Advancements in High-Resolution Computed Tomography: Revolutionising Bone Health Micro-Research
Abstract
1. Introduction
2. X-Ray-Based High-Resolution CT Modalities
2.1. Laboratory Micro-CT
2.1.1. Principles and System Types
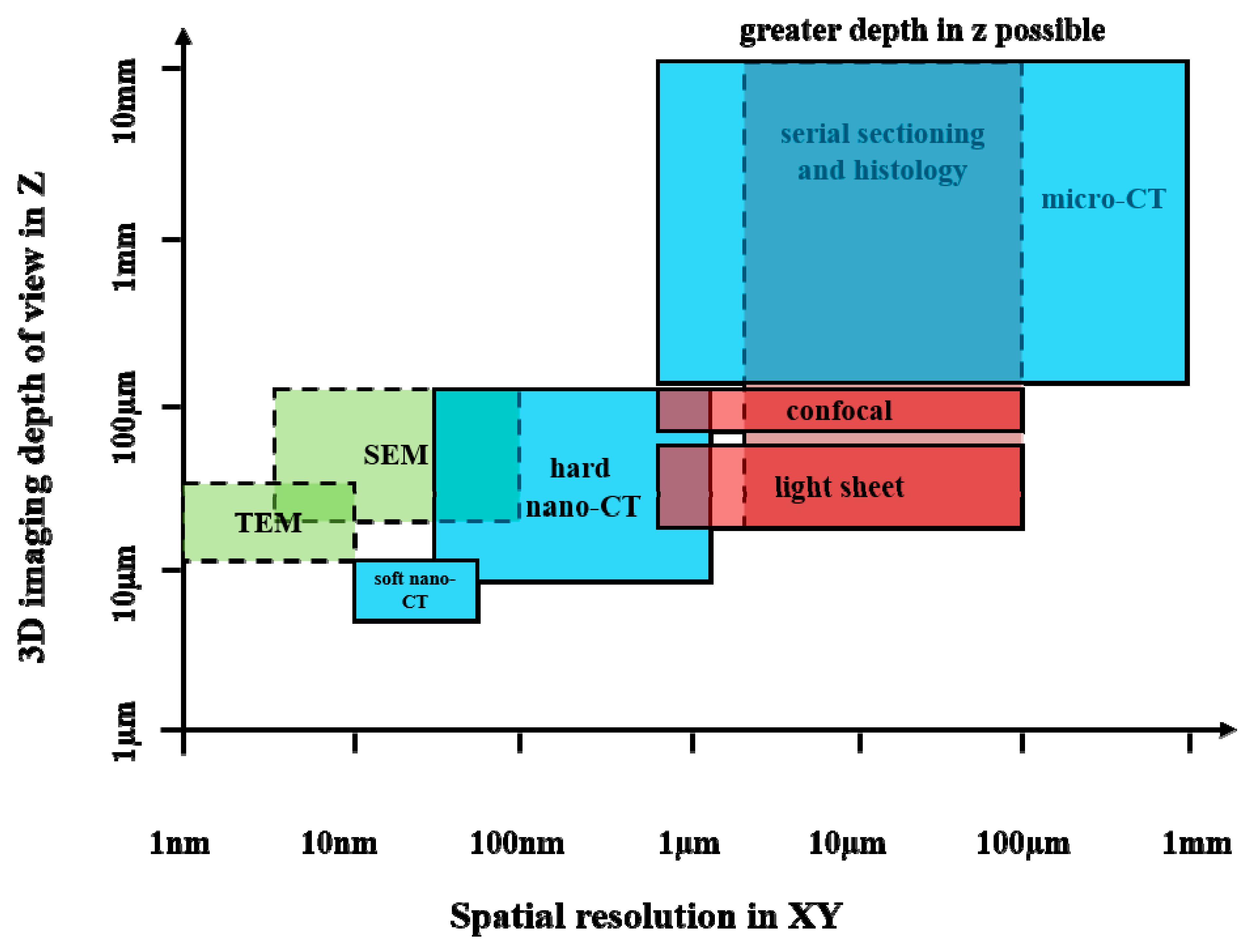
2.1.2. Applications in Life Sciences
2.1.3. Strengths and Limitations of Micro-CT
2.1.4. Comparison with Other Imaging Modalities
2.1.5. Special Topic: Micro-CT in Paleoradiology and Evolutionary Biology
2.1.6. Recent Desktop-Scanner Advances (Ex Vivo)
2.2. SR Micro-CT
2.2.1. Principles
2.2.2. Bone Applications
2.2.3. Strengths and Limitations of SR Micro-CT
2.3. High-Resolution Peripheral Quantitative Computed Tomography (HR-pQCT)
2.3.1. Principles and System Evolution
2.3.2. Reporting and Metrics (Harmonised with Micro-CT)
2.3.3. Clinical Applications and Integration with FE/ML
2.3.4. Surgical Planning, Density Mapping, and Robotics
3. Computational Methods: Finite Element Analysis and Artificial Intelligence
3.1. Finite Element Analysis
Beyond Strength: Transport and Poromechanics from Microstructure
3.2. Artificial Intelligence in HR-pQCT
4. Discussion
5. Conclusions
- Micro-CT remains the research gold standard, providing unparalleled resolution for ex vivo and preclinical studies, though it is limited in direct clinical use due to radiation and cost.
- HR-pQCT is the clinical frontier, offering in vivo assessment of trabecular and cortical bone microarchitecture, but its adoption is constrained by availability, motion artefacts, and lack of standardisation.
- FEA and ML add biomechanical and predictive layers, enhancing risk assessment and personalisation, but depend on high-quality datasets and rigorous validation.
- Future progress hinges on accessibility, cost reduction, and protocol harmonisation to translate these advances into everyday clinical care.
