EEG-Based Neurofeedback in Athletes and Non-Athletes: A Scoping Review of Outcomes and Methodologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection Process
2.5. Data Charting Process and Data Items
2.6. Synthesis of Results
3. Results
3.1. Selection of Sources of Evidence
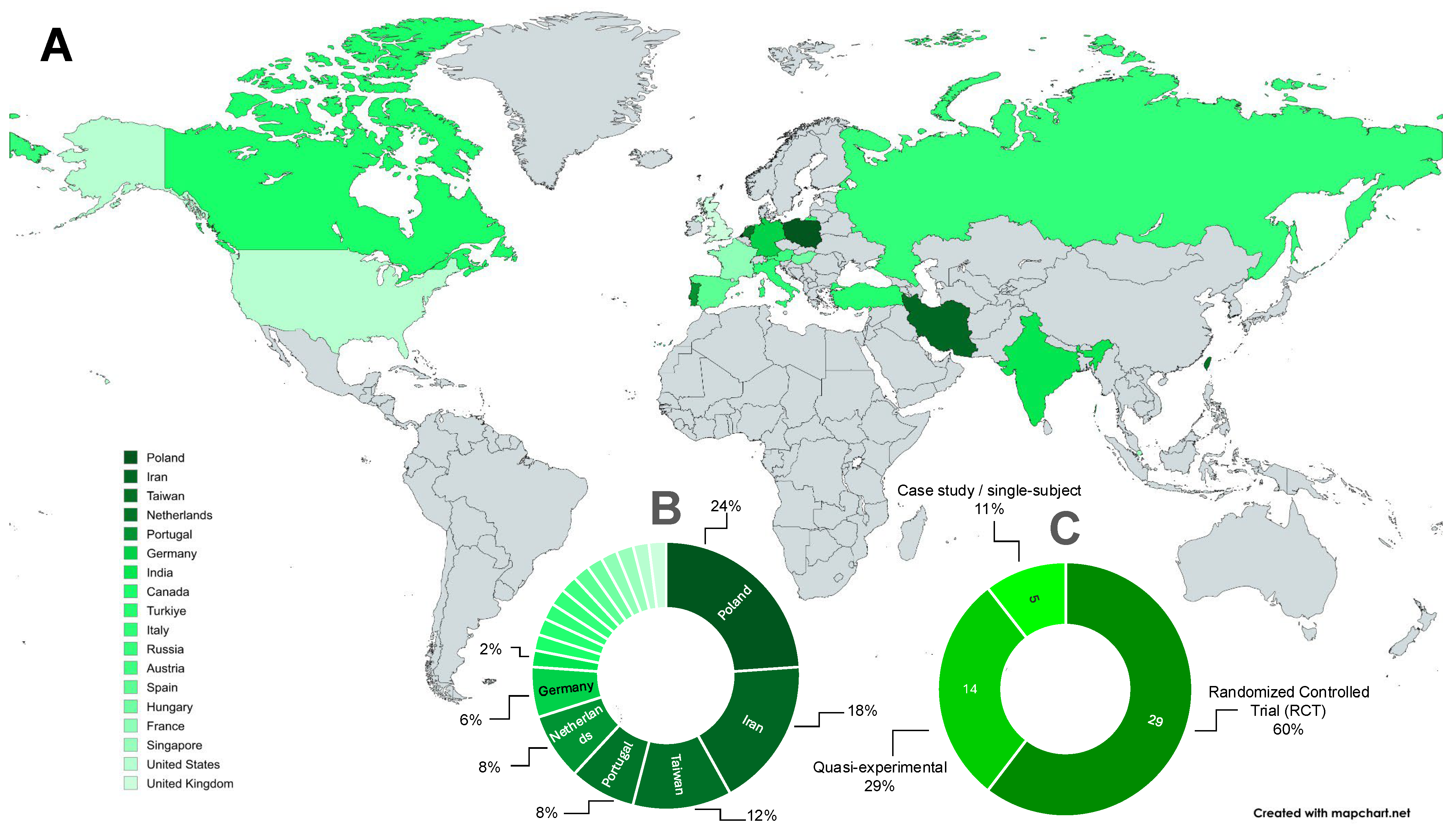
3.2. Characteristics of Included Studies
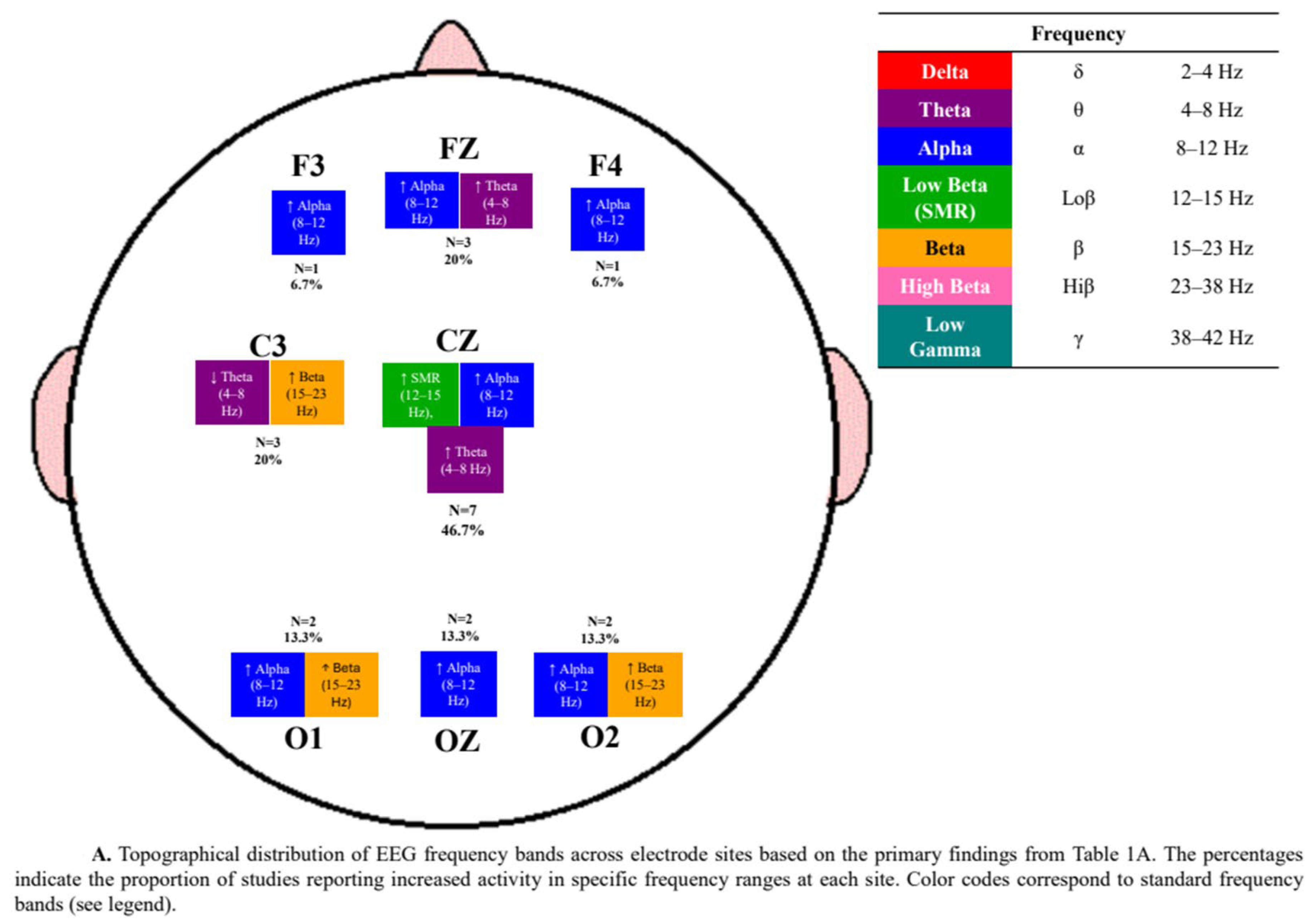
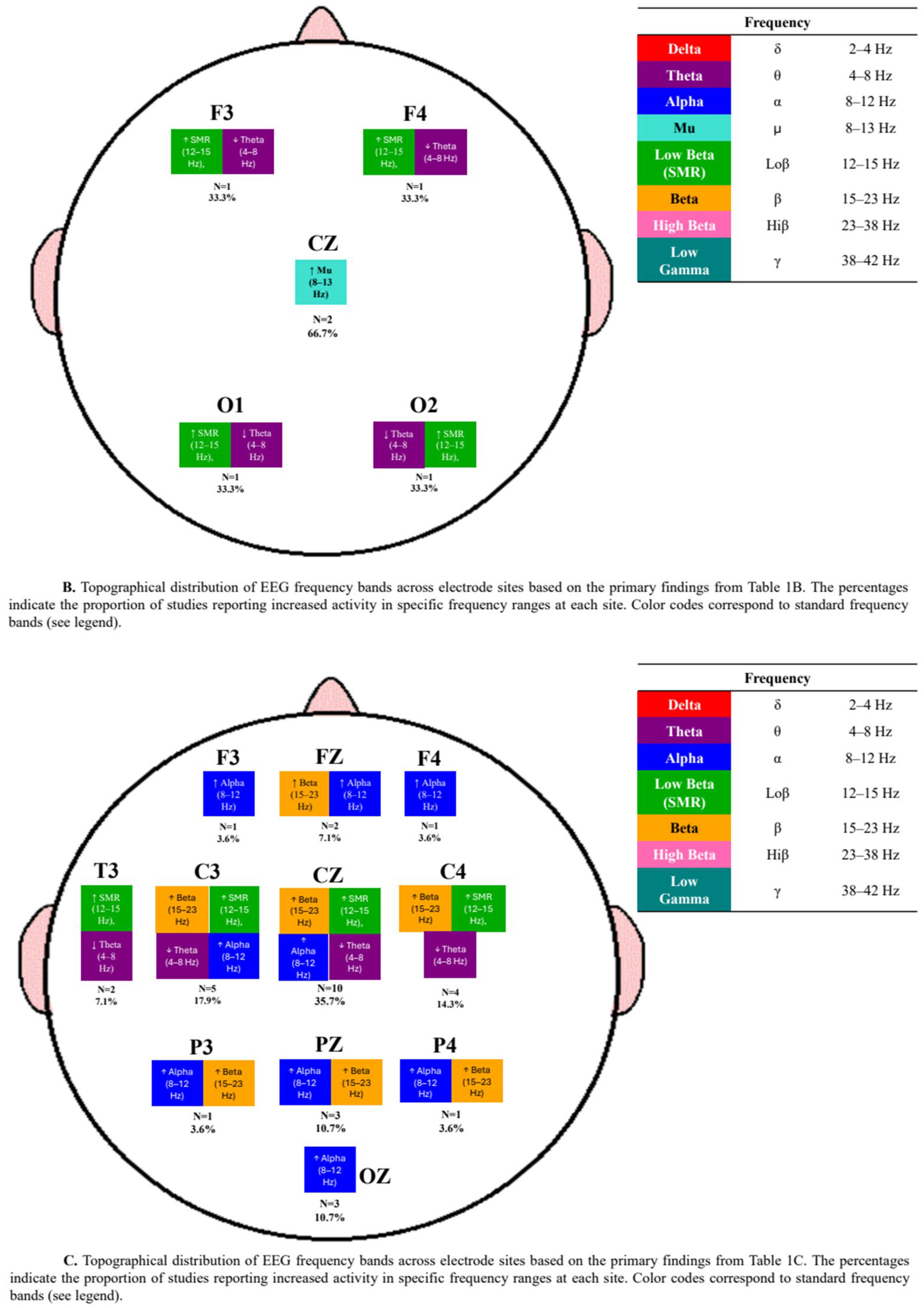
3.3. Neurophysiological Outcomes
3.4. Cognitive Outcomes
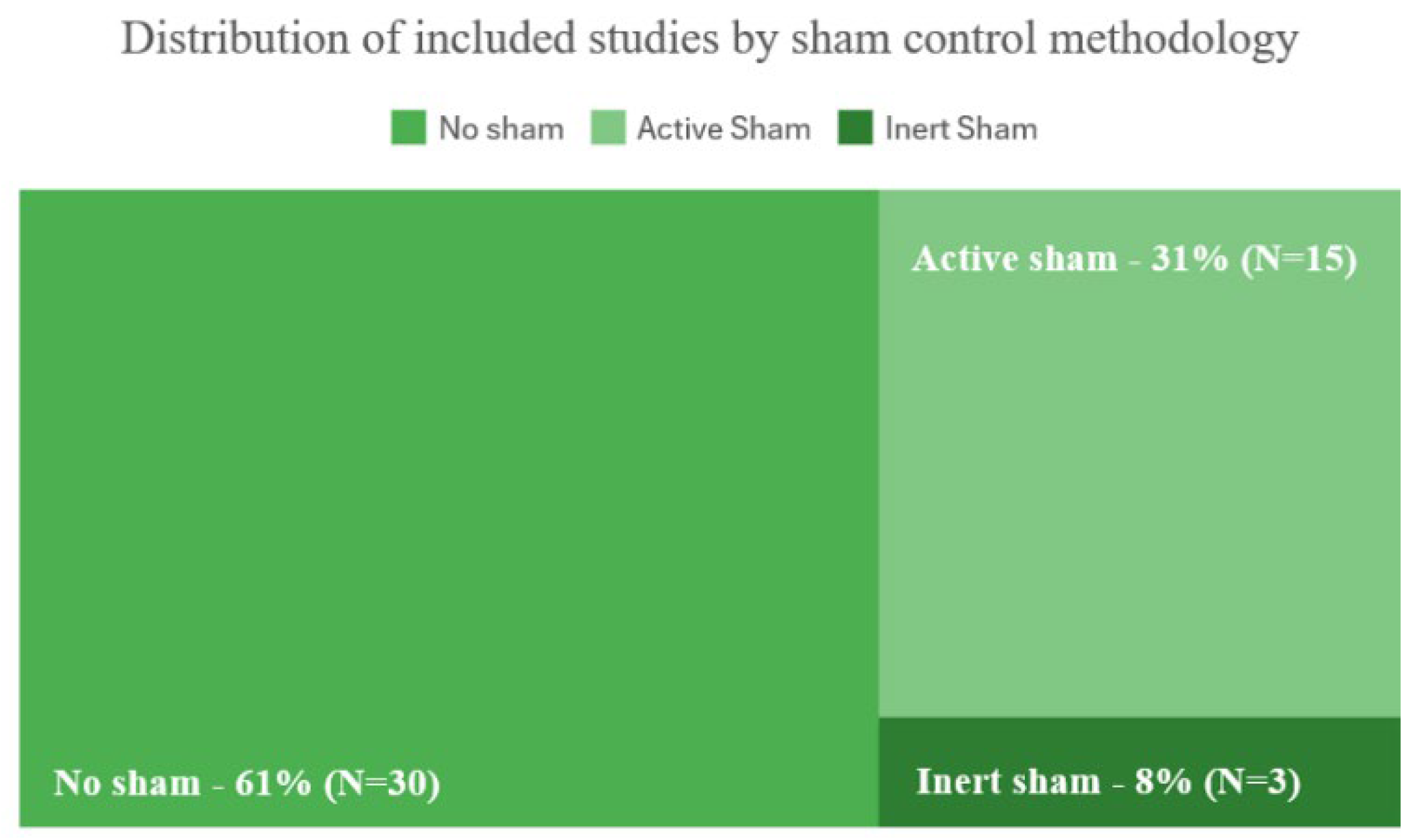
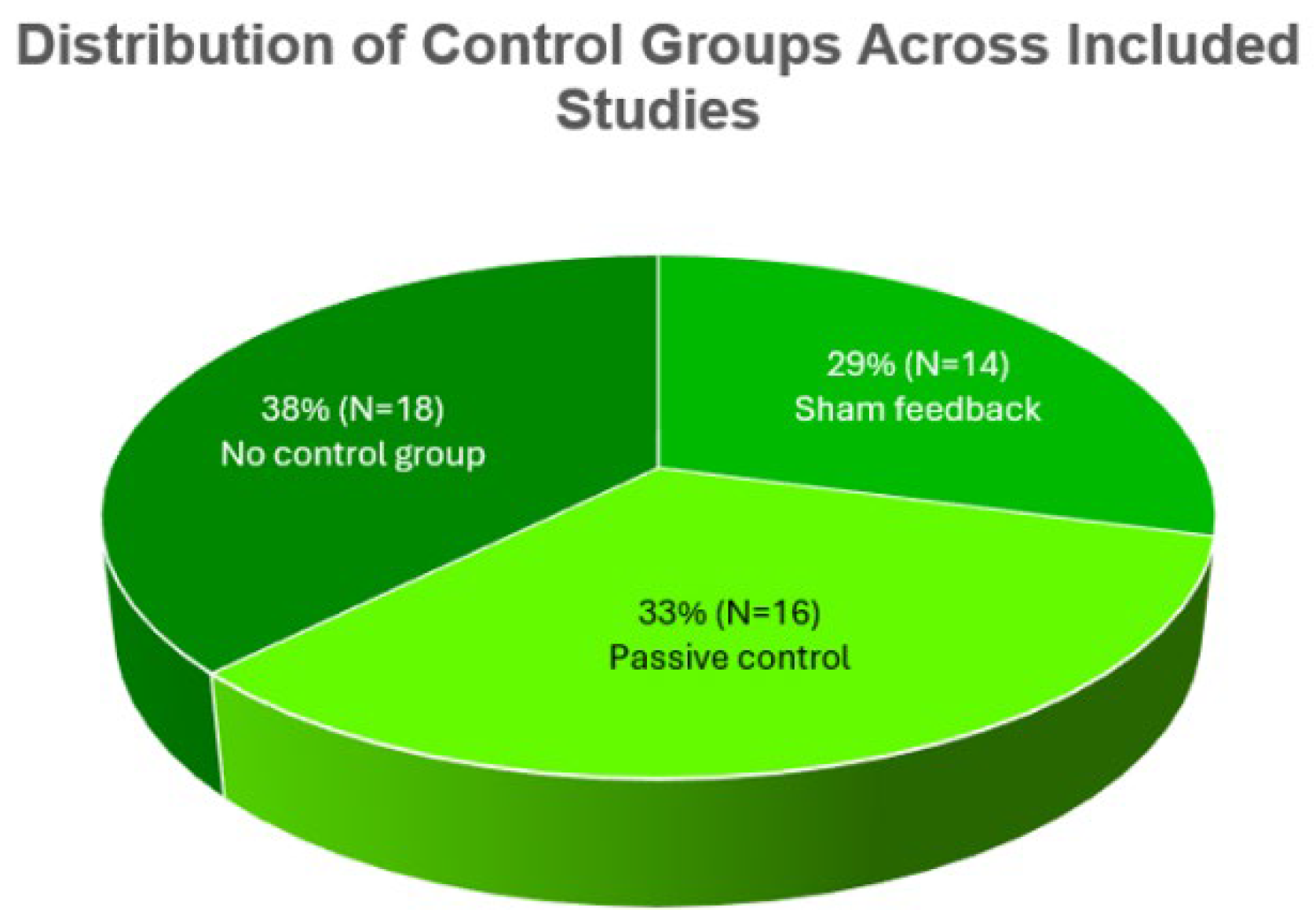
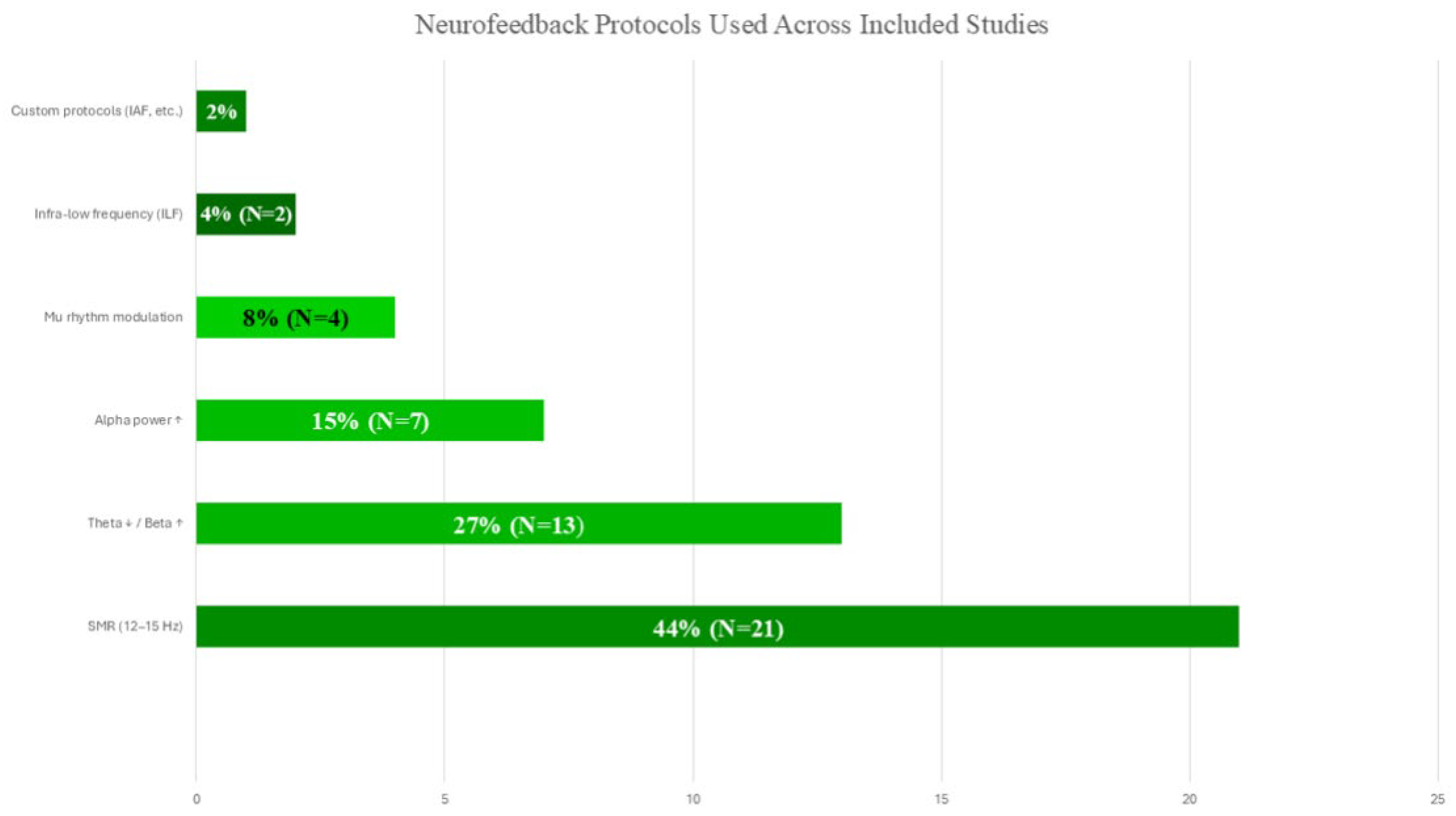
3.5. Methodological Features
3.6. Transparency and Reproducibility
4. Discussion
4.1. Neurophysiological and Cognitive Outcomes
4.2. The Role of Sham Controls
4.3. Electrode and Frequency Variability in Neurofeedback Protocols
4.4. Ecological Validity and Implementation Challenges
4.5. Considerations for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schomer, D.L.; Da Silva, F.H.L. (Eds.) Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, 7th ed.; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Gruzelier, J.H. EEG-neurofeedback for optimising performance. I: A review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 2014, 44, 124–141. [Google Scholar] [CrossRef]
- Hammond, D.C. What is Neurofeedback: An Update. J. Neurother. 2011, 15, 305–336. [Google Scholar] [CrossRef]
- Tosti, B.; Corrado, S.; Mancone, S.; Di Libero, T.; Carissimo, C.; Cerro, G.; Rodio, A.; Furtado da Silva, V.; Coimbra, D.R.; Andrade, A.; et al. Neurofeedback training protocols in sports: A systematic review of recent advances in performance, anxiety, and emotional regulation. Brain Sci. 2024, 14, 1036. [Google Scholar] [CrossRef] [PubMed]
- Rydzik, Ł.; Wsącz, W.; Ambrozy, T.; Javdanekh, N.; Rydzak, K.; Koparska, M. The use of neurofeedback in sports training: Systematic review. Brain Sci. 2023, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Landers, D.M.; Petruzzello, S.J.; Salazar, W.; Crews, D.J.; Kubitz, K.A.; Gannon, T.L.; Han, M. The influence of electrocortical biofeedback on performance in pre-elite archers. Med. Sci. Sports Exerc. 1991, 23, 123–129. [Google Scholar] [CrossRef]
- Paktaş, Y. The effect of neurofeedback training on the perceptual-motor abilities of basketball athletes. Pak. J. Med. Health Sci. 2021, 15, 791–793. [Google Scholar]
- Gołaś, A.; Nitychoruk, M.; Żak, M.; Kowalczyk, M.; Ignatjeva, A.; Maszczyk, A. Optimizing visual processing efficiency using neurofeedback training in judo athletes. Arch. Budo Sci. Martial Arts Extrem. Sports 2019, 15, 105–112. [Google Scholar]
- Krawczyk, M.; Kowalczyk, M.; Żak, M.; Daros, K.; Gozdowski, P. Zmiany aktywności fal mózgowych pod wpływem treningu neurofeedback u zawodników judo. Ogrody Nauk. I Szt. 2019, 9, 388–399. [Google Scholar] [CrossRef]
- Maszczyk, A.; Dobrakowski, P.; Nitychoruk, M.; Zak, M.; Kowalczyk, M.; Toborek, M. The Effect of Neurofeedback Training on the Visual Processing Efficiency in Judo Athletes. J. Hum. Kinet. 2020, 71, 219–227. [Google Scholar] [CrossRef]
- Rijken, N.H.; Soer, R.; de Maar, E.; Prins, H.; Teeuw, W.B.; Peuscher, J.; Oosterveld, F.G. Increasing Performance of Professional Soccer Players and Elite Track and Field Athletes with Peak Performance Training and Biofeedback: A Pilot Study. Appl. Psychophysiol. Biofeedback 2016, 41, 421–430. [Google Scholar] [CrossRef]
- Liu, Y.S.; Subramaniam, S.C.H.; Sourina, O.; Shah, E.; Chua, J.; Ivanov, K. Neurofeedback training for rifle shooters to improve cognitive ability. In Proceedings of the 2017 International Conference on Cyberworlds (CW), Chester, UK, 20–22 September 2017; pp. 186–189. [Google Scholar]
