Design and Fabrication of Customised Diabetic Insoles for Optimised Foot Pressure Distribution Using Finite Element Analysis and Additive Manufacturing Technology
Abstract
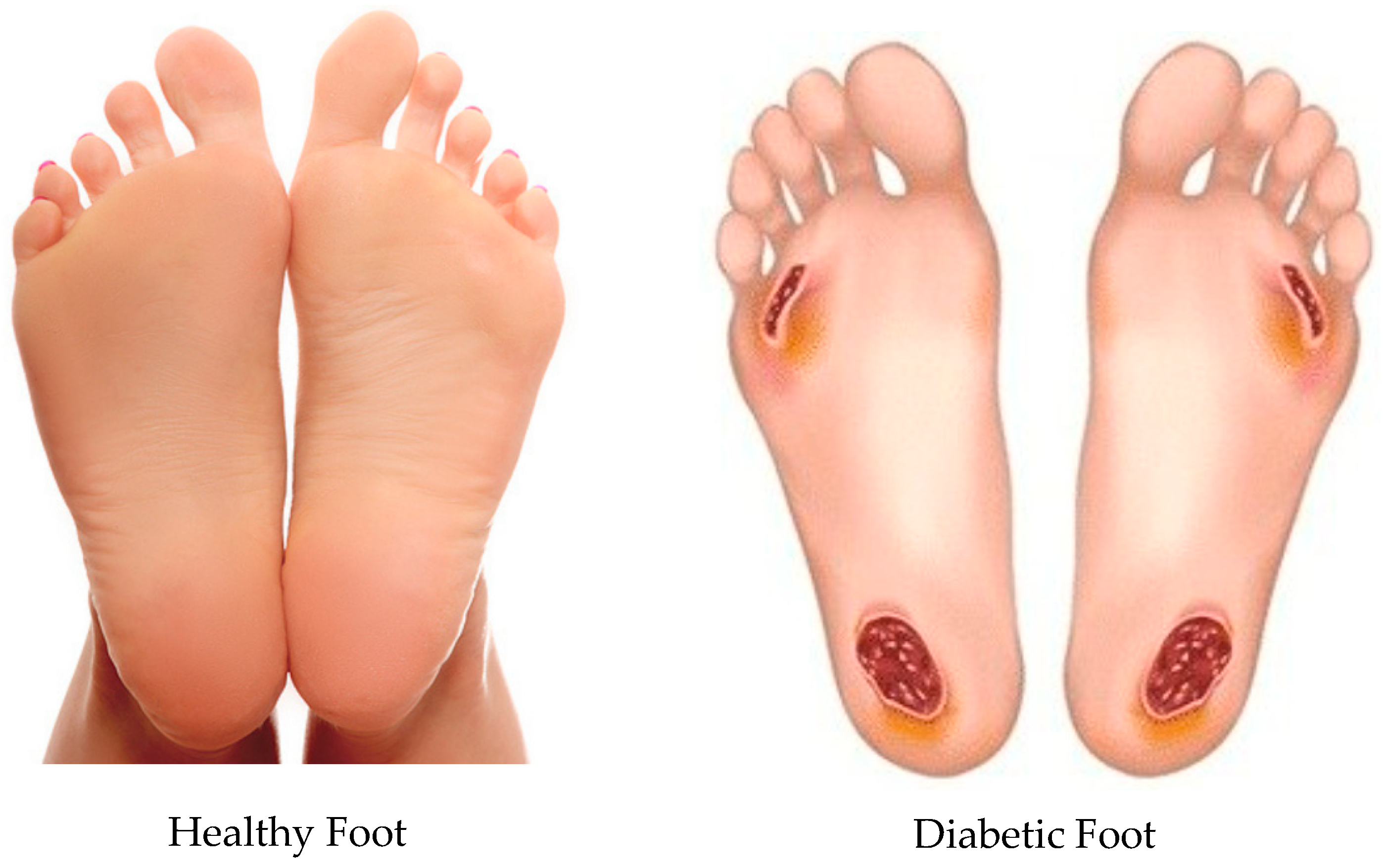
1. Introduction
2. Materials and Methods
2.1. Design and Simulation Procedure
- i.
- CAD Modelling: The insole models are created using Autodesk Inventor based on the UK size 6 dimensions. Different versions are modelled: one with no cutouts, one with circular ulcer relief zones, and one with irregular ulcer cutouts.
- ii.
- Human Foot Modelling: A simplified model of the human foot is developed and matched to the insole dimensions. Three versions are made: a normal foot, one with circular ulcers, and another with irregular ulcers.
- iii.
- Assembly of Foot and Insole: Each foot version is assembled with the corresponding version of the insole model in Autodesk Inventor for further simulation of realistic loading scenarios.
- iv.
- Material Selection: Materials are selected based on biomechanical needs such as shock absorption, pressure relief, and comfort. EVA foams are the main choice due to its frequent use in diabetic footwear. PU has also been selected as the material of the insole.
- v.
- FEA Simulation: Once models are assembled and materials assigned, static structural analysis is conducted in ANSYS Workbench to evaluate stress distribution and overall performance under applied pressure simulating the walking and stance loading conditions.
2.1.1. Insole Design and Material Selection
2.1.2. CAD Modelling of Insoles
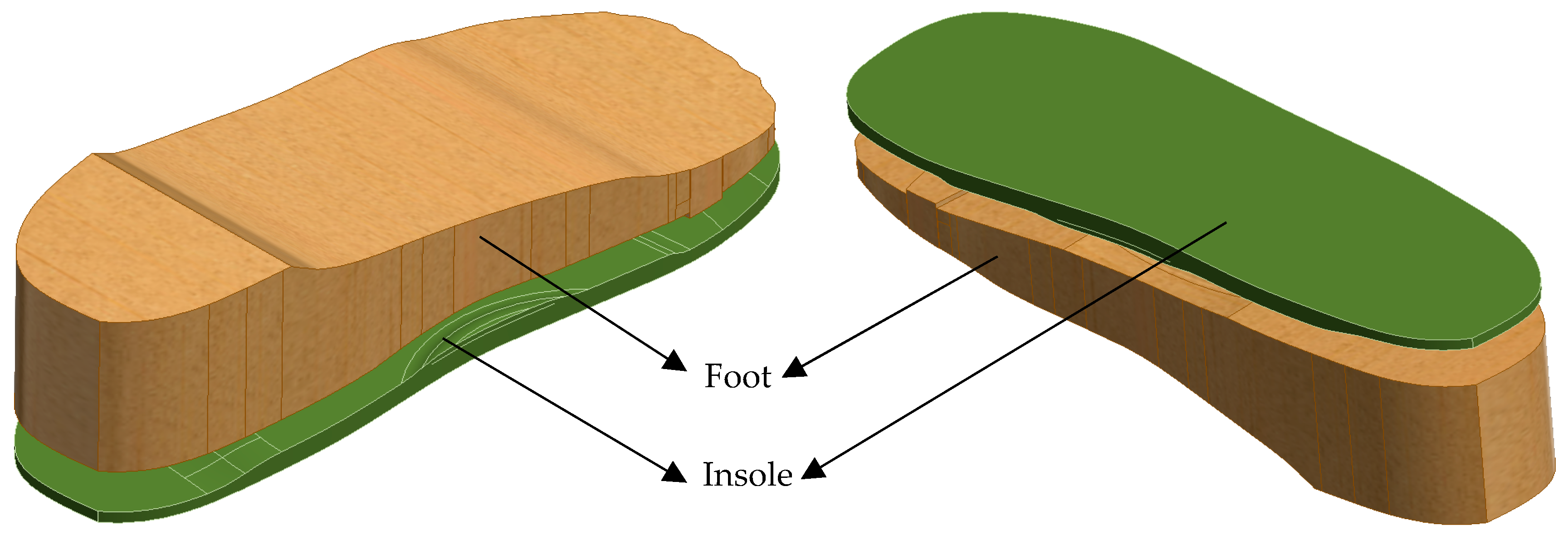
2.2. CAD Modelling Workflow
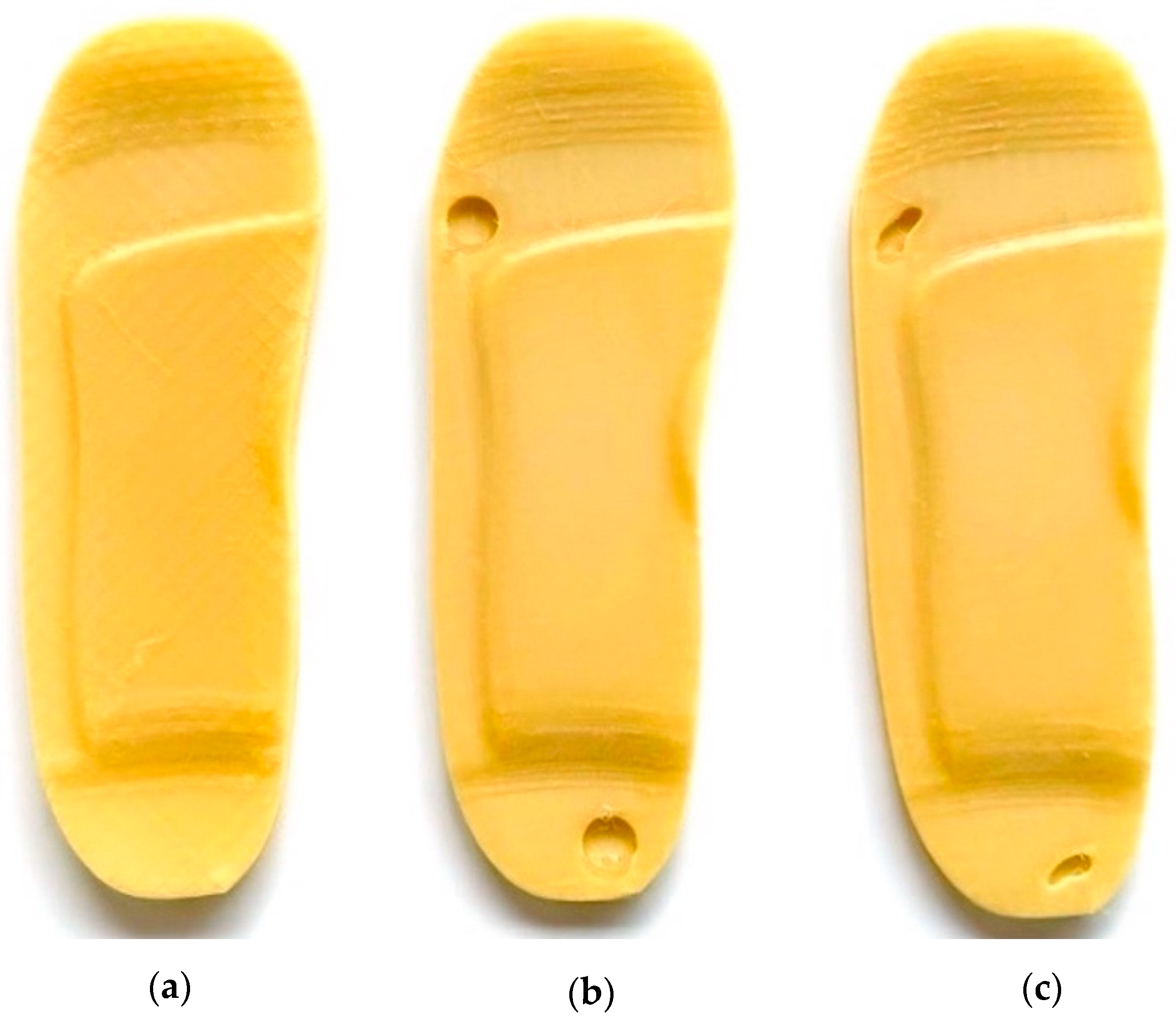
- i.