6. Future Perspectives
6.1. Multimodal Imaging
6.2. Contrast Agents
6.3. Artificial Intelligence and Machine Learning
6.4. Three-Dimensional Printing and Regenerative Medicine
6.5. Global Health and Accessibility
6.6. Standardisation and Translation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Zhou, H.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int. J. Biol. Sci. 2019, 15, 776–787. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Qin, L.; Chen, S.; Huo, S.; Li, J.; Zhang, F.; Yi, W.; Mei, Y.; Xiao, G. Bone-derived factors mediate crosstalk between skeletal and extra-skeletal organs. Bone Res. 2025, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Banaganapalli, B.; Fallatah, I.; Alsubhi, F.; Shetty, P.J.; Awan, Z.; Elango, R.; Shaik, N.A. Paget’s disease: A review of the epidemiology, etiology, genetics, and treatment. Front. Genet. 2023, 14, 2023. [Google Scholar] [CrossRef]
- Rendina, D.; Falchetti, A.; Diacinti, D.; Bertoldo, F.; Merlotti, D.; Giannini, S.; Cianferotti, L.; Girasole, G.; Di Monaco, M.; Gonnelli, S.; et al. Diagnosis and treatment of Paget’s disease of bone: Position paper from the Italian Society of Osteoporosis, Mineral Metabolism and Skeletal Diseases (SIOMMMS). J. Endocrinol. Investig. 2024, 47, 1335–1360. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Boutroy, S.; Bouxsein, M.L.; Munoz, F.; Delmas, P.D. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J. Clin. Endocrinol. Metab. 2005, 90, 6508–6515. [Google Scholar] [CrossRef]
- Stock, S.R. Recent advances in X-ray microtomography applied to materials. Int. Mater. Rev. 2008, 53, 129–181. [Google Scholar] [CrossRef]
- Elliott, J.C.; Dover, S.D. X-ray microtomography. J. Microsc. 1982, 126, 211–213. [Google Scholar] [CrossRef]
- Rawson, S.D.; Maksimcuka, J.; Withers, P.J.; Cartmell, S.H. X-ray computed tomography in life sciences. BMC Biol. 2020, 18, 21. [Google Scholar] [CrossRef]
- Thompson, A.C.; Llacer, J.; Campbell Finman, L.; Hughes, E.B.; Otis, J.N.; Wilson, S.; Zeman, H.D. Computed tomography using synchrotron radiation. Nucl. Instrum. Methods Phys. Res. 1984, 222, 319–323. [Google Scholar] [CrossRef]
- Withers, P.J.; Bouman, C.; Carmignato, S.; Cnudde, V.; Grimaldi, D.; Hagen, C.K.; Maire, E.; Manley, M.; Du Plessis, A.; Stock, S.R. X-ray computed tomography. Nat. Rev. Methods Primers 2021, 1, 18. [Google Scholar] [CrossRef]
- Claro, P.I.C.; Borges, E.P.B.S.; Schleder, G.R.; Archilha, N.L.; Pinto, A.; Carvalho, M.; Driemeier, C.E.; Fazzio, A.; Gouveia, R.F. From micro- to nano- and time-resolved x-ray computed tomography: Bio-based applications, synchrotron capabilities, and data-driven processing. Appl. Phys. Rev. 2023, 10, 021302. [Google Scholar] [CrossRef]
- Withers, P.J. X-ray nanotomography. Mater. Today 2007, 10, 26–34. [Google Scholar] [CrossRef]
- Hwu, Y.; Margaritondo, G.; Chiang, A.-S. Q&A: Why use synchrotron x-ray tomography for multi-scale connectome mapping? BMC Biol. 2017, 15, 122. [Google Scholar] [CrossRef]
- Dierolf, M.; Menzel, A.; Thibault, P.; Schneider, P.; Kewish, C.M.; Wepf, R.; Bunk, O.; Pfeiffer, F. Ptychographic X-ray computed tomography at the nanoscale. Nature 2010, 467, 436–439. [Google Scholar] [CrossRef]
- Rosenblatt, F. The perceptron: A probabilistic model for information storage and organization in the brain. Psychol. Rev. 1958, 65, 386–408. [Google Scholar] [CrossRef]
- Gardner, M.W.; Dorling, S.R. Artificial neural networks (the multilayer perceptron)—A review of applications in the atmospheric sciences. Atmos. Environ. 1998, 32, 2627–2636. [Google Scholar] [CrossRef]
- Ajit, A.; Acharya, K.; Samanta, A. A review of convolutional neural networks. In Proceedings of the 2020 International Conference on Emerging Trends in Information Technology and Engineering (ic-ETITE), Vellore, India, 24–25 February 2020; pp. 1–5. [Google Scholar]
- Koivu, A.; Kakko, J.-P.; Mäntyniemi, S.; Sairanen, M. Quality of randomness and node dropout regularization for fitting neural networks. Expert Syst. Appl. 2022, 207, 117938. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Guo, M.-H.; Xu, T.-X.; Liu, J.-J.; Liu, Z.-N.; Jiang, P.-T.; Mu, T.-J.; Zhang, S.-H.; Martin, R.R.; Cheng, M.-M.; Hu, S.-M. Attention mechanisms in computer vision: A survey. Comput. Vis. Media 2022, 8, 331–368. [Google Scholar] [CrossRef]
- Borovec, J.; Švihlík, J.; Kybic, J.; Habart, D. Supervised and unsupervised segmentation using superpixels, model estimation, and graph cut. J. Electron. Imaging 2017, 26, 061610. [Google Scholar] [CrossRef]
- Paddock, S.W.; Eliceiri, K.W. Laser scanning confocal microscopy: History, applications, and related optical sectioning techniques. Methods Mol. Biol. 2014, 1075, 9–47. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef]
- Elliott, A.D. Confocal Microscopy: Principles and Modern Practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef]
- Ueda, H.R.; Ertürk, A.; Chung, K.; Gradinaru, V.; Chédotal, A.; Tomancak, P.; Keller, P.J. Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci. 2020, 21, 61–79. [Google Scholar] [CrossRef]
- Courson, J.A.; Landry, P.T.; Do, T.; Spehlmann, E.; Lafontant, P.J.; Patel, N.; Rumbaut, R.E.; Burns, A.R. Serial Block-Face Scanning Electron Microscopy (SBF-SEM) of Biological Tissue Samples. J. Vis. Exp. 2021, 169, e62045. [Google Scholar] [CrossRef]
- Lewczuk, B.; Szyryńska, N. Field-Emission Scanning Electron Microscope as a Tool for Large-Area and Large-Volume Ultrastructural Studies. Animals 2021, 11, 3390. [Google Scholar] [CrossRef]
- Leung, S. Treatment of pediatric genitourinary malignancy with interstitial brachytherapy: Peter MacCallum Cancer Institute experience with four cases. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 393–398. [Google Scholar] [CrossRef]