- Mikicin, M.; Mroz, A.; Karczewska-Lindinger, M.; Malinowska, K.; Mastalerz, A.; Kowalczyk, M. Effect of the Neurofeedback-EEG Training During Physical Exercise on the Range of Mental Work Performance and Individual Physiological Parameters in Swimmers. Appl. Psychophysiol. Biofeedback 2020, 45, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Assadourian, S.; Branco Lopes, A.; Saj, A. Improvement in peripheral visual attentional performance in professional soccer players following a single neurofeedback training session. Rev. Neuropsychol. 2022, 14, 133–138. [Google Scholar] [CrossRef]
- Lo, L.C.; Hatfield, B.D.; Janjigian, K.; Wang, Y.S.; Fong, D.Y.; Hung, T.M. The Effect of Left Temporal EEG Neurofeedback Training on Cerebral Cortical Activity and Precision Cognitive-Motor Performance. Res. Q. Exerc. Sport 2024, 96, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Norouzi, E. Effect of neurofeedback training on self-talk and performance in elite and non-elite volleyball players. Med. Dello Sport 2017, 70, 344–353. [Google Scholar] [CrossRef]
- Salimnejad, Z.; Zandi, H.; Arsham, S. Effect of Bio-Neural Feedback Exercises on the Performance of Female Rugby Players. Int. J. Mot. Control Learn. 2019, 1, 10–18. [Google Scholar] [CrossRef]
- Bussalb, A.; Congedo, M.; Barthélemy, Q.; Ojeda, D.; Acquaviva, E.; Delorme, R.; Mayaud, L. Clinical and experimental factors influencing the efficacy of neurofeedback in ADHD: A meta-analysis. Front. Psychiatry 2019, 10, 35. [Google Scholar] [CrossRef]
- Thibault, R.; Raz, A. The psychology of neurofeedback: Clinical intervention even if applied placebo. Am. Psychol. 2017, 72, 679–688. [Google Scholar] [CrossRef]
- Ros, T.; Enriquez-Geppert, S.; Zotev, V.; Young, K.D.; Wood, G.; Whitfield-Gabrieli, S.; Wan, F.; Vuilleumier, P.; Vialatte, F.; Van De Ville, D.; et al. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist). Brain 2020, 143, 1674–1685. [Google Scholar] [CrossRef]
- Nichols, T.; Das, S.; Eickhoff, S.; Evans, A.; Glatard, T.; Hanke, M.; Kriegeskorte, N.; Milham, M.; Poldrack, R.; Poline, J.-B.; et al. Best practices in data analysis and sharing in neuroimaging using MRI. Nat. Neurosci. 2017, 20, 299–303. [Google Scholar] [CrossRef]
- Poldrack, R.; Baker, C.; Durnez, J.; Gorgolewski, K.; Matthews, P.; Munafo, M.; Nichols, T.; Poline, J.-B.; Vul, E.; Yarkoni, T. Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 2017, 18, 115–126. [Google Scholar] [CrossRef]
- Donoghue, T.; Haller, M.; Peterson, E.; Varma, P.; Sebastian, P.; Gao, R.; Noto, T.; Lara, A.; Wallis, J.; Knight, R.; et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 2020, 23, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Ring, C.; Cooke, A.; Kavussanu, M.; McIntyre, D.; Masters, R. Investigating the efficacy of neurofeedback training for expediting expertise and excellence in sport. Psychol. Sport Exerc. 2015, 16, 118–127. [Google Scholar] [CrossRef]
- van Boxtel, G.J.M.; Denissen, A.; de Groot, J.A.; Neleman, M.S.; Vellema, J.; Hart de Ruijter, E.M. Alpha Neurofeedback Training in Elite Soccer Players Trained in Groups. Appl. Psychophysiol. Biofeedback 2024, 49, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Chueh, T.Y.; Yu, C.L.; Wang, K.P.; Kao, S.C.; Gentili, R.J.; Hatfield, B.D.; Hung, T.M. Effect of a single session of sensorimotor rhythm neurofeedback training on the putting performance of professional golfers. Scand. J. Med. Sci. Sports 2024, 34, e14540. [Google Scholar] [CrossRef]
- Wu, J.H.; Tu, Y.C.; Chang, C.Y.; Chueh, T.Y.; Gentili, R.J.; Hatfield, B.D.; Hung, T.M. A single session of sensorimotor rhythm neurofeedback enhances long-game performance in professional golfers. Biol. Psychol. 2024, 192, 108844. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Rogala, J.; Jurewicz, K.; Paluch, K.; Kublik, E.; Cetnarski, R.; Wróbel, A. The Do’s and Don’ts of Neurofeedback Training: A Review of the Controlled Studies Using Healthy Adults. Front. Hum. Neurosci. 2016, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Trullinger, M.; Novian, A.; Russell-Chapin, L.; Pradhan, D. Perspectives on Type III Statistical Errors: Exaggerating the Effects of Placebo in Neurofeedback. NeuroRegulation 2019, 6, 38–41. [Google Scholar] [CrossRef]
