. Spatiotemporal Gait Patterns during Over Ground Locomotion in Major Depression Compared with Healthy Controls. J. Psychiatr. Res. 2000, 34, 277–283. [Google Scholar] [CrossRef]
- Bennabi, D.; Vandel, P.; Papaxanthis, C.; Pozzo, T.; Haffen, E. Psychomotor retardation in depression: A systematic review of diagnostic, pathophysiologic, and therapeutic implications. Biomed. Res. Int. 2013, 2013, 158746. [Google Scholar] [CrossRef]
- Martinot, M.; Bragulat, V.; Artiges, E.; Dollé, F.; Hinnen, F.; Jouvent, R.; Martinot, J. Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am. J. Psychiatry 2001, 158, 314–316. [Google Scholar] [CrossRef]
- Van Cauwenberge, M.G.A.; Bouckaert, F.; Vansteelandt, K.; Adamson, C.; De Winter, F.L.; Sienaert, P.; Van den Stock, J.; Dols, A.; Rhebergen, D.; Stek, M.L.; et al. A longitudinal study of the association between basal ganglia volumes and psychomotor symptoms in subjects with late-life depression undergoing ECT. Transl. Psychiatry 2021, 11, 199. [Google Scholar] [CrossRef]
- Rubino, F.A. Gait Disorders. Neurologist 2002, 8, 254–262. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, P.; He, J.; Zhang, G.; Zhou, W.; Chen, Q. Machine Learning Algorithms Predict Breast Cancer Incidence Risk: A Data-Driven Retrospective Study Based on Biochemical Biomarkers. BMC Cancer 2025, 25, 1061. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Chen, C.-H.; Shi, S.; Chung, C.-R.; Wen, Y.-H.; Wu, M.-H.; Lebowitz, M.S.; Zhou, J.; Lu, J.-J. Improving Multi-Tumor Biomarker Health Check-Up Tests with Machine Learning Algorithms. Cancers 2020, 12, 1442. [Google Scholar] [CrossRef]
- Yang, S.; Jang, H.; Park, I.K.; Lee, H.S.; Lee, K.Y.; Oh, G.E.; Park, C.; Kang, J. Machine-Learning Algorithms Using Systemic Inflammatory Markers to Predict the Oncologic Outcomes of Colorectal Cancer after Surgery. Ann. Surg. Oncol. 2023, 30, 8717–8726. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Chen, L.; Mao, X.; Wang, X.; Liu, J.; Huang, Y.; Qi, H.; Chen, L.; Shi, H.; et al. SNPs and blood inflammatory marker featured machine learning for predicting the efficacy of fluorouracil-based chemotherapy in colorectal cancer. Sci. Rep. 2024, 14, 27700. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guan, Y.; Yu, H.; Zhang, Y.; Tao, J.; Zhang, W.; Yao, Y. Predictive model using systemic inflammation markers to assess neoadjuvant chemotherapy efficacy in breast cancer. Front. Oncol. 2025, 15, 1552802. [Google Scholar] [CrossRef]
- Fatima, G.; Al-Amran, F.G.; Yousif, M.G. Automated Detection of Persistent Inflammatory Biomarkers in Post-COVID-19 Patients Using Machine Learning Techniques. arXiv 2023, arXiv:2309.15838. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Q.; Sun, F.; Shi, H. Deep Learning and Inflammatory Markers Predict Early Response to Immunotherapy in Unresectable NSCLC: A Multicenter Study. Biomol. Biomed. 2025, 25, 2252–2268. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Huang, J.; Sher, A.F.; Singh, G.; Chen, C.; Prasanna, P. ImmunoDiff: An anatomy-aware diffusion model integrating imaging and clinical biomarkers for immunotherapy response prediction in lung cancer. arXiv 2025, arXiv:2505.23675. [Google Scholar] [CrossRef]