- Niemann, B.; Rudolph, D.; Schmahl, G. X-ray microscopy with synchrotron radiation. Appl. Opt. 1976, 15, 1883–1884. [Google Scholar] [CrossRef]
- Sasov, A. X-Ray Nanotomography; SPIE: Bellingham, WA, USA, 2004; Volume 5535. [Google Scholar]
- Smith, D.; Starborg, T. Serial block face scanning electron microscopy in cell biology: Applications and technology. Tissue Cell 2019, 57, 111–122. [Google Scholar] [CrossRef]
- Shearer, T.; Bradley, R.S.; Hidalgo-Bastida, L.A.; Sherratt, M.J.; Cartmell, S.H. Three-dimensional visualisation of soft biological structures by X-ray computed micro-tomography. J. Cell Sci. 2016, 129, 2483–2492. [Google Scholar] [CrossRef]
- Burnett, T.L.; Kelley, R.; Winiarski, B.; Contreras, L.; Daly, M.; Gholinia, A.; Burke, M.G.; Withers, P.J. Large volume serial section tomography by Xe Plasma FIB dual beam microscopy. Ultramicroscopy 2016, 161, 119–129. [Google Scholar] [CrossRef]
- McDermott, G.; Le Gros, M.A.; Knoechel, C.G.; Uchida, M.; Larabell, C.A. Soft X-ray tomography and cryogenic light microscopy: The cool combination in cellular imaging. Trends Cell Biol. 2009, 19, 587–595. [Google Scholar] [CrossRef]
- Truong, T.V.; Supatto, W.; Koos, D.S.; Choi, J.M.; Fraser, S.E. Deep and fast live imaging with two-photon scanned light-sheet microscopy. Nat. Methods 2011, 8, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.C.; Shelmerdine, S.C.; Simcock, I.C.; Sebire, N.J.; Arthurs, O.J. Early clinical applications for imaging at microscopic detail: Microfocus computed tomography (micro-CT). Br. J. Radiol. 2017, 90, 20170113. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; McAteer, J.A.; Evan, A.P.; Lingeman, J.E. Micro-computed tomography for analysis of urinary calculi. Urol. Res. 2010, 38, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Yamada, A.; Ninomiya, T.; Kato, T.; Masuda, Y. Micro-computed tomography newly developed for in vivo small animal imaging. Oral Radiol. 2005, 21, 14–18. [Google Scholar] [CrossRef]
- Wöss, C.; Unterberger, S.H.; Degenhart, G.; Akolkar, A.; Traxl, R.; Kuhn, V.; Schirmer, M.; Pallua, A.K.; Tappert, R.; Pallua, J.D. Comparison of structure and composition of a fossil Champsosaurus vertebra with modern Crocodylidae vertebrae: A multi-instrumental approach. J. Mech. Behav. Biomed. Mater. 2020, 104, 103668. [Google Scholar] [CrossRef]
- Schmidt, V.M.; Zelger, P.; Woess, C.; Pallua, A.K.; Arora, R.; Degenhart, G.; Brunner, A.; Zelger, B.; Schirmer, M.; Rabl, W.; et al. Application of Micro-Computed Tomography for the Estimation of the Post-Mortem Interval of Human Skeletal Remains. Biology 2022, 11, 1105. [Google Scholar] [CrossRef]
- Obata, Y.; Bale, H.A.; Barnard, H.S.; Parkinson, D.Y.; Alliston, T.; Acevedo, C. Quantitative and qualitative bone imaging: A review of synchrotron radiation microtomography analysis in bone research. J. Mech. Behav. Biomed. Mater. 2020, 110, 103887. [Google Scholar] [CrossRef] [PubMed]
- Puce, S.; Pica, D.; Mancini, L.; Brun, F.; Peverelli, A.; Bavestrello, G. Three-dimensional analysis of the canal network of an Indonesian Stylaster (Cnidaria, Hydrozoa, Stylasteridae) by means of X-ray computed microtomography. Zoomorphology 2011, 130, 85–95. [Google Scholar] [CrossRef]
- Soviero, V.M.; Leal, S.C.; Silva, R.C.; Azevedo, R.B. Validity of MicroCT for in vitro detection of proximal carious lesions in primary molars. J. Dent. 2012, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Bemmann, M.; Schulz-Kornas, E.; Hammel, J.U.; Hipp, A.; Moosmann, J.; Herrel, A.; Rack, A.; Radespiel, U.; Zimmermann, E.; Kaiser, T.M.; et al. Movement analysis of primate molar teeth under load using synchrotron X-ray microtomography. J. Struct. Biol. 2021, 213, 107658. [Google Scholar] [CrossRef]
- Laperre, K.; Depypere, M.; van Gastel, N.; Torrekens, S.; Moermans, K.; Bogaerts, R.; Maes, F.; Carmeliet, G. Development of micro-CT protocols for in vivo follow-up of mouse bone architecture without major radiation side effects. Bone 2011, 49, 613–622. [Google Scholar] [CrossRef]
- Langer, M.; Peyrin, F. 3D X-ray ultra-microscopy of bone tissue. Osteoporos. Int. 2016, 27, 441–455. [Google Scholar] [CrossRef]
- Zimmermann, E.A.; Schaible, E.; Bale, H.; Barth, H.D.; Tang, S.Y.; Reichert, P.; Busse, B.; Alliston, T.; Ager, J.W.; Ritchie, R.O. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc. Natl. Acad. Sci. USA 2011, 108, 14416–14421. [Google Scholar] [CrossRef]
- Zelaya, V.M.; Archilha, N.L.; Calasans, M.; Rossi, A.L.; Farina, M.; Santisteban, T.S.; Rossi, A.M. Characterization of Zn-Substituted Carbonated Apatite Implanted in Rat Tibia by Synchrotron Radiation X-Ray Microtomography and 3D Image Analysis. Microsc. Microanal. 2018, 24, 536–537. [Google Scholar] [CrossRef]
- du Plessis, A.; Broeckhoven, C.; Guelpa, A.; le Roux, S.G. Laboratory x-ray micro-computed tomography: A user guideline for biological samples. Gigascience 2017, 6, gix027. [Google Scholar] [CrossRef]
- Ghani, M.U.; Zhou, Z.; Ren, L.; Li, Y.; Zheng, B.; Yang, K.; Liu, H. Investigation of spatial resolution characteristics of an in vivo micro computed tomography system. Nucl. Instrum. Methods Phys. Res. A 2016, 807, 129–136. [Google Scholar] [CrossRef]
- Langer, M.; Pacureanu, A.; Suhonen, H.; Grimal, Q.; Cloetens, P.; Peyrin, F. X-Ray Phase Nanotomography Resolves the 3D Human Bone Ultrastructure. PLoS ONE 2012, 7, e35691. [Google Scholar] [CrossRef] [PubMed]
- Metscher, B.D. MicroCT for comparative morphology: Simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 2009, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Dunmore-Buyze, P.J.; Tate, E.; Xiang, F.L.; Detombe, S.A.; Nong, Z.; Pickering, J.G.; Drangova, M. Three-dimensional imaging of the mouse heart and vasculature using micro-CT and whole-body perfusion of iodine or phosphotungstic acid. Contrast Media Mol. Imaging 2014, 9, 383–390. [Google Scholar] [CrossRef]