- Dekker, M.K.; van den Berg, B.R.; Denissen, A.J.; Sitskoorn, M.M.; van Boxtel, G.J. Feasibility of eyes open alpha power training for mental enhancement in elite gymnasts. J. Sports Sci. 2014, 32, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.Y.; Huang, C.J.; Chang, Y.K.; Koester, D.; Schack, T.; Hung, T.M. Sensorimotor Rhythm Neurofeedback Enhances Golf Putting Performance. J. Sport Exerc. Psychol. 2015, 37, 626–636. [Google Scholar] [CrossRef]
- Maszczyk, A.; Golas, A.; Pietraszewski, P.; Kowalczyk, M.; Cieszczyk, P.; Kochanowicz, A.; Smolka, W.; Zajac, A. Neurofeedback for the enhancement of dynamic balance of judokas. Biol. Sport 2018, 35, 99–102. [Google Scholar] [CrossRef]
- Kober, S.E.; Ninaus, M.; Witte, M.; Buchrieser, F.; Grossinger, D.; Fischmeister, F.P.S.; Neuper, C.; Wood, G. Triathletes are experts in self-regulating physical activity—But what about self-regulating neural activity? Biol. Psychol. 2022, 173, 108406. [Google Scholar] [CrossRef]
- Afrash, S.; Saemi, E.; Gong, A.; Doustan, M. Neurofeedback training and motor learning: The enhanced sensorimotor rhythm protocol is better or the suppressed alpha or the suppressed mu? BMC Sports Sci. Med. Rehabil. 2023, 15, 93. [Google Scholar] [CrossRef]
- Chen, T.-T.; Wang, K.-P.; Chang, W.-H.; Kao, C.-W.; Hung, T.-M. Effects of the function-specific instruction approach to neurofeedback training on frontal midline theta waves and golf putting performance. Psychol. Sport Exerc. 2022, 61, 102211. [Google Scholar] [CrossRef]
- Mottola, F.; Blanchfield, A.; Hardy, J.; Cooke, A. EEG neurofeedback improves cycling time to exhaustion. Psychol. Sport Exerc. 2021, 55, 101944. [Google Scholar] [CrossRef]
- Pourbehbahani, Z.; Saemi, E.; Cheng, M.Y.; Dehghan, M.R. Both Sensorimotor Rhythm Neurofeedback and Self-Controlled Practice Enhance Motor Learning and Performance in Novice Golfers. Behav. Sci. 2023, 13, 65. [Google Scholar] [CrossRef]
- Mirifar, A.; Keil, A.; Beckmann, J.; Ehrlenspiel, F. No Effects of Neurofeedback of Beta Band Components on Reaction Time Performance. J. Cogn. Enhanc. 2019, 3, 251–260. [Google Scholar] [CrossRef]
- Wang, K.P.; Frank, C.; Hung, T.M.; Schack, T. Neurofeedback training: Decreases in Mu rhythm lead to improved motor performance in complex visuomotor skills. Curr. Psychol. 2023, 42, 20860–20871. [Google Scholar] [CrossRef] [PubMed]
- Horvath, D.; Negyesi, J.; Racz, M.; Gyori, T.; Matics, Z.; Puskin, A.; Csipor, J.; Racz, L. Feasibility of a novel neurofeedback system: A parallel randomized single-blinded pilot study. Sci. Rep. 2023, 13, 17353. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.P.; Cheng, M.Y.; Elbanna, H.; Schack, T. A new EEG neurofeedback training approach in sports: The effects function-specific instruction of Mu rhythm and visuomotor skill performance. Front. Psychol. 2023, 14, 1273186. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; Kleinnijenhuis, M.; Fallahpour, K.; Breteler, R. Golf Performance Enhancement and Real-Life Neurofeedback Training Using Personalized Event-Locked EEG Profiles. J. Neurother. 2008, 11, 11–18. [Google Scholar] [CrossRef]
- Ziółkowski, A.; Graczyk, M.; Strzałkowska, A.; Włodarczyk, P.; Zarańska, B. Neuronal, cognitive and social indicators for the control of aggressive behaviors in sport. Acta Neuropsychol. 2012, 10, 537–546. [Google Scholar]
- Graczyk, M.; Pachalska, M.; Ziolkowski, A.; Manko, G.; Lukaszewska, B.; Kochanowicz, K.; Mirski, A.; Kropotov, I.D. Neurofeedback training for peak performance. Ann. Agric. Environ. Med. 2014, 21, 871–875. [Google Scholar] [CrossRef]
- Kao, S.-C.; Huang, C.-J.; Hung, T.-M. Neurofeedback Training Reduces Frontal Midline Theta and Improves Putting Performance in Expert Golfers. J. Appl. Sport Psychol. 2014, 26, 271–286. [Google Scholar] [CrossRef]