- Gorishniy, Y.; Rubachev, I.; Khrulkov, V.; Babenko, A. Revisiting Deep Learning Models for Tabular Data. Adv. Neural Inf. Process. Syst. 2021, 34, 18932–18943. [Google Scholar]
- Park, J.H.; Chun, M.; Bae, S.H.; Woo, J.; Chon, E.; Kim, H.J. Factors Influencing Psychological Distress among Breast Cancer Survivors Using Machine Learning Techniques. Sci. Rep. 2024, 14, 15052. [Google Scholar] [CrossRef]
- Zeinali, N.; Youn, N.; Albashayreh, A.; Fan, W.; Gilbertson White, S. Machine Learning Approaches to Predict Symptoms in People with Cancer: Systematic Review. JMIR Cancer 2024, 10, e52322. [Google Scholar] [CrossRef]
- Martinez, R.G.; van Dongen, D.M. Deep Learning Algorithms for the Early Detection of Breast Cancer: A Comparative Study with Traditional Machine Learning. Inform. Med. Unlocked 2023, 41, 101317. [Google Scholar] [CrossRef]
- Gao, L.; Cao, Y.; Cao, X.; Shi, X.; Lei, M.; Su, X.; Liu, Y. Machine Learning-Based Algorithms to Predict Severe Psychological Distress among Cancer Patients with Spinal Metastatic Disease. Spine J. 2023, 23, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Yala, A.; Lehman, C.; Schuster, T.; Portnoi, T.; Barzilay, R. A Deep Learning Mammography-Based Model for Improved Breast Cancer Risk Prediction. Radiology 2019, 292, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Parkhi, O.M.; Vedaldi, A.; Zisserman, A. Deep Face Recognition. In Proceedings of the British Machine Vision Conference (BMVC), Swansea, UK, 7–10 September 2015; Volume 1, p. 6. [Google Scholar]
- Barsoum, E.; Zhang, C.; Ferrer, C.C.; Zhang, Z. Training deep networks for facial expression recognition with crowd-sourced label distribution. arXiv 2016, arXiv:1608.01041. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Hasani, B.; Mahoor, M.H. AffectNet: A database for facial expression, valence, and arousal computing in the wild. IEEE Trans. Affect. Comput. 2019, 10, 18–31. [Google Scholar] [CrossRef]
- Baltrušaitis, T.; Robinson, P.; Morency, L.P. OpenFace: An open source facial behavior analysis toolkit. In Proceedings of the 2016 IEEE Winter Conference on Applications of Computer Vision (WACV), Lake Placid, NY, USA, 7–10 March 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–10. [Google Scholar]
- Dosovitskiy, A.; Beyer, L.; Kolesnikov, A.; Weissenborn, D.; Zhai, X.; Unterthiner, T.; Dehghani, M.; Minderer, M.; Heigold, G.; Gelly, S.; et al. An image is worth 16×16 words: Transformers for image recognition at scale. arXiv 2020, arXiv:2010.11929. [Google Scholar]
- Arnab, A.; Dehghani, M.; Heigold, G.; Sun, C.; Lučić, M.; Schmid, C. ViViT: A video vision transformer. arXiv 2021, arXiv:2103.15691. [Google Scholar]
- Li, X.; Yi, X.; Ye, J.; Zheng, Y.; Wang, Q. SFTNet: A microexpression-based method for depression detection. Comput Methods Programs Biomed. 2024, 243, 107923. [Google Scholar] [CrossRef]
- Chen, H.; Yang, L.; Li, T. Catching elusive depression via facial micro-expression recognition. arXiv 2023, arXiv:2307.15862. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, P.; Sharma, R. Action unit-based micro-expression recognition framework for driver emotion detection. Sci. Rep. 2025, 15, 1234. [Google Scholar] [CrossRef]