- Degenhardt, K.; Wright, A.C.; Horng, D.; Padmanabhan, A.; Epstein, J.A. Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining. Circulation. Cardiovasc. Imaging 2010, 3, 314–322. [Google Scholar] [CrossRef]
- Johnson, J.T.; Hansen, M.S.; Wu, I.; Healy, L.J.; Johnson, C.R.; Jones, G.M.; Capecchi, M.R.; Keller, C. Virtual histology of transgenic mouse embryos for high-throughput phenotyping. PLoS Genet. 2006, 2, e61. [Google Scholar] [CrossRef]
- Pallua, J.D.; Kuhn, V.; Pallua, A.F.; Pfaller, K.; Pallua, A.K.; Recheis, W.; Pöder, R. Application of micro-computed tomography to microstructure studies of the medicinal fungus Hericium coralloides. Mycologia 2015, 107, 227–238. [Google Scholar] [CrossRef]
- Palmer, A.J.; Brown, C.P.; McNally, E.G.; Price, A.J.; Tracey, I.; Jezzard, P.; Carr, A.J.; Glyn-Jones, S. Non-invasive imaging of cartilage in early osteoarthritis. Bone Jt. J. 2013, 95-B, 738–746. [Google Scholar] [CrossRef]
- Xie, L.; Lin, A.S.P.; Guldberg, R.E.; Levenston, M.E. Nondestructive assessment of sGAG content and distribution in normal and degraded rat articular cartilage via EPIC-μCT. Osteoarthr. Cartil. 2010, 18, 65–72. [Google Scholar] [CrossRef]
- Mashiatulla, M.; Moran, M.M.; Chan, D.; Li, J.; Freedman, J.D.; Snyder, B.D.; Grinstaff, M.W.; Plaas, A.; Sumner, D.R. Murine articular cartilage morphology and compositional quantification with high resolution cationic contrast-enhanced μCT. J. Orthop. Res. 2017, 35, 2740–2748. [Google Scholar] [CrossRef]
- Bolland, B.J.R.F.; Kanczler, J.M.; Dunlop, D.G.; Oreffo, R.O.C. Development of in vivo μCT evaluation of neovascularisation in tissue engineered bone constructs. Bone 2008, 43, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Metscher, B.D. X-ray microtomographic imaging of intact vertebrate embryos. Cold Spring Harb. Protoc. 2011, 2011, 1462–1471. [Google Scholar] [CrossRef]
- Martins de Souza e Silva, J.; Utsch, J.; Kimm, M.A.; Allner, S.; Epple, M.F.; Achterhold, K.; Pfeiffer, F. Dual-energy micro-CT for quantifying the time-course and staining characteristics of ex-vivo animal organs treated with iodine- and gadolinium-based contrast agents. Sci. Rep. 2017, 7, 17387. [Google Scholar] [CrossRef]
- Strotton, M.C.; Bodey, A.J.; Wanelik, K.; Darrow, M.C.; Medina, E.; Hobbs, C.; Rau, C.; Bradbury, E.J. Optimising complementary soft tissue synchrotron X-ray microtomography for reversibly-stained central nervous system samples. Sci. Rep. 2018, 8, 12017. [Google Scholar] [CrossRef]
- Fonseca, M.d.C.; Araujo, B.H.S.; Dias, C.S.B.; Archilha, N.L.; Neto, D.P.A.; Cavalheiro, E.; Westfahl, H.; da Silva, A.J.R.; Franchini, K.G. High-resolution synchrotron-based X-ray microtomography as a tool to unveil the three-dimensional neuronal architecture of the brain. Sci. Rep. 2018, 8, 12074. [Google Scholar] [CrossRef]
- Du, Z.; Hu, Y.; Ali Buttar, N.; Mahmood, A. X-ray computed tomography for quality inspection of agricultural products: A review. Food Sci. Nutr. 2019, 7, 3146–3160. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, A.S.; Karagiannidis, E.; Moysidis, D.V.; Sofidis, G.; Bompoti, A.; Stalikas, N.; Panteris, E.; Arvanitidis, C.; Herrmann, M.D.; Michaelson, J.S.; et al. Current clinical applications and potential perspective of micro-computed tomography in cardiovascular imaging: A systematic scoping review. Hell. J. Cardiol. 2021, 62, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.P.; Badea, C.T. Micro-CT of rodents: State-of-the-art and future perspectives. Phys. Medica 2014, 30, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.P.; Badea, C.T. Advances in micro-CT imaging of small animals. Phys. Medica 2021, 88, 175–192. [Google Scholar] [CrossRef]
- Akhter, M.P.; Recker, R.R. High resolution imaging in bone tissue research-review. Bone 2021, 143, 115620. [Google Scholar] [CrossRef]
- Ohs, N.; Collins, C.J.; Atkins, P.R. Validation of HR-pQCT against micro-CT for morphometric and biomechanical analyses: A review. Bone Rep. 2020, 13, 100711. [Google Scholar] [CrossRef]
- Vásárhelyi, L.; Kónya, Z.; Kukovecz, Á.; Vajtai, R. Microcomputed tomography–based characterization of advanced materials: A review. Mater. Today Adv. 2020, 8, 100084. [Google Scholar] [CrossRef]
- van Rietbergen, B.; Ito, K. A survey of micro-finite element analysis for clinical assessment of bone strength: The first decade. J. Biomech. 2015, 48, 832–841. [Google Scholar] [CrossRef]
- Meganck, J.A.; Kozloff, K.M.; Thornton, M.M.; Broski, S.M.; Goldstein, S.A. Beam hardening artifacts in micro-computed tomography scanning can be reduced by X-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone 2009, 45, 1104–1116. [Google Scholar] [CrossRef]
- Berghen, N.; Dekoster, K.; Marien, E.; Dabin, J.; Hillen, A.; Wouters, J.; Deferme, J.; Vosselman, T.; Tiest, E.; Lox, M.; et al. Radiosafe micro-computed tomography for longitudinal evaluation of murine disease models. Sci. Rep. 2019, 9, 17598. [Google Scholar] [CrossRef] [PubMed]
- Katsura, M.; Sato, J.; Akahane, M.; Kunimatsu, A.; Abe, O. Current and Novel Techniques for Metal Artifact Reduction at CT: Practical Guide for Radiologists. Radiographics 2018, 38, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Boone, S.; Martel, D.; Rajapakse, C.S.; Hallyburton, R.S.; Valko, M.; Honig, S.; Regatte, R.R. MRI assessment of bone structure and microarchitecture. J. Magn. Reson. Imaging 2017, 46, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Puri, T.; Frost, M.L.; Moore, A.E.B.; Choudhury, A.; Vinjamuri, S.; Mahajan, A.; Fynbo, C.; Vrist, M.; Theil, J.; Kairemo, K.; et al. Utility of a simplified [(18)F] sodium fluoride PET imaging method to quantify bone metabolic flux for a wide range of clinical applications. Front. Endocrinol. 2023, 14, 1236881. [Google Scholar] [CrossRef]