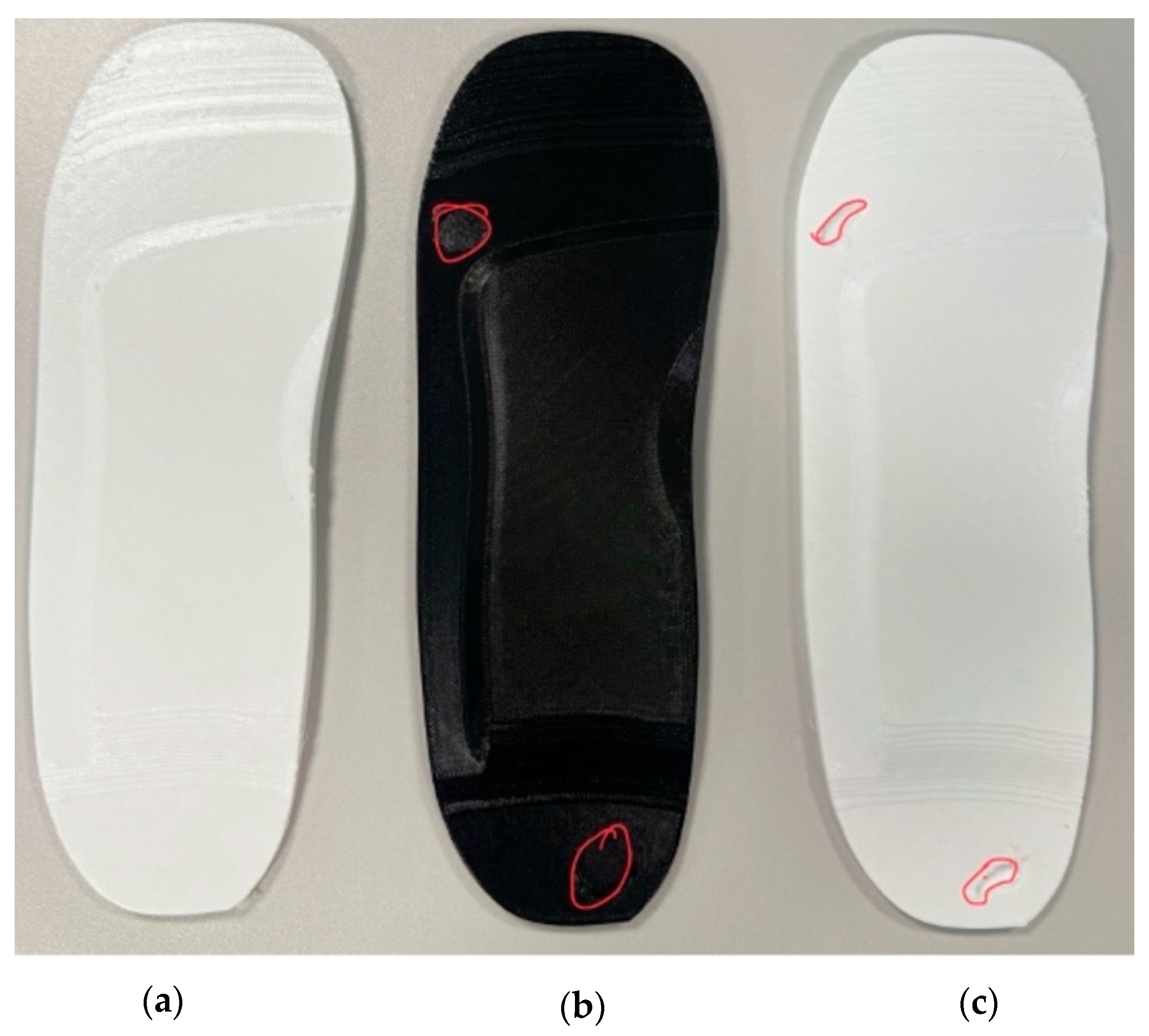
- Initial Insole Design Without Ulcer Cutouts
- A base insole is created using UK size 6 dimensions.
- The insole is extruded to a thickness.
- Functional features like arch support ( height) and heel cushioning ( recess) are incorporated.
- A heel cup is also added to stabilise the rearfoot when bearing weight (see Figure 2a).
- ii.
- Insole Design with Circular Ulcer Cutouts
- Based on medical literature and pressure mapping zones, two circular cutouts are made: one on the heel region and another on the ball of the foot (metatarsal area) as shown in Figure 2b.
- These cutouts are introduced to offload pressure from the ulcer zones during standing.
- The design is saved as a separate file to facilitate direct comparison with other models.
- iii.
- Insole Design with Irregular Ulcer Cutouts
- A more complex version of the insole is modelled by introducing irregularly shaped cutouts on the heel and forefoot areas as shown in Figure 2c.
- These are based on common ulcer geometries observed in diabetic patients and are manually shaped using spline profiles.
- This model is saved independently to evaluate the pressure redistribution effectiveness of more tailored insole designs.
- iv.
- Foot Model Development
- A simplified human foot is modelled to simulate pressure application (see Figure 3).
- Three variations in the foot are designed: normal foot (no ulcers), diabetic foot with circular ulcers (heel and ball), and diabetic foot with irregular ulcers (heel and ball).
- The foot model is sized to anatomically match the UK size 6 insole.
- v.
- CAD Assembly
- Each version of the foot is assembled with the corresponding insole in Autodesk Inventor as shown in Figure 4.
- These assemblies allow proper alignment for realistic boundary conditions and load application during FE simulations.
- These systematic design stages enable a controlled comparison of insole performance in ulcer management and foot pressure mitigation under diabetic conditions.
2.3. Materials Selection
2.4. Finite Element Analysis
- i.
- Importing the Geometry: The assembled CAD models (insole and foot) are exported as a STEP file from Autodesk Inventor and imported into the Static Structural module in ANSYS Workbench.
- ii.
- Assigning Material Properties: EVA1, EVA2, EVA3, and PU material properties, as listed in Table 1, are selected from the ANSYS library and applied to the imported insole models. In the Engineering Data section, a new material is defined with the linear elastic material properties listed in Table 2 to assign foot material properties to the imported foot model.
- iii.