- Mikicin, M.; Orzechowski, G.; Jurewicz, K.; Paluch, K.; Kowalczyk, M.; Wróbel, A. Brain-training for physical performance: A study of EEG-neurofeedback and alpha relaxation training in athletes. Acta Neurobiol. Exp. 2015, 75, 434–445. [Google Scholar]
- Mikicin, M. State of mind as a subjective mental sensation results from objective brain activity following neurofeedback-EEG and relaxation trainings. Acta Neuropsychol. 2016, 14, 17–33. [Google Scholar]
- Szczypińska, M.; Mikicin, M. Does attention training induce any changes in the level of the selected cognitive processes in handball players? J. Phys. Educ. Sport 2019, 19, 1445–1452. [Google Scholar] [CrossRef]
- Domingos, C.; Alves, C.; Sousa, E.; Rosa, A.; Pereira, J. Does Neurofeedback Training Improve Performance in Athletes? NeuroRegulation 2020, 7, 8–17. [Google Scholar] [CrossRef]
- Domingos, C.; da Silva Caldeira, H.; Miranda, M.; Melicio, F.; Rosa, A.C.; Pereira, J.G. The Influence of Noise in the Neurofeedback Training Sessions in Student Athletes. Int. J. Environ. Res. Public Health 2021, 18, 13223. [Google Scholar] [CrossRef] [PubMed]
- Domingos, C.; Peralta, M.; Prazeres, P.; Nan, W.; Rosa, A.; Pereira, J.G. Session frequency matters in neurofeedback training of athletes. Appl. Psychophysiol. Biofeedback 2021, 46, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Domingos, C.; Silva, C.M.D.; Antunes, A.; Prazeres, P.; Esteves, I.; Rosa, A.C. The Influence of an Alpha Band Neurofeedback Training in Heart Rate Variability in Athletes. Int. J. Environ. Res. Public Health 2021, 18, 12579. [Google Scholar] [CrossRef]
- Mikicin, M.; Orzechowski, G. Neuronal Activity in the Brain Changes During Exercise in Attention States, Warm-up, Submaximal Effort, and Recovery, After Neurofeedback-Eeg Training in Motion. Acta Neuropsychol. 2022, 20, 175–186. [Google Scholar] [CrossRef]
- Fuentes-Garcia, J.P.; Villafaina, S. Psychophysiological and Performance Effects of Biofeedback and Neurofeedback Interventions in a Top 100 Female Chess Player. Behav. Sci. 2024, 14, 1044. [Google Scholar] [CrossRef]
- Bakhtafrooz, S.; Kavyani, M.; Farsi, A.; Alboghebeish, S. The effect of infra low frequency-neurofeedback training on pistol shooting performance and attention in semi-skilled players. Front. Hum. Neurosci. 2025, 19, 1487737. [Google Scholar] [CrossRef]
- Paul, M.; Ganesan, S.; Sandhu, J.; Simon, J. Effect of sensory motor rhythm neurofeedback on psycho-physiological, electro-encephalographic measures and performance of archery players. Ibnosina J. Med. Biomed. Sci. 2012, 4, 32–39. [Google Scholar] [CrossRef]
- Rostami, R.; Sadeghi, H.; Karami, K.A.; Abadi, M.N.; Salamati, P. The Effects of Neurofeedback on the Improvement of Rifle Shooters’ Performance. J. Neurother. 2012, 16, 264–269. [Google Scholar] [CrossRef]
- Strizhkova, O.; Cherapkina, L.; Strizhkova, T. Neurofeedback course applying of high skilled gymnasts in competitive period. J. Hum. Sport Exerc. 2012, 7, S185–S193. [Google Scholar] [CrossRef][Green Version]
- Christie, S.; Bertollo, M.; Werthner, P. The Effect of an Integrated Neurofeedback and Biofeedback Training Intervention on Ice Hockey Shooting Performance. J. Sport Exerc. Psychol. 2020, 42, 34–47. [Google Scholar] [CrossRef]
- Shokri, A.; Nosratabadi, M. Comparison of Biofeedback and Combined Interventions on Athlete’s Performance. Appl. Psychophysiol. Biofeedback 2021, 46, 227–234. [Google Scholar] [CrossRef]
- Fardinia, M.; Shojaei, M.; Rahimi, A. The effect of neurofeedback training on the anxiety of elite female swimmers. Ann. Biol. Res. 2012, 3, 1020–1028. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


| (A) | ||||
| Authors (Year) | Sample | Discipline & Level | Protocol (Summary) | Main Findings |