- Bianchi, S. Ultrasound and bone: A pictorial review. J. Ultrasound 2020, 23, 227–257. [Google Scholar] [CrossRef]
- Peyrin, F.; Dong, P.; Pacureanu, A.; Langer, M. Micro- and nano-CT for the study of bone ultrastructure. Curr. Osteoporos. Rep. 2014, 12, 465–474. [Google Scholar] [CrossRef]
- Daube-Witherspoon, M.E.; Cherry, S.R. Scanner Design Considerations for Long Axial Field-of-View PET Systems. PET Clin. 2021, 16, 25–39. [Google Scholar] [CrossRef]
- Forney, M.C.; Delzell, P.B. Musculoskeletal ultrasonography basics. Clevel. Clin. J. Med. 2018, 85, 283–300. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Umetani, K.; Okamoto, T.; Saito, K.; Kawata, Y.; Niki, N. 36M-pixel synchrotron radiation micro-CT for whole secondary pulmonary lobule visualization from a large human lung specimen. Eur. J. Radiol. Open 2020, 7, 100262. [Google Scholar] [CrossRef] [PubMed]
- Douissard, P.A.; Cecilia, A.; Martin, T.; Chevalier, V.; Couchaud, M.; Baumbach, T.; Dupré, K.; Kühbacher, M.; Rack, A. A novel epitaxially grown LSO-based thin-film scintillator for micro-imaging using hard synchrotron radiation. J. Synchrotron Radiat. 2010, 17, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef]
- Kulpe, S.; Dierolf, M.; Günther, B.; Busse, M.; Achterhold, K.; Gleich, B.; Herzen, J.; Rummeny, E.; Pfeiffer, F.; Pfeiffer, D. K-edge Subtraction Computed Tomography with a Compact Synchrotron X-ray Source. Sci. Rep. 2019, 9, 13332. [Google Scholar] [CrossRef]
- Ma, S.; Boughton, O.; Karunaratne, A.; Jin, A.; Cobb, J.; Hansen, U.; Abel, R. Synchrotron Imaging Assessment of Bone Quality. Clin. Rev. Bone Min. Metab. 2016, 14, 150–160. [Google Scholar] [CrossRef]
- Salomé, M.; Peyrin, F.; Cloetens, P.; Odet, C.; Laval-Jeantet, A.M.; Baruchel, J.; Spanne, P. A synchrotron radiation microtomography system for the analysis of trabecular bone samples. Med. Phys. 1999, 26, 2194–2204. [Google Scholar] [CrossRef]
- Núñez, J.A.; Goring, A.; Hesse, E.; Thurner, P.J.; Schneider, P.; Clarkin, C.E. Simultaneous visualisation of calcified bone microstructure and intracortical vasculature using synchrotron X-ray phase contrast-enhanced tomography. Sci. Rep. 2017, 7, 13289. [Google Scholar] [CrossRef]
- Chappard, C.; Peyrin, F.; Bonnassie, A.; Lemineur, G.; Brunet-Imbault, B.; Lespessailles, E.; Benhamou, C.L. Subchondral bone micro-architectural alterations in osteoarthritis: A synchrotron micro-computed tomography study. Osteoarthr. Cartil. 2006, 14, 215–223. [Google Scholar] [CrossRef]
- Voide, R.; Schneider, P.; Stauber, M.; Wyss, P.; Stampanoni, M.; Sennhauser, U.; van Lenthe, G.H.; Müller, R. Time-lapsed assessment of microcrack initiation and propagation in murine cortical bone at submicrometer resolution. Bone 2009, 45, 164–173. [Google Scholar] [CrossRef]
- Cooper, D.M.L.; Erickson, B.; Peele, A.G.; Hannah, K.; Thomas, C.D.L.; Clement, J.G. Visualization of 3D osteon morphology by synchrotron radiation micro-CT. J. Anat. 2011, 219, 481–489. [Google Scholar] [CrossRef]
- Ito, M.; Nakamura, T.; Matsumoto, T.; Tsurusaki, K.; Hayashi, K. Analysis of trabecular microarchitecture of human iliac bone using microcomputed tomography in patients with hip arthrosis with or without vertebral fracture. Bone 1998, 23, 163–169. [Google Scholar] [CrossRef]
- Kazakia, G.J.; Burghardt, A.J.; Cheung, S.; Majumdar, S. Assessment of bone tissue mineralization by conventional x-ray microcomputed tomography: Comparison with synchrotron radiation microcomputed tomography and ash measurements. Med. Phys. 2008, 35, 3170–3179. [Google Scholar] [CrossRef] [PubMed]
- Larrue, A.; Rattner, A.; Peter, Z.-A.; Olivier, C.; Laroche, N.; Vico, L.; Peyrin, F. Synchrotron Radiation Micro-CT at the Micrometer Scale for the Analysis of the Three-Dimensional Morphology of Microcracks in Human Trabecular Bone. PLoS ONE 2011, 6, e21297. [Google Scholar] [CrossRef] [PubMed]
- Brock, G.R.; Kim, G.; Ingraffea, A.R.; Andrews, J.C.; Pianetta, P.; van der Meulen, M.C.H. Nanoscale Examination of Microdamage in Sheep Cortical Bone Using Synchrotron Radiation Transmission X-Ray Microscopy. PLoS ONE 2013, 8, e57942. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Lee, P.D.; Poologasundarampillai, G.; Yao, Z.; Rockett, P.; Devlin, A.H.; Mitchell, C.A.; Konerding, M.A.; Jones, J.R. Synchrotron X-ray microtomography for assessment of bone tissue scaffolds. J. Mater. Sci. Mater. Med. 2010, 21, 847–853. [Google Scholar] [CrossRef]
- Cancedda, R.; Cedola, A.; Giuliani, A.; Komlev, V.; Lagomarsino, S.; Mastrogiacomo, M.; Peyrin, F.; Rustichelli, F. Bulk and interface investigations of scaffolds and tissue-engineered bones by X-ray microtomography and X-ray microdiffraction. Biomaterials 2007, 28, 2505–2524. [Google Scholar] [CrossRef]
- Nuzzo, S.; Lafage-Proust, M.H.; Martin-Badosa, E.; Boivin, G.; Thomas, T.; Alexandre, C.; Peyrin, F. Synchrotron Radiation Microtomography Allows the Analysis of Three-Dimensional Microarchitecture and Degree of Mineralization of Human Iliac Crest Biopsy Specimens: Effects of Etidronate Treatment. J. Bone Miner. Res. 2002, 17, 1372–1382. [Google Scholar] [CrossRef]
- Tzaphlidou, M.; Speller, R.; Royle, G.; Griffiths, J. Preliminary estimates of the calcium/phosphorus ratio at different cortical bone sites using synchrotron microCT. Phys. Med. Biol. 2006, 51, 1849. [Google Scholar] [CrossRef]
- Djomehri, S.I.; Candell, S.; Case, T.; Browning, A.; Marshall, G.W.; Yun, W.; Lau, S.H.; Webb, S.; Ho, S.P. Mineral Density Volume Gradients in Normal and Diseased Human Tissues. PLoS ONE 2015, 10, e0121611. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nishikawa, K.; Tanaka, M.; Uesugi, K. In Vivo CT Quantification of Trabecular Bone Dynamics in Mice after Sciatic Neurectomy Using Monochromatic Synchrotron Radiation. Calcif. Tissue Int. 2011, 88, 432–441. [Google Scholar] [CrossRef]