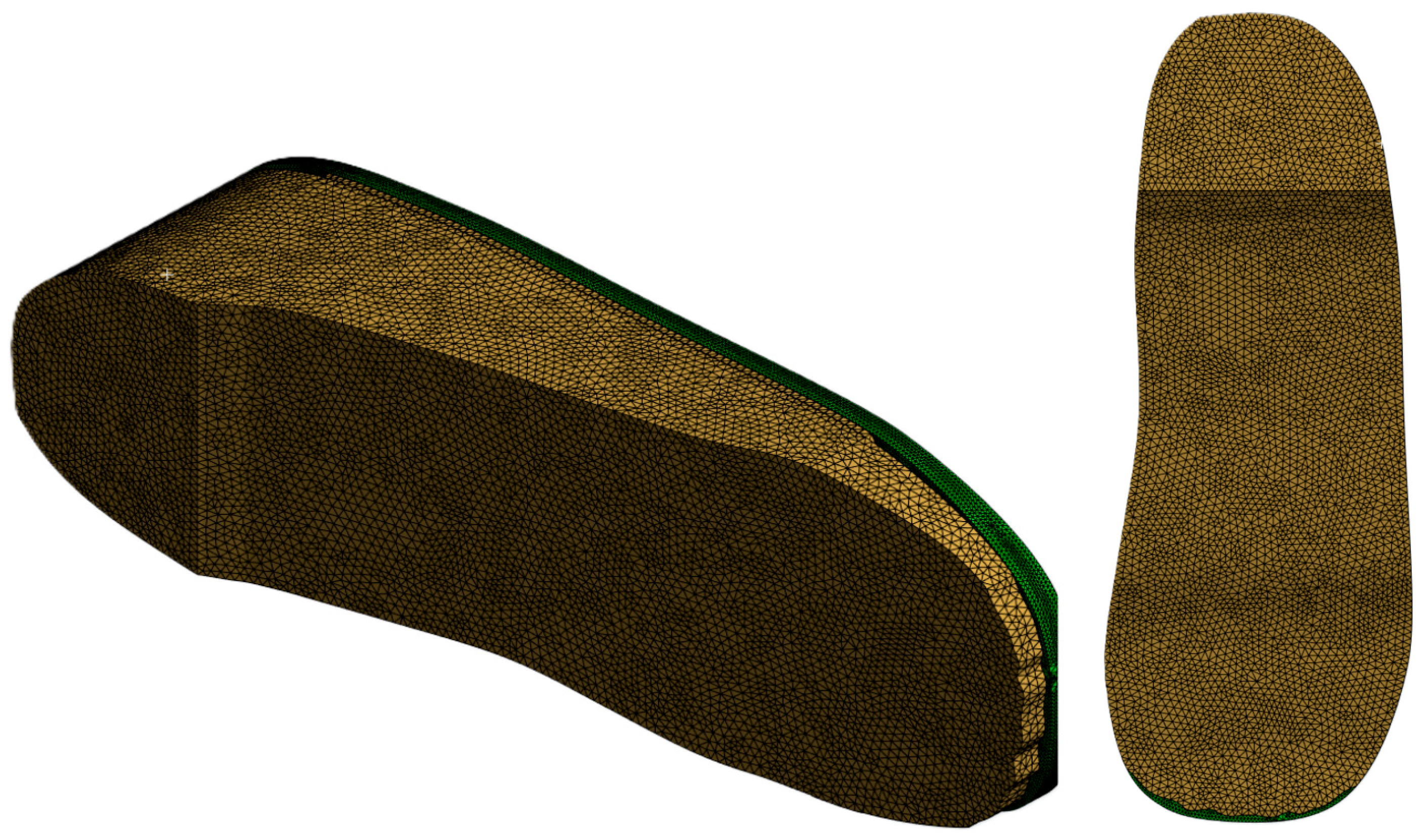
- Mesh Generation: The entire assembled insole and foot model is meshed with quadratic element type and appropriate mesh size is determined through a convergence study. Extra care is taken to ensure finer meshing around ulcer zones and contact areas to improve simulation accuracy (see Figure 5).
- iv.
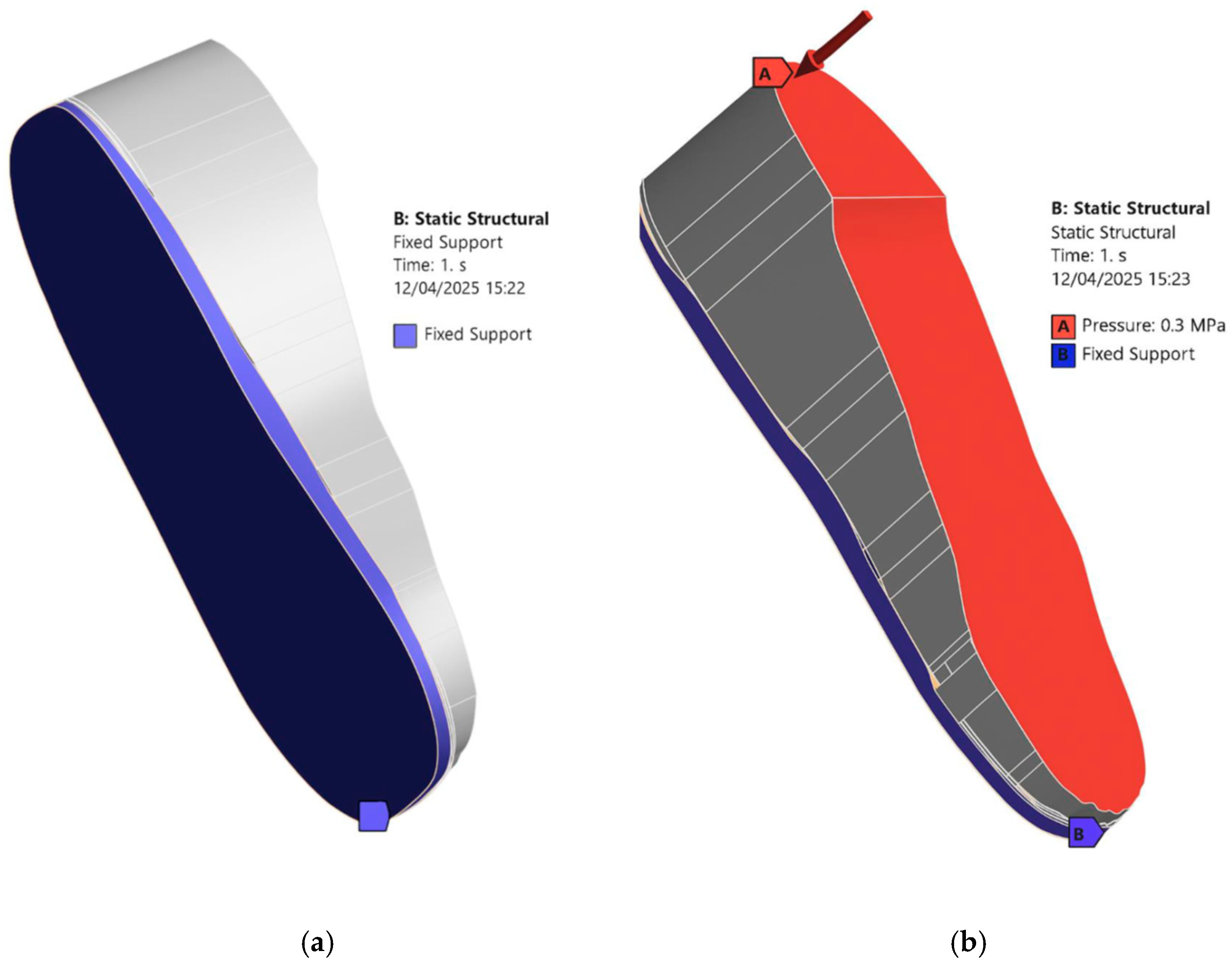
- Loading and Boundary Conditions: In the Static Structural module, a fixed support is applied to the bottom of the insole and side edges that would be in contact with the ground or shoe interior [see Figure 6a]. A pressure load is applied to the top surface of the foot as shown in Figure 6b. The magnitude of the applied pressure is set to 0.1 MPa, 0.2 MPa, and 0.3 MPa to represent different stance loading conditions. These values are chosen based on the magnitudes considered in the study of Ref. [23] to simulate plantar pressures applied to the diabetic feet under static loading conditions. Selecting this range allows us to stay within physiologically relevant loading scenarios and observe how the insole material and design influence the stress distribution. Figure 6 describes the applied loading and boundary conditions.
- v.
- Solution Setup: In the solution setup, the output parameter is Equivalent von Mises stress, which is used to evaluate stress distribution in the foot and insole. The simulation is then executed in ANSYS Workbench.