- Rejaibi, E.; Komaty, A.; Meriaudeau, F.; Agrebi, S.; Othmani, A. MFCC-based recurrent neural network for automatic clinical depression recognition and assessment from speech. Biomed. Signal Process. Control. 2022, 71, 103107. [Google Scholar] [CrossRef]
- Hershey, S.; Chaudhuri, S.; Ellis, D.P.; Gemmeke, J.F.; Jansen, A.; Moore, R.C.; Plakal, M.; Platt, D.; Saurous, R.A.; Seybold, B.; et al. Architectures for Large-Scale Audio Classification. In Proceedings of the 2017 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), New Orleans, LA, USA, 5–9 March 2017; pp. 131–135. [Google Scholar] [CrossRef]
- Gemmeke, J.F.; Ellis, D.P.; Freedman, D.; Jansen, A.; Lawrence, W.; Moore, R.C.; Plakal, M.; Ritter, M. Audio Set: An Ontology and Human-Labeled Dataset for Audio Events. In Proceedings of the 2017 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), New Orleans, LA, USA, 5–9 March 2017; pp. 776–780. [Google Scholar]
- Baevski, A.; Zhou, Y.; Mohamed, A.; Auli, M. wav2vec 2.0: A Framework for Self-Supervised Learning of Speech Representations. Adv. Neural Inf. Process. Syst. 2020, 33, 12449–12460. [Google Scholar]
- Hsu, W.N.; Bolte, B.; Tsai, Y.H.H.; Lakhotia, K.; Salakhutdinov, R.; Mohamed, A. Hubert: Self-Supervised Speech Representation Learning by Masked Prediction of Hidden Units. IEEE/ACM Trans. Audio Speech Lang. Process. 2021, 29, 3451–3460. [Google Scholar] [CrossRef]
- Dong, L.; Xu, S.; Xu, B. Speech-Transformer: A No-Recurrence Sequence-to-Sequence Model for Speech Recognition. In Proceedings of the 2018 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Calgary, AB, Canada, 15–20 April 2018; pp. 5884–5888. [Google Scholar] [CrossRef]
- Menne, F.; Dörr, F.; Schräder, J.; Tröger, J.; Habel, U.; König, A.; Wagels, L. The Voice of Depression: Speech Features as Biomarkers for Major Depressive Disorder. BMC Psychiatry 2024, 24, 794. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhu, Z.; Jing, X. Deep Learning for Depression Recognition from Speech. Mob. Netw. Appl. 2024, 29, 1212–1227. [Google Scholar] [CrossRef]
- Cao, Z.; Simon, T.; Wei, S.-E.; Sheikh, Y. Realtime Multi-Person 2D Pose Estimation Using Part Affinity Fields. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 7291–7299. [Google Scholar]
- Bazarevsky, V.; Grishchenko, I.; Raveendran, K.; Zhu, T.; Zhang, F.; Grundmann, M. BlazePose: On-Device Real-Time Body Pose Tracking. arXiv 2020, arXiv:2006.10204. [Google Scholar] [CrossRef]
- Shotton, J.; Fitzgibbon, A.; Cook, M.; Sharp, T.; Finocchio, M.; Moore, R.; Kipman, A.; Blake, A. Real-time human pose recognition in parts from a single depth image. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Colorado Springs, CO, USA, 20–25 June 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 1297–1304. [Google Scholar]
- Tahouri, M.A.; Alecu, A.A.; Denis, L.; Munteanu, A. Lossless and near-lossless L-infinite compression of depth video data. Sensors 2025, 25, 1403. [Google Scholar] [CrossRef]