- Sarve, H.; Lindblad, J.; Borgefors, G.; Johansson, C.B. Extracting 3D information on bone remodeling in the proximity of titanium implants in SRμCT image volumes. Comput. Methods Programs Biomed. 2011, 102, 25–34. [Google Scholar] [CrossRef]
- Neldam, C.A.; Sporring, J.; Rack, A.; Lauridsen, T.; Hauge, E.-M.; Jørgensen, H.L.; Jørgensen, N.R.; Feidenhansl, R.; Pinholt, E.M. Synchrotron radiation μCT and histology evaluation of bone-to-implant contact. J. Cranio-Maxillofac. Surg. 2017, 45, 1448–1457. [Google Scholar] [CrossRef]
- Bernhardt, R.; Kuhlisch, E.; Schulz, M.C.; Eckelt, U.; Stadlinger, B. Comparison of bone-implant contact and bone-implant volume between 2D-histological sections and 3D-SRµCT slices. Eur. Cells Mater. 2012, 23, 237–247, discussion 247–238. [Google Scholar] [CrossRef]
- Weiss, P.; Obadia, L.; Magne, D.; Bourges, X.; Rau, C.; Weitkamp, T.; Khairoun, I.; Bouler, J.M.; Chappard, D.; Gauthier, O.; et al. Synchrotron X-ray microtomography (on a micron scale) provides three-dimensional imaging representation of bone ingrowth in calcium phosphate biomaterials. Biomaterials 2003, 24, 4591–4601. [Google Scholar] [CrossRef]
- Neldam, C.A.; Lauridsen, T.; Rack, A.; Lefolii, T.T.; Jørgensen, N.R.; Feidenhans’l, R.; Pinholt, E.M. Application of high resolution synchrotron micro-CT radiation in dental implant osseointegration. J. Cranio-Maxillofac. Surg. 2015, 43, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.P.; Kimmel, D.B.; Lappe, J.M.; Recker, R.R. Effect of Macroanatomic Bone Type and Estrogen Loss on Osteocyte Lacunar Properties in Healthy Adult Women. Calcif. Tissue Int. 2017, 100, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, A.; Peyrin, F.; Fernandez, S.; Laredo, J.D.; de Vernejoul, M.C.; Chappard, C. Cortical measurements of the tibia from high resolution peripheral quantitative computed tomography images: A comparison with synchrotron radiation micro-computed tomography. Bone 2014, 63, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Krucker, T.; Meyer, E.; Ulmann-Schuler, A.; Weber, B.; Stampanoni, M.; Müller, R. Simultaneous 3D visualization and quantification of murine bone and bone vasculature using micro-computed tomography and vascular replica. Microsc. Res. Tech. 2009, 72, 690–701. [Google Scholar] [CrossRef]
- Schneider, P.; Meier, M.; Wepf, R.; Müller, R. Towards quantitative 3D imaging of the osteocyte lacuno-canalicular network. Bone 2010, 47, 848–858. [Google Scholar] [CrossRef]
- Schneider, P.; Stauber, M.; Voide, R.; Stampanoni, M.; Donahue, L.R.; Müller, R. Ultrastructural Properties in Cortical Bone Vary Greatly in Two Inbred Strains of Mice as Assessed by Synchrotron Light Based Micro- and Nano-CT. J. Bone Miner. Res. 2007, 22, 1557–1570. [Google Scholar] [CrossRef]
- Carter, Y.; Thomas, C.D.L.; Clement, J.G.; Peele, A.G.; Hannah, K.; Cooper, D.M.L. Variation in osteocyte lacunar morphology and density in the human femur—A synchrotron radiation micro-CT study. Bone 2013, 52, 126–132. [Google Scholar] [CrossRef]
- Carter, Y.; Thomas, C.D.L.; Clement, J.G.; Cooper, D.M.L. Femoral osteocyte lacunar density, volume and morphology in women across the lifespan. J. Struct. Biol. 2013, 183, 519–526. [Google Scholar] [CrossRef]
- Carter, Y.; Suchorab, J.L.; Thomas, C.D.L.; Clement, J.G.; Cooper, D.M.L. Normal variation in cortical osteocyte lacunar parameters in healthy young males. J. Anat. 2014, 225, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Fritton, S.P.; Gailani, G.; Benalla, M.; Cowin, S.C. Advances in assessment of bone porosity, permeability and interstitial fluid flow. J. Biomech. 2013, 46, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Weber, L.; Pacureanu, A.; Langer, M.; Olivier, C.; Cloetens, P.; Peyrin, F. Evaluation of phase retrieval approaches in magnified X-ray phase nano computerized tomography applied to bone tissue. Opt. Express 2018, 26, 11110–11124. [Google Scholar] [CrossRef] [PubMed]
- Mazy, L.; Kerckhofs, G. A review of in-situ mechanical testing combined with X-ray microfocus computed tomography: Application and current challenges for biological tissues. Tomogr. Mater. Struct. 2025, 8, 100062. [Google Scholar] [CrossRef]
- Dall’Ara, E.; Bodey, A.J.; Isaksson, H.; Tozzi, G. A practical guide for in situ mechanical testing of musculoskeletal tissues using synchrotron tomography. J. Mech. Behav. Biomed. Mater. 2022, 133, 105297. [Google Scholar] [CrossRef]
- Goff, E.; Buccino, F.; Bregoli, C.; McKinley, J.P.; Aeppli, B.; Recker, R.R.; Shane, E.; Cohen, A.; Kuhn, G.; Müller, R. Large-scale quantification of human osteocyte lacunar morphological biomarkers as assessed by ultra-high-resolution desktop micro-computed tomography. Bone 2021, 152, 116094. [Google Scholar] [CrossRef]
- Peña Fernández, M.; Cipiccia, S.; Dall’Ara, E.; Bodey, A.J.; Parwani, R.; Pani, M.; Blunn, G.W.; Barber, A.H.; Tozzi, G. Effect of SR-microCT radiation on the mechanical integrity of trabecular bone using in situ mechanical testing and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2018, 88, 109–119. [Google Scholar] [CrossRef]
- Croton, L.C.P.; Ruben, G.; Morgan, K.S.; Paganin, D.M.; Kitchen, M.J. Ring artifact suppression in X-ray computed tomography using a simple, pixel-wise response correction. Opt. Express 2019, 27, 14231–14245. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Ruegsegger, E.; Ruegsegger, P. Peripheral QCT: A low-risk procedure to identify women predisposed to osteoporosis. Phys. Med. Biol. 1989, 34, 741. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, H.H.; Sievänen, H. Inaccuracies Inherent in Dual-Energy X-Ray Absorptiometry In Vivo Bone Mineral Density Can Seriously Mislead Diagnostic/Prognostic Interpretations of Patient-Specific Bone Fragility. J. Bone Miner. Res. 2001, 16, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Force, U.S.P.S.T.; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 2521–2531. [Google Scholar] [CrossRef]