3. Results and Discussion
3.1. Validation
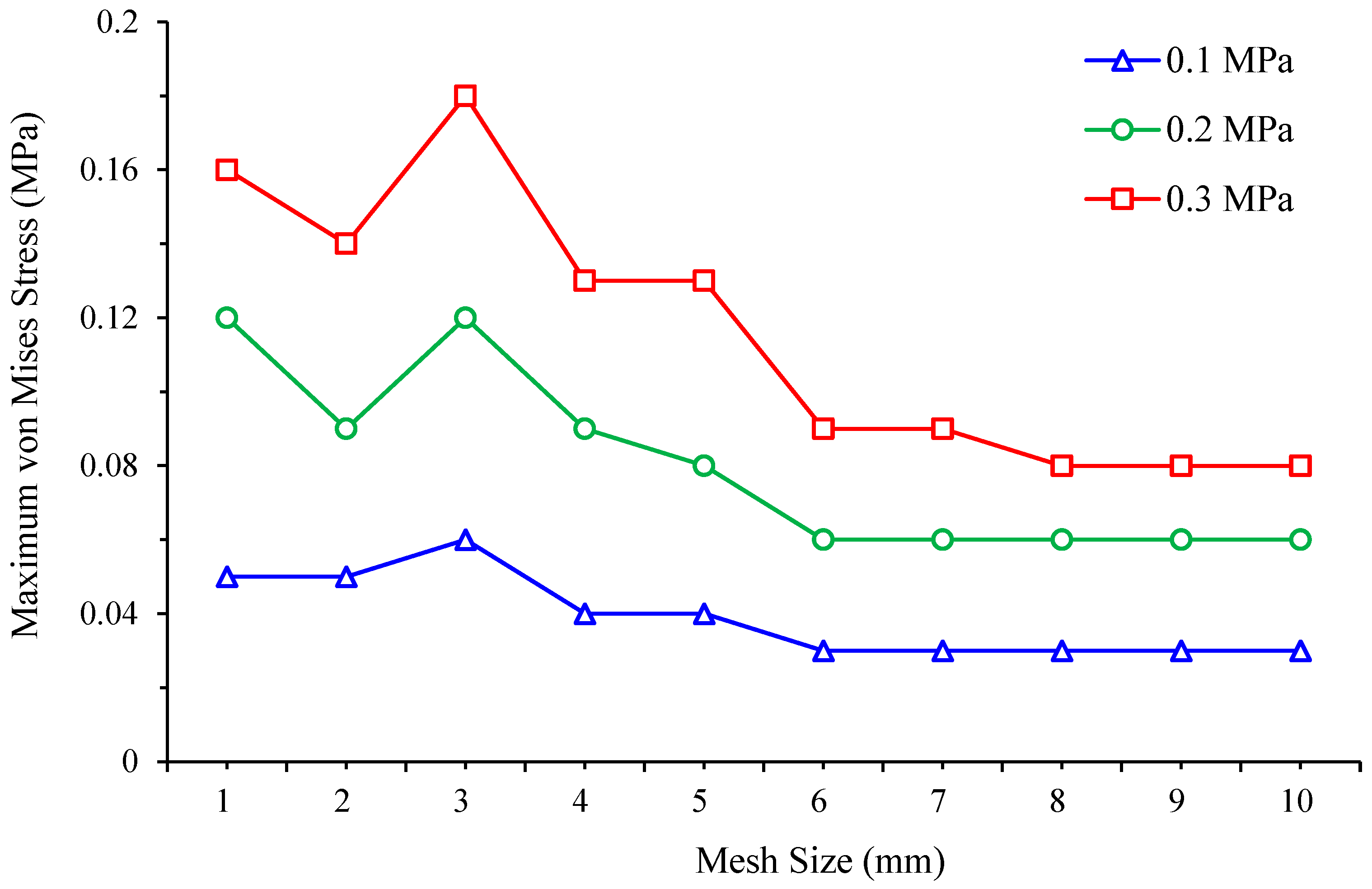
3.2. Mesh Convergence Study
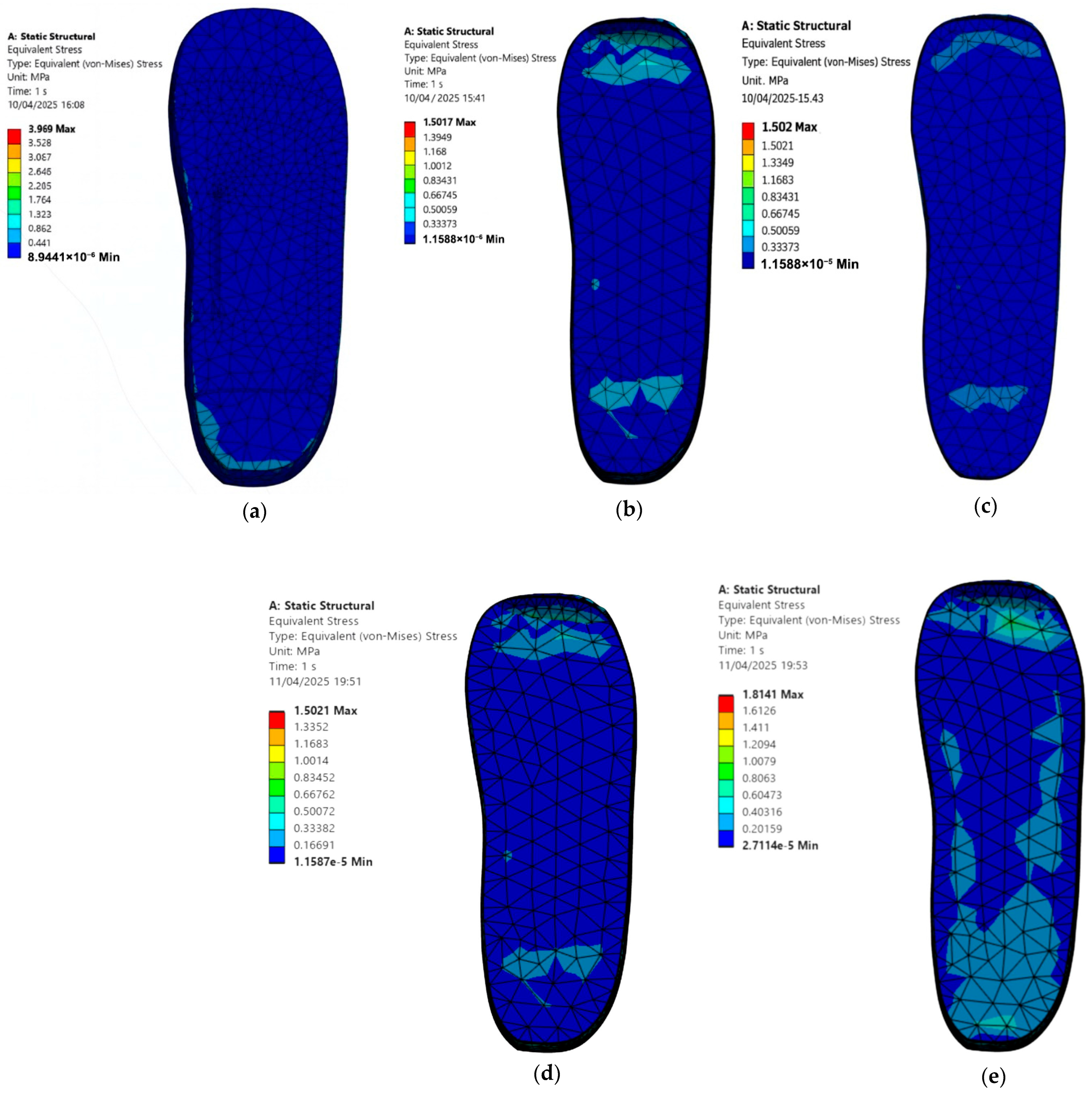
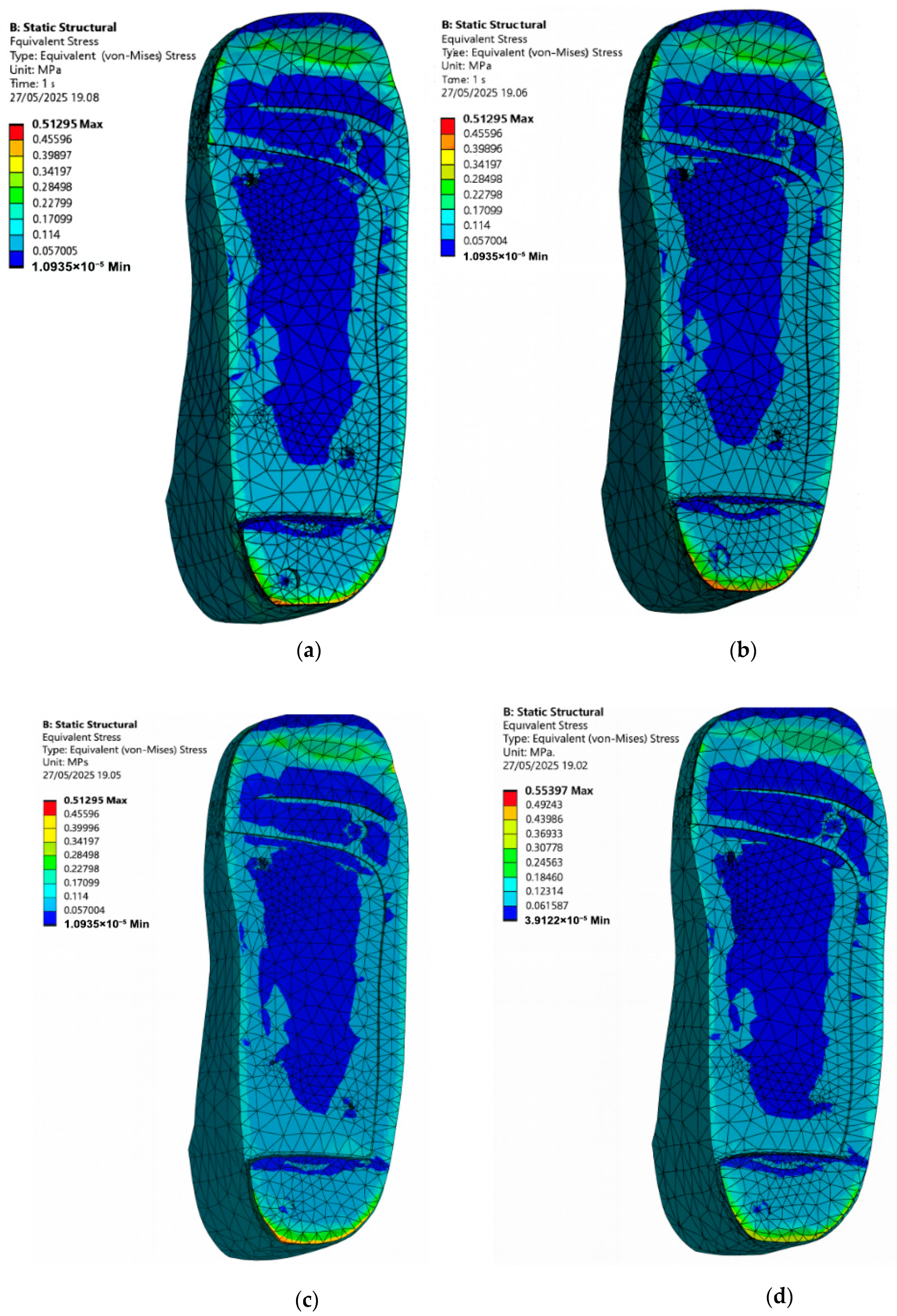
3.3. Diabetic Foot Stresses in a Barefoot Without Ulcer Conditions and with Custom Insoles
- Cohort A, 0.3 MPa:
- Barefoot: 3.97 MPa.