| [6] | 24 pre-elite archers | Archery—pre-elite with competition experience | RCT with 3 groups: correct feedback, incorrect (active sham), and control; feedback modeled on slow cortical potential (SCP) paradigm | Significant improvement in performance in performance in the correct feedback group; performance decrement in incorrect feedback group; no significant change in control |
| [35] | 12 elite gymnasts | Gymnastics—national/international | Double-blind RCT: Alpha-band NFB vs. Sham (active control with random beta-band feedback) | Trend toward improved mental balance, physical shape, and reduced sleep complaints (ns) |
| [36] | 16 pre-elite/elite golfers | Golf—national/international | RCT: SMR-based NFB vs. Sham; pre-post assessment of EEG and performance | Increased putting accuracy, consistency, and SMR power in NFB group; no change in control |
| [37] | 18 elite judokas | Judo—national/international | Double-blind RCT: NFB targeting θ/β ratio vs. Sham; pre-post design | Significant gains in balance and β-power (p < 0.05); no significant change in sham group |
| [7] | 30 male basketball players | Basketball—competitive athletes (level not specified) | Double-blind quasi-experimental design: generic NFB protocol (EEG at Cz/Fz/parietal); control with sham feedback | Improved reaction time, balance, attention and reduced anxiety compared with controls (p < 0.05) |
| [38] | 26 triathletes (elite endurance) and 25 controls | Triathlon—elite endurance athletes | Double-blind RCT: SMR-NFB vs. Sham (single session); EEG and MRI metrics | Real NFB group: ↑ SMR power (p = 0.02), ↑ gray/white matter volumes (p < 0.001); no change in sham |
| [39] | 64 novice golfers | Golf—no prior competitive experience | RCT: SMR, alpha, mu NFB vs. Sham; pre-post and retention tests | SMR & alpha groups: ↑ putting accuracy and EEG power short- and long-term; mu group ≈ sham |
| [40] | 36 skilled golfers | Golf—competitive amateurs | Single-session RCT: FSI-NFB vs. TI vs. Sham; FMT measured | FSI-NFB improved putting success and reduced FMT power (p < 0.05); sham and TI showed no improvement |
| [41] | 40 recreational cyclists | Cycling—recreational | Crossover RCT: NFL-NFB vs. NFR-NFB vs. Sham; endurance and EEG metrics assessed | NFB increased endurance (+30%, p < 0.05), frontal alpha asymmetry, and HR/RPE; no changes in lactate or cadence |
| [42] | 40 novice golfers | Golf—no prior experience | RCT: SMR-NFB vs. Sham; with self-controlled and yoked conditions; pre-post follow-up design | SMR-NFB group: ↑ putting accuracy (p < 0.05), ↑ SMR power, ↑ self-control; no additive effect beyond feedback |
| [43] | 38 male soccer players (14–23 y; ≥4 y training) | Soccer—competitive athletes | RCT: TBR-NFB and SMR-NFB vs. Sham; 10 sessions; outcomes: attention, reaction time, EEG power | No significant improvement in attention or reaction time; no EEG changes reported |
| [8] | 12 elite judo athletes (Polish Judo Assoc.) | Judo—elite athletes | RCT: β↑/θ↓ NFB vs. Sham; 15 sessions across 2 cycles | NFB group showed ↑ reaction speed and β power, ↓ θ power; sham group showed no change |
| [24] | 24 recreational golfers (all male) | Golf—novice athletes | RCT: high-alpha/theta NFB vs. Sham; 3 sessions; putting accuracy task | NFB group: ↓ frontal high-alpha power; no selective performance improvement; both groups performed similarly under pressure |
| [9] | 12 elite judokas | Judo—elite national | RCT: β↑/θ↓ NFB vs. Sham; 15 sessions in 2 training cycles | NFB group: ↑ reaction speed and β power, ↓ θ power; sham group unchanged |
| [10] | 12 elite judokas | Judo—elite international | RCT: β↑/θ↓ NFB vs. Sham; 15 sessions (2 cycles); system: Deymed TruScan (Deymed Diagnostic-Hronov, Czech Republic) | NFB group: ↑ reaction speed and β power, ↓ θ power; no EEG change in sham |
| (B) | ||||
| Authors (Year) | Sample | Discipline & Level | Protocol (Summary) | Main Finding |