- Borah, B.; Dufresne, T.E.; Chmielewski, P.A.; Johnson, T.D.; Chines, A.; Manhart, M.D. Risedronate preserves bone architecture in postmenopausal women with osteoporosis as measured by three-dimensional microcomputed tomography. Bone 2004, 34, 736–746. [Google Scholar] [CrossRef]
- Borah, B.; Dufresne, T.E.; Ritman, E.L.; Jorgensen, S.M.; Liu, S.; Chmielewski, P.A.; Phipps, R.J.; Zhou, X.; Sibonga, J.D.; Turner, R.T. Long-term risedronate treatment normalizes mineralization and continues to preserve trabecular architecture: Sequential triple biopsy studies with micro-computed tomography. Bone 2006, 39, 345–352. [Google Scholar] [CrossRef]
- Boutroy, S.; Khosla, S.; Sornay-Rendu, E.; Zanchetta, M.B.; McMahon, D.J.; Zhang, C.A.; Chapurlat, R.D.; Zanchetta, J.; Stein, E.M.; Bogado, C.; et al. Microarchitecture and Peripheral BMD are Impaired in Postmenopausal White Women with Fracture Independently of Total Hip T-Score: An International Multicenter Study. J. Bone Miner. Res. 2016, 31, 1158–1166. [Google Scholar] [CrossRef]
- Du, J.; Brooke-Wavell, K.; Paggiosi, M.A.; Hartley, C.; Walsh, J.S.; Silberschmidt, V.V.; Li, S. Characterising variability and regional correlations of microstructure and mechanical competence of human tibial trabecular bone: An in-vivo HR-pQCT study. Bone 2019, 121, 139–148. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, F.; Wu, F.; Squibb, K.; Thomson, R.; Winzenberg, T.; Jones, G. Familial resemblance in trabecular and cortical volumetric bone mineral density and bone microarchitecture as measured by HRpQCT. Bone 2018, 110, 76–83. [Google Scholar] [CrossRef]
- Whittier, D.E.; Boyd, S.K.; Burghardt, A.J.; Paccou, J.; Ghasem-Zadeh, A.; Chapurlat, R.; Engelke, K.; Bouxsein, M.L. Guidelines for the assessment of bone density and microarchitecture in vivo using high-resolution peripheral quantitative computed tomography. Osteoporos. Int. 2020, 31, 1607–1627. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, J.A.; Boyd, S.K. Improved reproducibility of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med. Eng. Phys. 2008, 30, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.P.; Lappe, J.M.; Davies, K.M.; Recker, R.R. Transmenopausal changes in the trabecular bone structure. Bone 2007, 41, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Hsieh, Y.-F.; Müller, R.; Bouxsein, M.L.; Baylink, D.J.; Rosen, C.J.; Grynpas, M.D.; Donahue, L.R.; Beamer, W.G. Genetic Regulation of Cortical and Trabecular Bone Strength and Microstructure in Inbred Strains of Mice. J. Bone Miner. Res. 2000, 15, 1126–1131. [Google Scholar] [CrossRef]
- Laib, A.; Kumer, J.L.; Majumdar, S.; Lane, N.E. The temporal changes of trabecular architecture in ovariectomized rats assessed by MicroCT. Osteoporos. Int. 2001, 12, 936–941. [Google Scholar] [CrossRef]
- Layton, M.W.; Goldstein, S.A.; Goulet, R.W.; Feldkamp, L.A.; Kubinski, D.J.; Bole, G.G. Examination of subchondral bone architecture in experimental osteoarthritis by microscopic computed axial tomography. Arthritis Rheum. 1988, 31, 1400–1405. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Hormuzdi, S.G.; Meganck, J.A.; Bornstein, P. Mice with a disruption of the thrombospondin 3 gene differ in geometric and biomechanical properties of bone and have accelerated development of the femoral head. Mol. Cell. Biol. 2005, 25, 5599–5606. [Google Scholar] [CrossRef]
- Akhter, M.P.; Alvarez, G.K.; Cullen, D.M.; Recker, R.R. Disuse-related decline in trabecular bone structure. Biomech. Model. Mechanobiol. 2011, 10, 423–429. [Google Scholar] [CrossRef]
- Dubrow, S.A.; Hruby, P.M.; Akhter, M.P. Gender specific LRP5 influences on trabecular bone structure and strength. J. Musculoskelet. Neuronal Interact. 2007, 7, 166–173. [Google Scholar]
- Akhter, M.P.; Jung, L.K.L. Decreased bone strength in HLA-B27 transgenic rat model of spondyloarthropathy. Rheumatology 2007, 46, 1258–1262. [Google Scholar] [CrossRef]
- Akhter, M.P.; Lund, A.D.; Gairola, C.G. Bone Biomechanical Property Deterioration Due to Tobacco Smoke Exposure. Calcif. Tissue Int. 2005, 77, 319–326. [Google Scholar] [CrossRef]
- Manolides, A.S.; Cullen, D.M.; Akhter, M.P. Effects of glucocorticoid treatment on bone strength. J. Bone Miner. Metab. 2010, 28, 532–539. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Myers, K.S.; Shultz, K.L.; Donahue, L.R.; Rosen, C.J.; Beamer, W.G. Ovariectomy-induced bone loss varies among inbred strains of mice. J. Bone Miner. Res. 2005, 20, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Babij, P.; Zhao, W.; Small, C.; Kharode, Y.; Yaworsky, P.J.; Bouxsein, M.L.; Reddy, P.S.; Bodine, P.V.; Robinson, J.A.; Bhat, B.; et al. High Bone Mass in Mice Expressing a Mutant LRP5 Gene. J. Bone Miner. Res. 2003, 18, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.P.; Wells, D.J.; Short, S.J.; Cullen, D.M.; Johnson, M.L.; Haynatzki, G.R.; Babij, P.; Allen, K.M.; Yaworsky, P.J.; Bex, F.; et al. Bone biomechanical properties in LRP5 mutant mice. Bone 2004, 35, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.M.; Bab, I.; Fish, S.; Müller, R.; Uchiyama, T.; Gronowicz, G.; Nahounou, M.; Zhao, Q.; White, D.W.; Chorev, M.; et al. Human Parathyroid Hormone 1–34 Reverses Bone Loss in Ovariectomized Mice. J. Bone Miner. Res. 2001, 16, 1665–1673. [Google Scholar] [CrossRef]
- Barbier, A.; Martel, C.; de Vernejoul, M.C.; Tirode, F.; Nys, M.; Mocaer, G.; Morieux, C.; Murakami, H.; Lacheretz, F. The visualization and evaluation of bone architecture in the rat using three-dimensional X-ray microcomputed tomography. J. Bone Min. Metab. 1999, 17, 37–44. [Google Scholar] [CrossRef]