- EVA1: 1.50 MPa.
- EVA2: 1.50 MPa.
- EVA3: 1.50 MPa.
- PU: 1.81 MPa.
- Cohort B, 0.1 MPa:
- EVA1: 0.50 MPa.
- EVA2: 0.50 MPa.
- EVA3: 0.50 MPa.
- PU: 0.64 MPa.
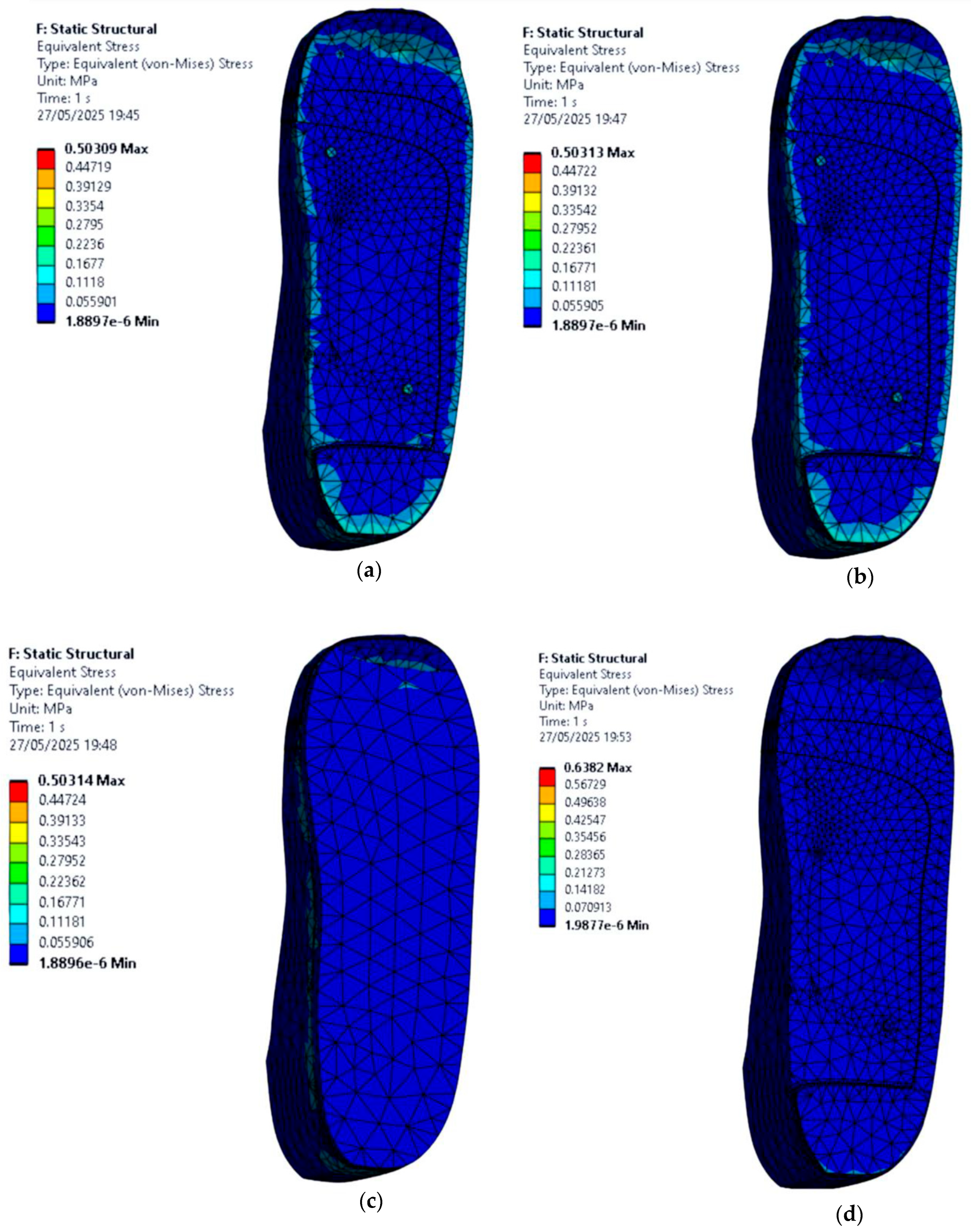
3.4. Stress Distribution on the Foot with Circular Ulcers Standing on Customised Insoles (Cohort A, 0.3 MPa)
- EVA1: 0.55 MPa.
- EVA2: 0.55 MPa.
- EVA3: 0.55 MPa.
- PU: 0.58 MPa.
- EVA1: 0.51 MPa.
- EVA2: 0.51 MPa.
- EVA3: 0.51 MPa.
- PU: 0.55 MPa.
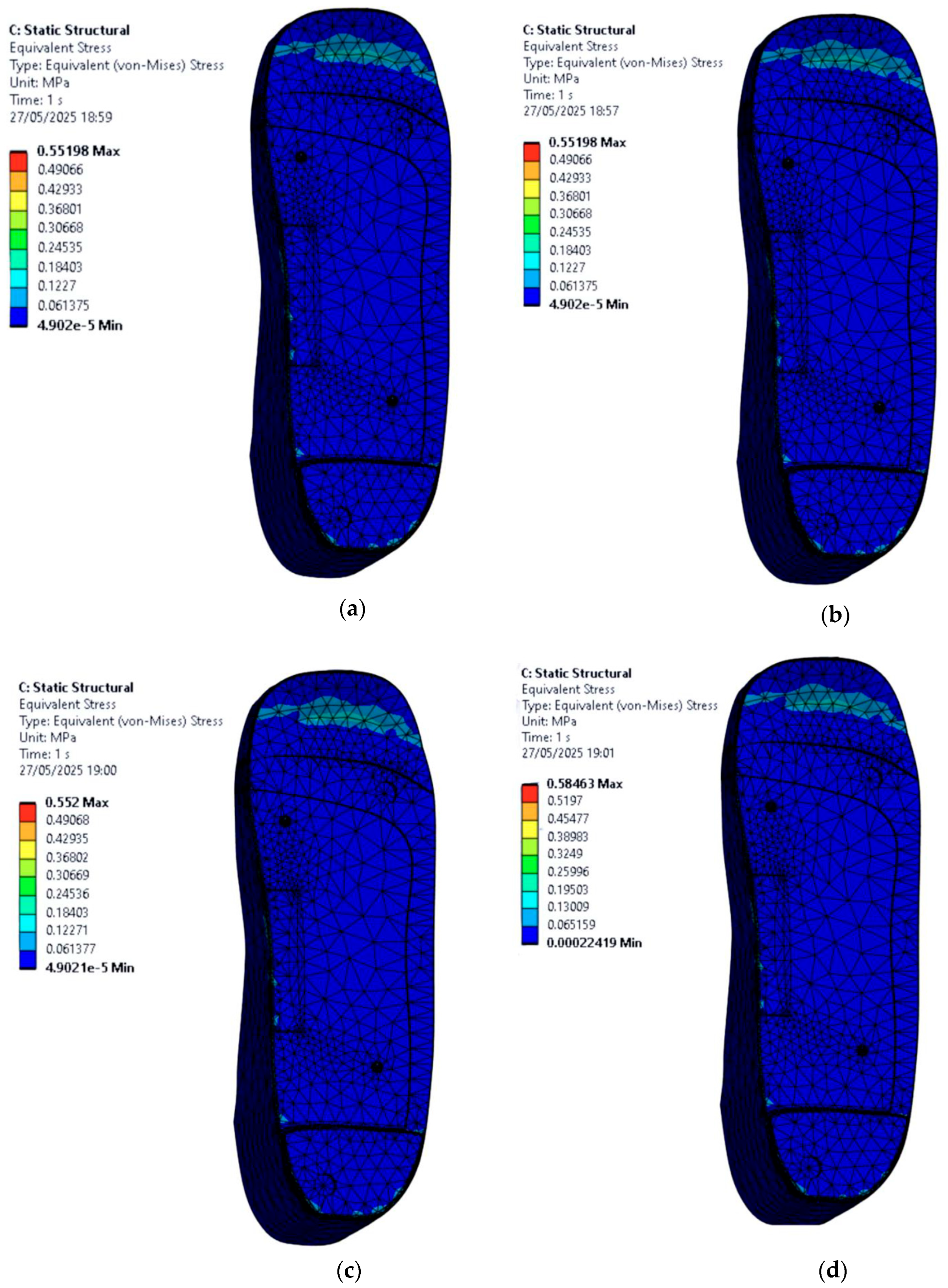
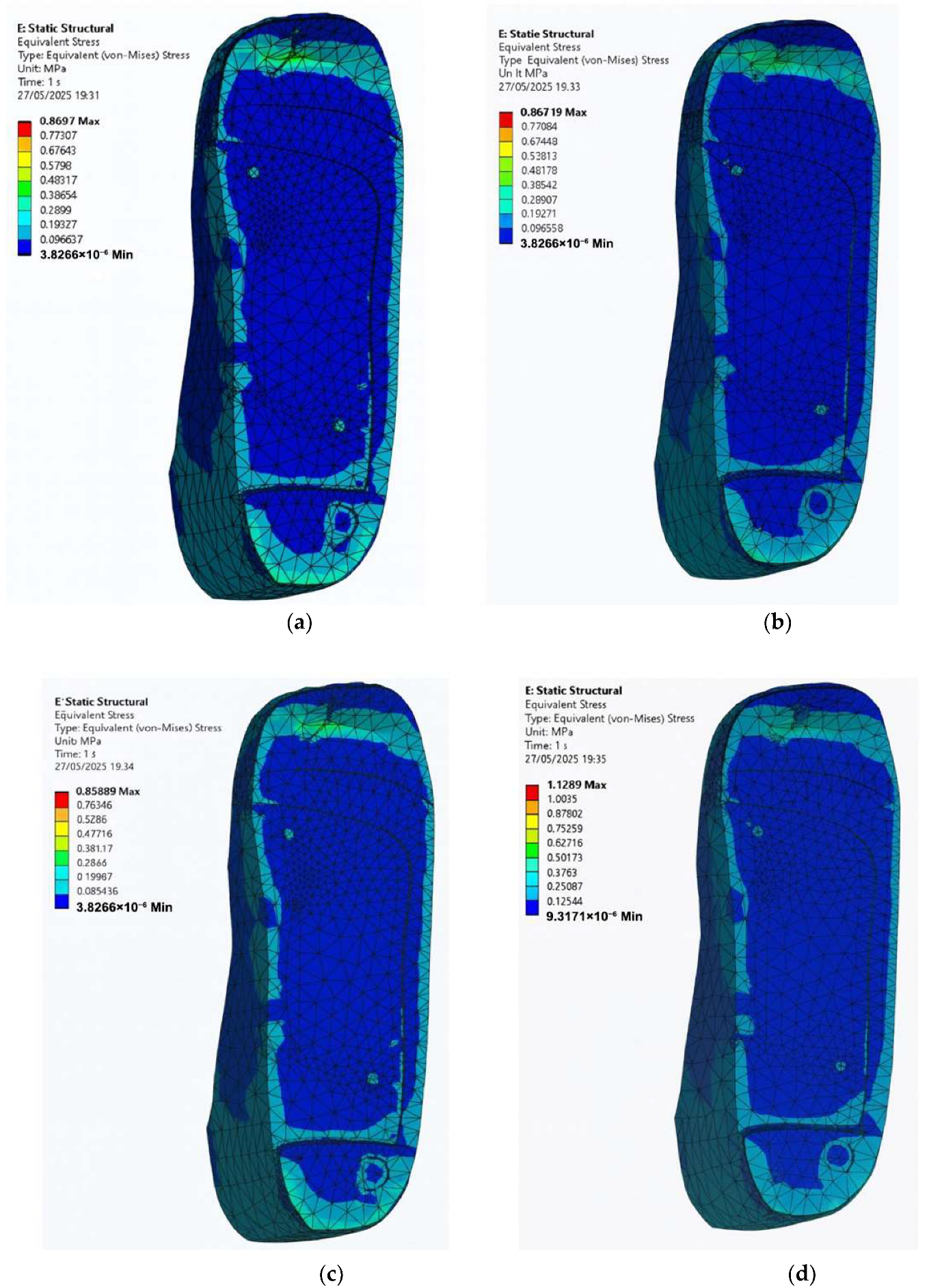
3.5. Stress Distribution on the Foot with Irregular Ulcers Standing on Customised Insoles (Cohort A, 0.3 MPa)
- EVA1: 0.87 MPa.
- EVA2: 0.87 MPa.
- EVA3: 0.86 MPa.
- PU: 1.13 MPa.
- EVA1: 0.63 MPa.
- EVA2: 0.63 MPa.
- EVA3: 0.63 MPa.
- PU: 0.66 MPa.
3.6. Effect of Insole Material Properties on Foot Stress
4. Customised Insole Prototyping Using Additive Manufacturing Technology
- Technology: Fused Filament Fabrication (FFF) or Fused Deposition Modelling (FDM).
- Material: flexible EVA and PU (FLEX/117) filaments.
- Layer height: 0.2 mm.
- Print time: approximately 8 h per specimen.
5. Conclusions and Perspective
- Customised Insole Geometry and Plantar Stress Reduction: When the diabetic foot was simulated in a barefoot condition on a hard and flat ground surface, the peak von Mises stress reached 3.97 MPa. When supported by a customised insole made of EVA1, the stress dropped to 1.51 MPa, representing a 61.96% reduction. This confirms that using insoles can meaningfully offload pressure in high-risk regions such as the heel and forefoot, which is critical for ulcer prevention.
- Ulcer Isolation Cutouts and Local Pressure Relief: For feet with irregular ulcers, adding cutouts at the heel and forefoot regions offloaded stress around the ulcer zones. The peak stress reduced from 0.87 MPa to 0.63 MPa, a 27.58% reduction. While not drastic, this validates the principle that small geometric modifications can improve clinical outcomes, especially when paired with compliant materials.
- Material Selection and Stress Reduction: Among all tested materials, EVA1 (Shore A65) consistently produced the lowest stress values. For example, in the non-ulcer foot condition, EVA1 (1.50 MPa) reduced stress by 17.12% compared to PU (1.81 MPa), which showed the highest values. This demonstrates that softer and more elastic materials are better at absorbing shock and redistributing pressure in diabetic feet.
- Three-Dimensional Printing for Effective and Accessible Prototyping: All three insole models, including the standard design, the circular-cutout design, and the irregular-cutout design, were successfully printed using flexible EVA and PU filaments with the FDM additive manufacturing technique. Each specimen required approximately 8 h of print time, and the process was carried out entirely at the additive manufacturing laboratory of Anglia Ruskin University. This demonstrates that simulation-led workflows can be practically and affordably translated into physical prototypes using standard additive manufacturing technologies.