- Franco, A.; Russo, M.; Amboni, M.; Ponsiglione, A.M.; Di Filippo, F.; Romano, M.; Ricciardi, C. The Role of Deep Learning and Gait Analysis in Parkinson’s Disease: A Systematic Review. Sensors 2024, 24, 5957. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Wang, M.; Bi, J.; Lin, S.; Wang, Q.; Zheng, Y. Multimodal Depression Recognition and Analysis: Facial Expression and Body Posture Changes via Emotional Stimuli. J. Affect. Disord. 2025, 381, 44–54. [Google Scholar] [CrossRef]
- Alhanai, T.; Ghassemi, M.; Glass, J. Detecting Depression with Audio/Text Sequence Modeling of Interviews. In Proceedings of the Interspeech, Hyderabad, India, 2–6 September 2018; pp. 1716–1720. [Google Scholar]
- Abbate, S.; Avvenuti, M.; Corsini, P.; Light, J.; Vecchio, A. Monitoring of Human Movements for Fall Detection and Activities Recognition in Elderly Care Using Wireless Sensor Network: A Survey. In Wireless Sensor Networks: Application—Centric Design; IntechOpen Limited: London, UK, 2010. [Google Scholar] [CrossRef]
- Zheng, C.; Zhu, S.; Mendieta, M.; Yang, T.; Chen, C.; Ding, Z. 3D Human Pose Estimation with Spatial and Temporal Transformers. In Proceedings of the IEEE/CVF International Conference on Computer Vision (ICCV), Montreal, QC, Canada, 10–17 October 2021; pp. 11656–11665. [Google Scholar]
- Bertasius, G.; Wang, H.; Torresani, L. Is Space-Time Attention All You Need for Video Understanding? arXiv 2021, arXiv:2102.05095. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, J. Spatiotemporal Decoupling Attention Transformer for 3D Skeleton-Based Driver Action Recognition. Complex Intell. Syst. 2025, 11, 195. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Yan, W.J.; Li, X.; Wang, S.J.; Zhao, G.; Liu, Y.J.; Chen, Y.H.; Fu, X. CASME II: An Improved Spontaneous Micro-Expression Database and the Baseline Evaluation. PLoS ONE 2014, 9, e86041. [Google Scholar] [CrossRef]
- Davison, A.K.; Lansley, C.; Costen, N.; Tan, K.; Yap, M.H. SAMM: A Spontaneous Micro-Facial Movement Dataset. IEEE Trans. Affective Comput. 2018, 9, 116–129. [Google Scholar] [CrossRef]
- Georgescu, T.; Cândea, V. Réseaux Télémédecine: La Nouvelle Approche dans les Sciences Médicales - appliqués das la médecine d’urgence. Bul. Inf. AOSR 2009, 13. Available online: https://www.aosr.ro/wp-content/uploads/2021/10/Raport-AOSR-2009.pdf (accessed on 2 November 2025).
- Georgescu, T.; Bobu, A. NET-DD Systems Authors, 2016–2025. Available online: www.net-dd.com (accessed on 10 November 2025).

| Category | Examples | Clinical Relevance | References |
|---|---|---|---|
| Tumor markers | CEA, CA-125, CA-19-9, PSA, AFP, LDH | Early detection, monitoring tumor burden | [33,34,35,36,37,38,39] |
| Genetic markers | BRCA1, BRCA2, RB1, MEN1, MEN2A | Hereditary cancer risk | [38] |
| Inflammatory markers | IL-6, TNF-α, CRP, ESR, fibrinogen | Chronic inflammation, cancer progression | [41,42,43,44,45,46] |
| Domain | Key Features | Tools/Methods | Clinical Relevance | References |
|---|---|---|---|---|
| Stress and anxiety | Perceived stress, state/trait anxiety | PSS, STAI | Links to immunosuppression and oncogenesis | [59,60,61,62,63,64] |
| Depression | Mood, facial expressivity, voice | HADS, MADRS, digital voice/facial analysis | Predictor of cancer outcomes | [82,83,84,85,86,87,88,89,90,91,92,93] |