| [44] | 30 novice golfers (15 F, 15 M) | Golf—no prior experience | RCT: ↑ Mu-NFB vs. ↓ Mu-NFB vs. Sham; single session (BioTrace+ system. Mind Media, Herten, The Netherlands); pre–post design | ↓ Mu-NFB group: ↓ Mu power, ↓ MRE, ↑ putting accuracy (p = 0.006); ↑Mu-NFB showed no significant changes; sham had no effect |
| [45] | 31 young athletes (multi-sport) | Multi-sport—regular training | Single-blind RCT: SMR/Theta NFB vs. Sham; 12 sessions over 4 weeks (NeuroTracker platform, NeuroTrackerX Inc., Montreal, QC, Canada) | All groups improved cognitive performance (p < 0.05), but no between-group differences and no EEG changes |
| [46] | 30 novice golfers (14 F, 16 M) | Golf—no prior experience | Double-blind RCT: FSI-NFB vs. TI-NFB vs. Sham; single session (BioTrace+) | FSI-NFB group: ↓ putting accuracy (p = 0.013), slight ↑ Mu power (ns), positive Mu–error correlation (r = 0.319, p = 0.043); TI and sham showed no changes |
| (C) | ||||
| Authors (Year) | Sample | Discipline & Level | Study Design | Electrode & Protocol |
| [47] | 6 amateur golfers (3 F, 3 M; avg handicap 12.3) | Golf—amateur | Quasi-experimental within-subject ABAB design; same athletes performed alternating blocks with and without event-locked NFB (Fpz channel) | NFB blocks led to ↑ putting success (+25%) compared to no-NFB blocks; no sham group included |
| [48] | 1 elite javelin thrower (25 y, Olympic level) | Javelin—elite | Case study using alpha-NFB (C3/C4; ProComp Infiniti, Thought Technology Ltd., Montreal, QC, Canada); ERP assessment (NOGO task) | Improved attention and social behavior; ↓ aggression; ↑ ERP changes and reaction time |
| [49] | 1 elite javelin thrower (male, 25 years old, Olympic-level athlete) | Javelin—elite Olympic athlete (2012 London Olympics participant) | Single-subject pre–post case study; Alpha/Low-Beta NFB (C3/C4; ProComp Infiniti); ERP and cognitive testing | ↑ ERP amplitudes and β power; improved cognitive control and emotional regulation |
| [50] | 3 expert golfers (20–25 y, >10 y exp.) | Golf—elite | Case study with multiple baseline design (3 athletes); EEG at Fz (4–8 Hz) downregulation; NeuroTek training (NeuroTek, Goshen, KY, USA) | 2/3 participants: ↑ putting performance and ↓ θ power; mixed results for anxiety and confidence |
| [51] | 35 semi-professional athletes (25 M, 10 F; mixed sports) | Multi-sport—semi-pro | Quasi-experimental: 20 NFB sessions (alpha, beta1, theta) vs. control; pre–post design | EG showed ↑ α and β1 power, faster reaction time, improved mental performance; control group unchanged; no sham group |
| [52] | 73 student athletes (40 M, 33 F; 18–25 y; swimming, fencing, track & field, taekwondo, judo) | Multi-sport—national-level | Quasi-experimental: 20 NFB sessions (SMR, beta1, theta) vs. control; 7-month program | EG: ↑ SMR & β1, ↓ θ, ↑ mental readiness/performance; CG: no change |
| [11] | 21 elite athletes (soccer & track & field; 16–38 y) | Soccer & Track & Field—elite | Quasi-experimental: alpha↑ NFB (C3/C4); 4 coached + daily home sessions (5 weeks) | Soccer group: ↑ alpha (5/7 EEG sites), ↓ LF/HF, ↑ emotional stability, concentration & sleep; Track & Field: ↑ recovery (RESTQ), sustained LF/HF balance |
| [12] | 5 elite female rifle shooters | Rifle Shooting—elite international | Single-subject design using β1/θ NFB at P8 (Emotiv 14-channel EEG System, San Francisco, CA, USA); 6–7 sessions; attention and shooting accuracy assessed | 3/5 athletes improved shooting accuracy and attention; 2 remained stable; no adverse effects reported |
| [53] | 18 elite handball players (9 M, 9 F; 1st/2nd league) | Handball—elite | Quasi-experimental NFB (↑SMR, ↑β1, ↓θ, ↓β2) using DigiTrack system (ELMIKO Medical, Warsaw, Poland); 20 sessions over 10 weeks; outcomes: attention, sensorimotor coordination, peripheral perception | Improved attention, ↑ sensorimotor coordination, ↑ peripheral perception (mainly in males) |