- Advanced Foot Modelling with Hyper-Elastic Tissue: This study used a simplified linear elastic foot material. Future work should implement hyper-elastic models (e.g., Ogden or Mooney–Rivlin) to better capture the non-linear behaviour of plantar soft tissue under compression.
- Patient-Specific Foot Geometry: The current design used a generic UK size 6 foot. Using 3D scans of actual patient feet would allow for customised insole shapes and ulcer relief zones, improving performance and clinical relevance.
- Dual-Material Design Concepts: While this study focused on a single-material approach, performance could be enhanced through composite or dual-material 3D printing, where soft materials (like EVA) are used for comfort zones and firmer materials (like PU or nylon) provide arch support and stability.
- Alternative Prototyping Techniques Beyond FDM: While the FDM technique was practical and effective, other manufacturing techniques may provide improved accuracy and durability:
- ✓
- Stereolithography (SLA) allows for high-detail prototypes with better surface finish.
- ✓
- Selective Laser Sintering (SLS) can process flexible or composite materials.
- ✓
- Injection Moulding is ideal for large-scale production with consistent material properties, although less suited for rapid prototyping due to tooling costs.
- Sensor Feedback for Physical Validation: Future studies should integrate thin-film pressure sensors into insole prototypes to validate simulation results with actual pressure measurements through physical testing under dynamic loading such as walking trials.
- Optimisation of Ulcer Isolation Shapes: Only one size and shape of cutout was assessed. Future research should experiment with different depths, contours, and geometries, potentially using AI or machine learning to automate patient-specific optimisation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Facts & Figures. Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 24 April 2025).
- Baig, M.S.; Banu, A.; Zehravi, M.; Rana, R.; Burle, S.S.; Khan, S.L.; Islam, F.; Siddiqui, F.A.; Massoud, E.E.S.; Rahman, M.H.; et al. An Overview of Diabetic Foot Ulcers and Associated Problems with Special Emphasis on Treatments with Antimicrobials. Life 2022, 12, 1054. [Google Scholar] [CrossRef]
- Raghav, A.; Khan, Z.A.; Labala, R.K.; Ahmad, J.; Noor, S.; Mishra, B.K. Financial Burden of Diabetic Foot Ulcers to World: A Progressive Topic to Discuss Always. Ther. Adv. Endocrinol. 2018, 9, 29–31. [Google Scholar] [CrossRef]
- Holroyd, J. Healthy Heels: How We Manage Plantar Fasciitis; The Healthy Body Company: Jordan Springs, Australia, 2021. [Google Scholar]
- Diabetic Foot Infection—Causes and Treatment. Available online: https://www.vpslakeshorehospital.com (accessed on 27 October 2025).