| Personality traits | Type C, low self-efficacy, low coherence | Freiburg Inventory, Self-Efficacy Scale | Modulate immune function, stress response | [94,95,96,97,98,99,100,101] |
| Modality | Model(s) | Data Type | References |
|---|---|---|---|
| Biomarkers | MLP, Random Forest, XGBoost | Structured tabular | [139,140,141,142,143,144,145,146] |
| Psychometrics | Logistic Regression, MLP, RNN | Questionnaire data | [147,148,149,150,151] |
| Facial Analysis | CNN, ViT, ViViT | Image/video | [152,153,154,155,156,157,158,159,160,161,162] |
| Voice | wav2vec2.0, CNN-RNN hybrids | Audio | [162,163,164,165,166,167,168,169] |
| Gait/Posture | OpenPose, ViViT, LSTM | Video skeletal data | [170,171,172,173,174,175,176,177,178,179,180] |
| TM | GM | IM | PT | Total Score | Risk Index (%) | |
|---|---|---|---|---|---|---|
| Patient 1: TG | 3/19 | 5/24 | 3/17 | 4/6 | 15/66 | X1 |
| Patient 2: AB | 6/19 | 6/24 | 6/17 | 5/6 | 23/66 | X2 |
| Patient 3: SN | 4/19 | 4/24 | 4/17 | 6/6 | 18/66 | X3 |
| Patient 4: CE | 5/19 | 4/24 | 5/17 | 3/6 | 17/66 | X4 |
| TM | GM | IM | PT | Total Score | Risk Index (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Relevance | Score | Relevance | Score | Relevance | Score | Relevance | Score | Relevance | ||
| TG | 3/19 | R1.1 | 5/24 | R1.2 | 3/17 | R1.3 | 4/6 | R1.4 | 15/66 | R1 | Y1 |
| AB | 6/19 | R2.1 | 6/24 | R2.2 | 6/17 | R2.3 | 5/6 | R2.4 | 23/66 | R2 | Y2 |
| SN | 4/19 | R3.1 | 4/24 | R3.2 | 4/17 | R3.3 | 6/6 | R3.4 | 18/66 | R3 | Y3 |
| CE | 5/19 | R4.1 | 4/24 | R4.2 | 5/17 | R4.3 | 3/6 | R4.4 | 17/66 | R4 | Y4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bubulac, L.; Georgescu, T.; Zivari, M.; Popescu-Spineni, D.-M.; Albu, C.-C.; Bobu, A.; Nemeth, S.T.; Bogdan-Andreescu, C.-F.; Gurghean, A.; Alecu, A.A. An Integrative Review of Computational Methods Applied to Biomarkers, Psychological Metrics, and Behavioral Signals for Early Cancer Risk Detection. Bioengineering 2025, 12, 1259. https://doi.org/10.3390/bioengineering12111259
Bubulac L, Georgescu T, Zivari M, Popescu-Spineni D-M, Albu C-C, Bobu A, Nemeth ST, Bogdan-Andreescu C-F, Gurghean A, Alecu AA. An Integrative Review of Computational Methods Applied to Biomarkers, Psychological Metrics, and Behavioral Signals for Early Cancer Risk Detection. Bioengineering. 2025; 12(11):1259. https://doi.org/10.3390/bioengineering12111259
Chicago/Turabian StyleBubulac, Lucia, Tudor Georgescu, Mirela Zivari, Dana-Maria Popescu-Spineni, Cristina-Crenguţa Albu, Adrian Bobu, Sebastian Tiberiu Nemeth, Claudia-Florina Bogdan-Andreescu, Adriana Gurghean, and Alin Adrian Alecu. 2025. "An Integrative Review of Computational Methods Applied to Biomarkers, Psychological Metrics, and Behavioral Signals for Early Cancer Risk Detection" Bioengineering 12, no. 11: 1259. https://doi.org/10.3390/bioengineering12111259
APA StyleBubulac, L., Georgescu, T., Zivari, M., Popescu-Spineni, D.-M., Albu, C.-C., Bobu, A., Nemeth, S. T., Bogdan-Andreescu, C.-F., Gurghean, A., & Alecu, A. A. (2025). An Integrative Review of Computational Methods Applied to Biomarkers, Psychological Metrics, and Behavioral Signals for Early Cancer Risk Detection. Bioengineering, 12(11), 1259. https://doi.org/10.3390/bioengineering12111259