| [54] | 45 participants (15 athletes, 15 non-athletes, 15 controls; 18–44 y) | Multi-sport—athletes & non-athletes | RCT: athletes-NFB, athletes-control, non-athletes-NFB, non-athletes-control; 12–15 sessions; EEG at Cz (Vertex 823 system—Meditron Eletrônica, São Paulo, Brazil) | Athletes-NFB group: ↑ reaction time and IAB; non-athletes-NFB: ↑ SAB/IAB; control groups: no change |
| [13] | 7 elite swimmers (~20.6 y) | Swimming—elite | Pre–post single-group design (no control); NFB at C3/C4 (TruScan Flex 30); β (20–30 Hz) inhibition and SMR enhancement; 20 sessions (6 × 5 min) during exercise across 4 months | ↑ mental work capacity, ↑ EMG signal consistency; no changes in VO2max or anaerobic performance |
| [55] | 45 student athletes (7 F, ages 18–35; ≥5 y practice) | Multi-sport—student athletes | RCT: noisy vs. silent-room NFB vs. control; 12 sessions; working memory and reaction time tasks | Noisy-NFB group: ↑ working memory (p = 0.005) and faster reaction time; silent NFB and control: no effect |
| [56] | 45 male student athletes (18–34 y; ≥5 y practice) | Multi-sport—student athletes | RCT: 3×/week vs. 2×/week NFB vs. control; 12 sessions; EEG at Cz (Meditron Vertex 823) | 3×/week group: ↑ IAB and faster reaction time; 2×/week and control: no significant changes |
| [57] | 30 male student athletes (18–34 y; ≥5 y practice) | Multi-sport—student athletes | Quasi-experimental: 3×/week vs. 2×/week NFB; 12 sessions; HRV and IAB measured | 3×/week group: ↑ IAB and HRV; 2×/week: no change |
| [14] | 15 male professional soccer players (17.6 y; U17/U19/N2 levels) | Soccer—pro youth | Single-group RCT (no sham); EEG at P3/P4; 7 × 3-min sessions (Spectre Biotech, Suresnes, France) | ↑ attention and reaction (+30%, +27%, p < 0.01); effect maintained at 1-month follow-up (+20%) |
| [58] | 20 athletes (10 track & field, 10 swimmers; 18–25 y) | Multi-sport—elite athletes | Quasi-experimental: β2-NFB at C3/C4; 20 sessions over 4 months; EEG and recovery measures | ↑ EEG (θ–β) modulation during attention tasks, ↑ effort and recovery; control group: no change |
| [59] | 1 elite female chess player (ELO > 2350; Top 100 worldwide) | Chess—elite athlete | Case study: SMR↑/θ↓ NFB + BFB; 14 sessions + 6/12-month follow-up | ↑ chess performance (+38% puzzle rush, +20–25 ELO), ↓ anxiety, ↑ HRV and regulation control; sustained gains at 6–12 months |
| [25] | 41 elite soccer players (26 F, 15 M; 2 Dutch pro teams; ~20 s) | Soccer—elite national | Crossover quasi-experimental study: α↑ NFB (BrainBit music-feedback system, BrainBit Inc., Rancho Santa Margarita, CA, USA); 20 sessions over 4–6 weeks; cognitive tasks included (N-back, PVT, mental rotation) | EG: ↑ α power (+34%, p < 0.001), ↑ task switching and mental rotation performance; slight ↑ in control/flow; no effect on N-back or PVT |
| [26] | 44 professional golfers (20 F, 24 M; PGA/LPGA; mean age 26.8 y) | Golf—professional | Crossover RCT: SMR-NFB vs. no-training control; single session (~2.5 h); EEG at Cz (12–15 Hz; ProComp5 Infiniti) | NFB group: ↑ putting accuracy and SMR power (p < 0.01), ↑ attention/motor control, ↑ relaxation (p < 0.01); control: no change |
| [27] | 17 professional female golfers (PGA of Taiwan/LPGA; mean age ~24.6 y) | Golf—professional | Crossover RCT: SMR-NFB vs. no-training control; single session (~2.5 h) using ProComp5 Infiniti; EEG at Cz | NFB group: ↑ swing accuracy (To Pin, p = 0.04), ↑ SMR power, ↑ motor control and relaxation; control group: no change |
| [60] | 20 semi-skilled pistol shooters (10 M, 10 F; mean age 28–40 y) | Shooting—semi-skilled national level | RCT: ILF-NFB vs. control; 20 sessions over 7 weeks; EEG at T3/T4/P4/Fp1 | ILF-NFB group: ↑ shooting accuracy (p = 0.005), ↑ attention network efficiency (ANT, p < 0.01); control: no change |
| [61] | 24 university archers (16 M, 8 F; ~22 y; 4 y experience) | Archery—university competitive | RCT: SMR-NFB vs. control; 12 sessions over 4 weeks (ProComp5 Infiniti); EEG at Cz | SMR-NFB group: ↑ pleasure and arousal (p < 0.05), ↓ SMR/θ ratio (p < 0.05); no performance improvement; control group: ↓ precision over time |