- Owings, T.M.; Woerner, J.L.; Frampton, J.D.; Cavanagh, P.R.; Botek, G. Custom Therapeutic Insoles Based on Both Foot Shape and Plantar Pressure Measurement Provide Enhanced Pressure Relief. Diabetes Care 2008, 31, 839–844. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, Q.; Pu, F.; Boone, D.; Zhang, M. A Review of the Application of Additive Manufacturing in Prosthetic and Orthotic Clinics from a Biomechanical Perspective. Engineering 2020, 6, 1258–1266. [Google Scholar] [CrossRef]
- Rahmati, S.; Kheirollahi, H.; Azari, A. Design and Analysis of a New Dental Implant Using Finite Element Method. Int. J. Adv. Des. Manuf. Technol. 2013, 6, 23–32. [Google Scholar]
- Kheirollahi, H.; Luo, Y. Assessment of Hip Fracture Risk Using Cross-Section Strain Energy Determined by QCT-Based Finite Element Modeling. BioMed Res. Int. 2015, 2015, 413839. [Google Scholar] [CrossRef]
- Kheirollahi, H.; Luo, Y. Identification of High Stress and Strain Regions in Proximal Femur during Single-Leg Stance and Sideways Fall Using QCT-Based Finite Element Model. Int. J. Med. Health Biomed. Bioeng. Pharm. Eng. 2015, 9, 633–640. [Google Scholar]
- Kheirollahi Nataj Bisheh, H. Assessment of Hip Fracture Risk Using Cross-Section Strain Energy Determined from QCT-Based Finite Element Model; University of Manitoba: Winnipeg, Canada, 2015. [Google Scholar]
- Kheirollahi, H.; Luo, Y. Understanding Hip Fracture by QCT-Based Finite Element Modeling. J. Med. Biol. Eng. 2017, 37, 686–694. [Google Scholar] [CrossRef]
- Bisheh, H.; Luo, Y.; Rabczuk, T. Hip Fracture Risk Assessment Based on Different Failure Criteria Using QCT-Based Finite Element Modeling. Comput. Mater. Contin. 2020, 63, 567–591. [Google Scholar] [CrossRef]
- Bisheh, H.; Luo, Y.; Rabczuk, T. Parametric Study of Hip Fracture Risk Using QCT-Based Finite Element Analysis. Comput. Mater. Contin. 2021, 71, 1349–1369. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R.; Rab, S. Role of Additive Manufacturing Applications towards Environmental Sustainability. Adv. Ind. Eng. Polym. Res. 2021, 4, 312–322. [Google Scholar] [CrossRef]
- Ashry, H.R.; Lavery, L.A.; Murdoch, D.P.; Frolich, M.; Lavery, D.C. Effectiveness of Diabetic Insoles to Reduce Foot Pressures. J. Foot Ankle Surg. 1997, 36, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.S.; Stenhouse, E.A.; Bruce, G.; Zahra, D.; Jones, R.B. A Comparison of Customised and Prefabricated Insoles to Reduce Risk Factors for Neuropathic Diabetic Foot Ulceration: A Participant-Blinded Randomised Controlled Trial. J. Foot Ankle Res. 2012, 5, 31. [Google Scholar] [CrossRef]
- Raspovic, A.; Newcombe, L.; Lloyd, J.; Dalton, E. Effect of Customized Insoles on Vertical Plantar Pressures in Sites of Previous Neuropathic Ulceration in the Diabetic Foot. Foot 2000, 10, 133–138. [Google Scholar] [CrossRef]
- Chen, W.-P.; Ju, C.-W.; Tang, F.-T. Effects of Total Contact Insoles on the Plantar Stress Redistribution: A Finite Element Analysis. Clin. Biomech. 2003, 18, S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; Ulbrecht, J.S.; Cavanagh, P.R. Pressure Relief and Load Redistribution by Custom-Made Insoles in Diabetic Patients with Neuropathy and Foot Deformity. Clin. Biomech. 2004, 19, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Barani, Z.; Haghpanahi, M.; Katoozian, H. Three Dimensional Stress Analysis of Diabetic Insole: A Finite Element Approach. Technol. Health Care 2005, 13, 185–192. [Google Scholar] [CrossRef]
- Collings, R.; Freeman, J.; Latour, J.M.; Glasser, S.; Paton, J. Footwear and Insole Design Features to Prevent Foot Ulceration in People with Diabetes: A Systematic Review Protocol. JBI Evid. Synth. 2017, 15, 1824. [Google Scholar] [CrossRef]
- Chanda, A.; Unnikrishnan, V. Novel Insole Design for Diabetic Foot Ulcer Management. Proc. Inst. Mech. Eng. H 2018, 232, 1182–1195. [Google Scholar] [CrossRef]
- Mahesh, C.C.; Ramachandran, K.I. Finite Element Modelling of Functionally Graded Elastomers for the Application of Diabetic Footwear. Mater. Today Proc. 2018, 5, 16367–16377. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Bao, W.; Zhu, S.; Li, D.; Zhao, N.; Liu, C. Functional Gradient Structural Design of Customized Diabetic Insoles. J. Mech. Behav. Biomed. Mater. 2019, 94, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Barwick, A.; Butterworth, P.; Nancarrow, S. Footwear and Insole Design Features That Reduce Neuropathic Plantar Forefoot Ulcer Risk in People with Diabetes: A Systematic Literature Review. J. Foot Ankle Res. 2020, 13, 30. [Google Scholar] [CrossRef]
- Collings, R.; Freeman, J.; Latour, J.M.; Paton, J. Footwear and Insole Design Features for Offloading the Diabetic at Risk Foot—A Systematic Review and Meta-Analyses. Endocrinol. Diabetes Metab. 2021, 4, e00132. [Google Scholar] [CrossRef]
- Korada, H.; Maiya, A.; Rao, S.K.; Hande, M. Effectiveness of Customized Insoles on Maximum Plantar Pressure in Diabetic Foot Syndrome: A Systematic Review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1093–1099. [Google Scholar] [CrossRef]
- Niu, J.; Liu, J.; Zheng, Y.; Ran, L.; Chang, Z. Are Arch-Conforming Insoles a Good Fit for Diabetic Foot? Insole Customized Design by Using Finite Element Analysis. Hum. Factors Ergon. Manuf. Serv. Ind. 2020, 30, 303–310. [Google Scholar] [CrossRef]
- Bose, D.; Singh, G.; Gupta, S.; Chanda, A. Development of a Novel Customized Insole for Effective Pressure Offloading in Diabetic Patients. Prosthesis 2024, 6, 341–356. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, H.; Song, X.; Wu, Y.; Lyu, Y.; Zeng, W. Advancements in Diabetic Foot Insoles: A Comprehensive Review of Design, Manufacturing, and Performance Evaluation. Front. Bioeng. Biotechnol. 2024, 12, 1394758. [Google Scholar] [CrossRef]
- Singh, G.; Chanda, A. Finite Element Modeling of Diabetic Foot: A State-of-the-Art Review. Eng. Res. Express 2024, 6, 012507. [Google Scholar] [CrossRef]
- Ghazali, M.J.; Ren, X.; Rajabi, A.; Zamri, W.F.H.W.; Mohd Mustafah, N.; Ni, J. Finite Element Analysis of Cushioned Diabetic Footwear Using Ethylene Vinyl Acetate Polymer. Polymers 2021, 13, 2261. [Google Scholar] [CrossRef]
- Even-Tzur, N.; Weisz, E.; Hirsch-Falk, Y.; Gefen, A. Role of EVA Viscoelastic Properties in the Protective Performance of a Sport Shoe: Computational Studies. Bio-Med. Mater. Eng. 2006, 16, 289–299. [Google Scholar]
- Rahmati, S.; Kheirollahi, H.; Azari, A. Analysis and Fabrication of New Designed Dental Implant Using Rapid Prototyping Technology. In Innovative Developments in Design and Manufacturing; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Kheirollahi, H.; Abesi, F.; Rahmati, S. Comparison of CT and CBCT for Fabrication of Dentistry Models via Rapid Prototyping Technology. In Innovative Developments in Design and Manufacturing; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Kheirollahi, H.; Rahmati, S.; Abesi, F. A Novel Methodology in Design and Fabrication of Lingual Orthodontic Appliance Based on Rapid Prototyping Technologies. In Innovative Developments in Design and Manufacturing; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Kheirollahi, H.; Abbaszadeh, F. Application of Rapid Prototyping Technology in Dentistry. Int. J. Rapid Manuf. 2011, 2, 104–120. [Google Scholar] [CrossRef]
- Abbaszadeh, F.; Rahmati, S.; Kheirollahi, H.; Farahmand, F. Design for Manufacturing of Custom-Made Femoral Stem Using CT Data and Rapid Prototyping Technology. Int. J. Rapid Manuf. 2011, 2, 76–91. [Google Scholar] [CrossRef]
- Ravanbod, S.; Rahmani, K.; Karmel, S.; Pande, I.; Amel, H.; Branfoot, C.; Shahidi, A.M.; Alderson, A.; Bodaghi, M. From Coral to Control: Bio-Inspired, 3D-Printable Metamaterials with Tuneable Quasi-Zero Stiffness and Multi-Functional Bio-Composites. Mater. Des. 2025, 257, 114398. [Google Scholar] [CrossRef]






| Properties | EVA1 (Shore A65, 33% Vinyl Acetate) | EVA2 (Shore A85, 25% Vinyl Acetate) | EVA3 (Shore A95/D50, 12% Vinyl Acetate) | PU (Polyurethane, Flexible) |
|---|---|---|---|---|
| Density | 950 | 950 | 935 | 31.94 |
| Young’s modulus (Pa) | 48,990 | |||
| Poisson’s ratio | 0.4799 | 0.4799 | 0.4799 | 0.2793 |
| Bulk modulus (Pa) | 36,996 | |||
| Shear modulus (Pa) | 19,147 | |||
| Tensile ultimate strength (Pa) | ||||
| Tensile yield strength (Pa) | 4135 |
| Linear Elastic Parameters | Modulus of Elasticity (MPa) | Poisson’s Ratio |
|---|---|---|
| Diabetic foot model | 483 | 0.4 |
| Comparison Parameter | Present Study | Study of Ref. [23] |
|---|---|---|
| Applied pressure: standing position | 0.30 MPa | 0.30 MPa |
| Foot peak stress: barefoot on the hard ground versus on the insole | 3.97 MPa → 1.51 MPa (61.96% ↓) | 0.23 MPa → 0.17 MPa (26.08% ↓) |
| Foot peak stress with irregular ulcers: non-isolated insole versus isolated one | 0.87 MPa → 0.63 MPa (27.58% ↓) | 0.93 MPa → 0.08MPa (91.39% ↓) |
| Foot tissue model | Linear elastic (, ) | Hyper-elastic (Ogden model) |
| Insole materials used | EVA1, EVA2, EVA3, and PU | Multilayer soft foam |
| Simulation scope | Static structural + 3D prototyping | Static structural + 3D prototyping |
| Peak von Mises Stress (MPa) | ||||
|---|---|---|---|---|
| Pressure (MPa) | 0.1 | 0.2 | 0.3 | |
| Mesh Size (mm) | ||||
| 1 | 2.89 | 5.78 | 8.67 | |
| 2 | 2.13 | 4.26 | 6.39 | |
| 3 | 1.54 | 3.07 | 3.26 | |
| 4 | 1.09 | 2.18 | 3.26 | |
| 5 | 0.72 | 1.44 | 2.16 | |
| 6 | 0.60 | 1.21 | 1.81 | |
| 7 | 0.50 | 1.01 | 1.50 | |
| 8 | 0.51 | 1.02 | 1.52 | |
| 9 | 0.45 | 0.90 | 1.35 | |
| 10 | 0.43 | 0.87 | 1.30 | |
| Peak von Mises Stress (MPa) | ||||
|---|---|---|---|---|
| Pressure (MPa) | 0.1 | 0.2 | 0.3 | |
| Mesh Size (mm) | ||||
| 1 | 0.05 | 0.11 | 0.16 | |
| 2 | 0.05 | 0.09 | 0.14 | |
| 3 | 0.06 | 0.12 | 0.18 | |
| 4 | 0.04 | 0.09 | 0.13 | |
| 5 | 0.04 | 0.08 | 0.13 | |
| 6 | 0.03 | 0.06 | 0.09 | |
| 7 | 0.03 | 0.06 | 0.09 | |
| 8 | 0.03 | 0.06 | 0.08 | |
| 9 | 0.03 | 0.06 | 0.08 | |
| 10 | 0.03 | 0.06 | 0.10 | |
| Peak von Mises Stress (MPa) | ||||
|---|---|---|---|---|
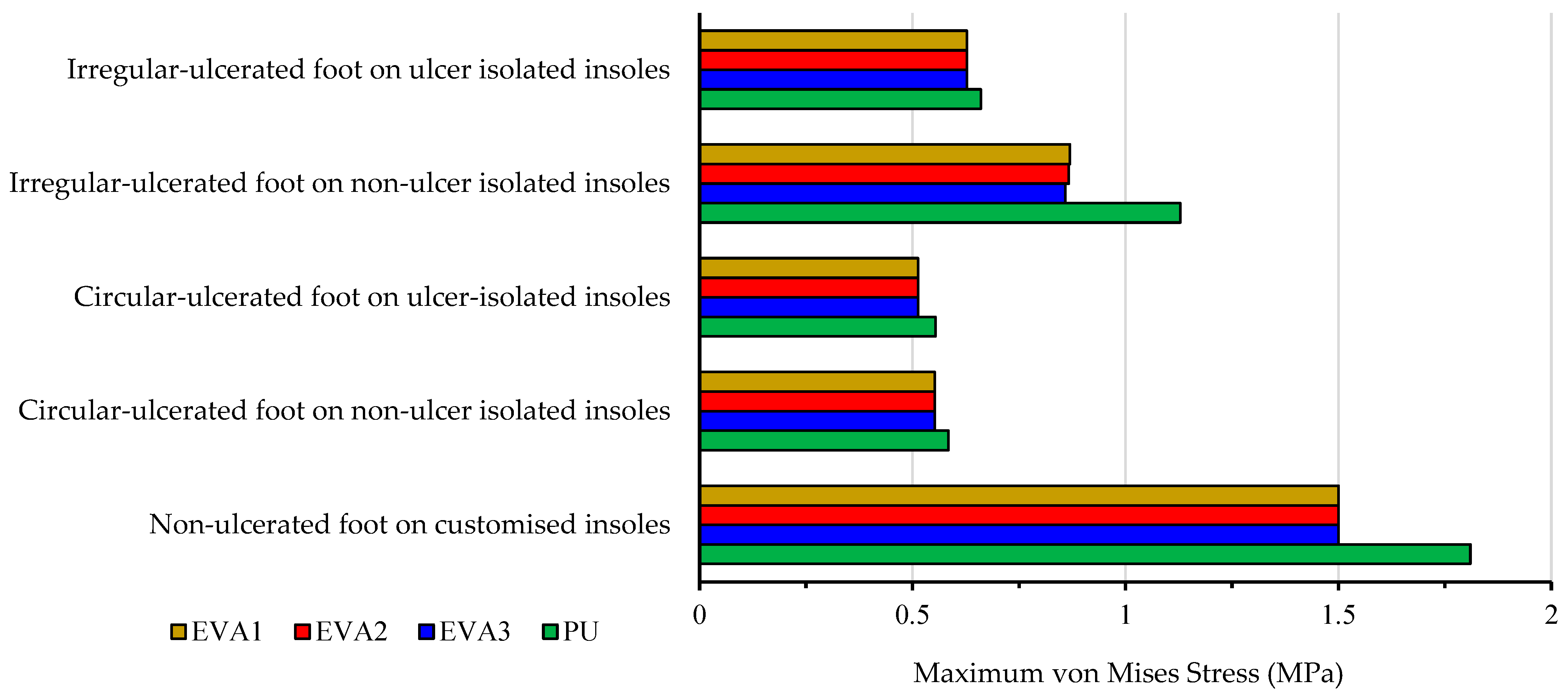
| Foot and Insole Conditions | EVA1 | EVA2 | EVA3 | PU |
| Non-ulcerated foot on customised insoles | 1.50 | 1.50 | 1.50 | 1.81 |
| Circular-ulcerated foot on non-ulcer isolated insoles | 0.55 | 0.55 | 0.55 | 0.58 |
| Circular-ulcerated foot on ulcer-isolated insoles | 0.51 | 0.51 | 0.51 | 0.55 |
| Irregular- ulcerated foot on non-ulcer isolated insoles | 0.87 | 0.87 | 0.86 | 1.13 |
| Irregular-ulcerated foot on ulcer isolated insoles | 0.63 | 0.63 | 0.63 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mala, J.; Bisheh, H. Design and Fabrication of Customised Diabetic Insoles for Optimised Foot Pressure Distribution Using Finite Element Analysis and Additive Manufacturing Technology. Bioengineering 2025, 12, 1217. https://doi.org/10.3390/bioengineering12111217
Mala J, Bisheh H. Design and Fabrication of Customised Diabetic Insoles for Optimised Foot Pressure Distribution Using Finite Element Analysis and Additive Manufacturing Technology. Bioengineering. 2025; 12(11):1217. https://doi.org/10.3390/bioengineering12111217
Chicago/Turabian StyleMala, Jafar, and Hossein Bisheh. 2025. "Design and Fabrication of Customised Diabetic Insoles for Optimised Foot Pressure Distribution Using Finite Element Analysis and Additive Manufacturing Technology" Bioengineering 12, no. 11: 1217. https://doi.org/10.3390/bioengineering12111217
APA StyleMala, J., & Bisheh, H. (2025). Design and Fabrication of Customised Diabetic Insoles for Optimised Foot Pressure Distribution Using Finite Element Analysis and Additive Manufacturing Technology. Bioengineering, 12(11), 1217. https://doi.org/10.3390/bioengineering12111217