| [62] | 24 expert rifle shooters (~30 y; ~7 y experience) | Shooting—expert, national/provincial | Pre–post design: NFB vs. control; 15 sessions over 5 weeks (ProComp P2 and P8 systems (Thought Technology Ltd., Montreal, QC, Canada; C3/Pz; SMR, β1↑, α↑) | NFB group: ↑ shot result (p = 0.001); no other EEG or behavioral changes; control: no improvement |
| [63] | 28 female gymnasts (15 NFB, 13 control) | Gymnastics—high-skilled, competitive period | Pre–post quasi-experimental design: α↑ NFB (F1/F2/P3/P4; Boslab-alpha (SRIMBB RAMS, Novosibirsk, Russia)); 15 sessions before competition | NFB group: ↑ α power (p < 0.05), ↑ vestibular stability, ↑ memorization speed, ↓ self-estimation bias; control: no change in attention/anxiety |
| [15] | 20 national-level pistol shooters (3 F, 17 M) | Pistol Shooting—national-level experts | RCT: α↑ NFB vs. control; 16 sessions (Peak Achievement Trainer) | NFB group: ↑ shooting accuracy (p < 0.001), ↑ temporal α power; no change in coherence; control: no improvement |
| [64] | 31 university ice hockey players (18 F, 13 M; ~21.7 y) | Ice Hockey—elite university athletes | RCT: SMR/θ/β1 NFB + BFB vs. control; 15 sessions over 4.5 months | NFB group: ↑ shooting accuracy (p = 0.018), ↑ SMR (p = 0.001); no SMR change during competition; control: slower improvement |
| [65] | 45 novice basketball players (3 groups, n = 15) | Basketball—novice athletes (1–3 y exp.) | RCT: BFB + NFB vs. BFB vs. control; 24 sessions over 8 weeks (ProComp Infiniti system) | BFB + NFB group: ↑ technical skills (lay-up, passing) and physiological indices (BFB, HRV); control: no improvement |
| [66] | 20 elite female swimmers (13–14 y; 5–6 y experience) | Swimming—elite | RCT: SMR↑, θ↓, high-β↓ NFB (videogame-based) vs. control; 12 sessions over 4 weeks | NFB group: ↓ anxiety (p < 0.01); control: no change; significant time × group interaction (p = 0.017) |
| [16] | 30 male volleyball players (15 elite, 15 non-elite; mean age 22.8 y) | Volleyball—elite & non-elite | Quasi-experimental: 1 NFB session (SMR↑ 12–15 Hz; T3, T4 sites; videogame feedback); comparison between elite and non-elite groups | ↓ self-talk (p < 0.05), ↑ serve performance (p < 0.01); greater gains observed in elite athletes |
| [17] | 24 female rugby players (16–25 y; n = 12 NFB, n = 12 control) | Rugby—female athletes | Quasi-experimental pre–post design: NFB vs. control; 15 sessions (3×/week, 40 min); α↑ at Pz and SMR↑ at C3 | NFB group: ↑ passing accuracy (p < 0.01, both sides); no change in shot accuracy; control: no improvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacarias, R.M.G.; Bulathwatta, D.T.; Bidzan-Bluma, I.; de Jesus, S.N.; Correia, J.M. EEG-Based Neurofeedback in Athletes and Non-Athletes: A Scoping Review of Outcomes and Methodologies. Bioengineering 2025, 12, 1202. https://doi.org/10.3390/bioengineering12111202
Zacarias RMG, Bulathwatta DT, Bidzan-Bluma I, de Jesus SN, Correia JM. EEG-Based Neurofeedback in Athletes and Non-Athletes: A Scoping Review of Outcomes and Methodologies. Bioengineering. 2025; 12(11):1202. https://doi.org/10.3390/bioengineering12111202
Chicago/Turabian StyleZacarias, Rui Manuel Guerreiro, Darshika Thejani Bulathwatta, Ilona Bidzan-Bluma, Saúl Neves de Jesus, and João Mendonça Correia. 2025. "EEG-Based Neurofeedback in Athletes and Non-Athletes: A Scoping Review of Outcomes and Methodologies" Bioengineering 12, no. 11: 1202. https://doi.org/10.3390/bioengineering12111202
APA StyleZacarias, R. M. G., Bulathwatta, D. T., Bidzan-Bluma, I., de Jesus, S. N., & Correia, J. M. (2025). EEG-Based Neurofeedback in Athletes and Non-Athletes: A Scoping Review of Outcomes and Methodologies. Bioengineering, 12(11), 1202. https://doi.org/10.3390/bioengineering12111202