- Christiansen, B.A.; Silva, M.J. The Effect of Varying Magnitudes of Whole-Body Vibration on Several Skeletal Sites in Mice. Ann. Biomed. Eng. 2006, 34, 1149–1156. [Google Scholar] [CrossRef]
- Squire, M.; Donahue, L.-R.; Rubin, C.; Judex, S. Genetic variations that regulate bone morphology in the male mouse skeleton do not define its susceptibility to mechanical unloading. Bone 2004, 35, 1353–1360. [Google Scholar] [CrossRef]
- Cunningham, H.C.; West, D.W.D.; Baehr, L.M.; Tarke, F.D.; Baar, K.; Bodine, S.C.; Christiansen, B.A. Age-dependent bone loss and recovery during hindlimb unloading and subsequent reloading in rats. BMC Musculoskelet. Disord. 2018, 19, 223. [Google Scholar] [CrossRef]
- Uthgenannt, B.A.; Silva, M.J. Use of the rat forelimb compression model to create discrete levels of bone damage in vivo. J. Biomech. 2007, 40, 317–324. [Google Scholar] [CrossRef]
- Naik, A.A.; Xie, C.; Zuscik, M.J.; Kingsley, P.; Schwarz, E.M.; Awad, H.; Guldberg, R.; Drissi, H.; Puzas, J.E.; Boyce, B.; et al. Reduced COX-2 Expression in Aged Mice Is Associated with Impaired Fracture Healing. J. Bone Miner. Res. 2009, 24, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Ricciardi, B.F.; Wright, T.M.; Bostrom, M.P.; van der Meulen, M.C.H. Pause Insertions During Cyclic In Vivo Loading Affect Bone Healing. Clin. Orthop. Relat. Res. 2008, 466, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Mason, Z.D.; Chien, K.B.; Pfeiffer, A.J.; Barnes, G.L.; Einhorn, T.A.; Gerstenfeld, L.C. Micro-computed tomography assessment of fracture healing: Relationships among callus structure, composition, and mechanical function. Bone 2009, 44, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Recker, R.R.; Bare, S.P.; Smith, S.Y.; Varela, A.; Miller, M.A.; Morris, S.A.; Fox, J. Cancellous and cortical bone architecture and turnover at the iliac crest of postmenopausal osteoporotic women treated with parathyroid hormone 1–84. Bone 2009, 44, 113–119. [Google Scholar] [CrossRef]
- Hordon, L.D.; Itoda, M.; Shore, P.A.; Shore, R.C.; Heald, M.; Brown, M.; Kanis, J.A.; Rodan, G.A.; Aaron, J.E. Preservation of thoracic spine microarchitecture by alendronate: Comparison of histology and microCT. Bone 2006, 38, 444–449. [Google Scholar] [CrossRef]
- Gabet, Y.; Müller, R.; Levy, J.; Dimarchi, R.; Chorev, M.; Bab, I.; Kohavi, D. Parathyroid hormone 1–34 enhances titanium implant anchorage in low-density trabecular bone: A correlative micro-computed tomographic and biomechanical analysis. Bone 2006, 39, 276–282. [Google Scholar] [CrossRef]
- Lewin, S.; Barba, A.; Persson, C.; Franch, J.; Ginebra, M.P.; Öhman-Mägi, C. Evaluation of bone formation in calcium phosphate scaffolds with μCT-method validation using SEM. Biomed. Mater. 2017, 12, 065005. [Google Scholar] [CrossRef]
- Hilldore, A.J.; Morgan, A.W.; Woodard, J.R.; Wagoner Johnson, A.J. The curve integration method is comparable to manual segmentation for the analysis of bone/scaffold composites using micro-CT. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88B, 271–279. [Google Scholar] [CrossRef]
- Cooper, D.; Turinsky, A.; Sensen, C.; Hallgrimsson, B. Effect of Voxel Size on 3D Micro-CT Analysis of Cortical Bone Porosity. Calcif. Tissue Int. 2007, 80, 211–219. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. Quantification of Bone Microarchitecture with the Structure Model Index. Comput. Methods Biomech. Biomed. Engin. 1997, 1, 15–23. [Google Scholar] [CrossRef]
- Müller, R.; Van Campenhout, H.; Van Damme, B.; Van der Perre, G.; Dequeker, J.; Hildebrand, T.; Rüegsegger, P. Morphometric Analysis of Human Bone Biopsies: A Quantitative Structural Comparison of Histological Sections and Micro-Computed Tomography. Bone 1998, 23, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Chappard, D.; Retailleau-Gaborit, N.; Legrand, E.; Baslé, M.F.; Audran, M. Comparison insight bone measurements by histomorphometry and microCT. J. Bone Miner. Res. 2005, 20, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, N.; Alsheghri, A.A.; Piché, N.; Gendron, M.; Desrosiers, C.; Morozova, I.; Sanchez Siles, J.M.; Gonzalez-Quevedo, D.; Tamimi, I.; Song, J.; et al. Altered topological blueprint of trabecular bone associates with skeletal pathology in humans. Bone Rep. 2020, 12, 100264. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.P.; Rosenberg, W.S.; Keaveny, T.M. Quantitative computed tomography-based finite element models of the human lumbar vertebral body: Effect of element size on stiffness, damage, and fracture strength predictions. J. Biomech. Eng. 2003, 125, 434–438. [Google Scholar] [CrossRef]
- Ulrich, D.; Hildebrand, T.; Van Rietbergen, B.; Müller, R.; Rüegsegger, P. The quality of trabecular bone evaluated with micro-computed tomography, FEA and mechanical testing. Stud. Health Technol. Inform. 1997, 40, 97–112. [Google Scholar]
- van den Bergh, J.P.; Szulc, P.; Cheung, A.M.; Bouxsein, M.; Engelke, K.; Chapurlat, R. The clinical application of high-resolution peripheral computed tomography (HR-pQCT) in adults: State of the art and future directions. Osteoporos. Int. 2021, 32, 1465–1485. [Google Scholar] [CrossRef]
- Rapagna, S.; Berahmani, S.; Wyers, C.E.; van den Bergh, J.P.W.; Reynolds, K.J.; Tozzi, G.; Janssen, D.; Perilli, E. Quantification of human bone microarchitecture damage in press-fit femoral knee implantation using HR-pQCT and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2019, 97, 278–287. [Google Scholar] [CrossRef]
- Michalak, G.J.; Walker, R.; Boyd, S.K. Concurrent Assessment of Cartilage Morphology and Bone Microarchitecture in the Human Knee Using Contrast-Enhanced HR-pQCT Imaging. J. Clin. Densitom. 2019, 22, 74–85. [Google Scholar] [CrossRef]
- Goetzen, M.; Hofmann-Fliri, L.; Arens, D.; Zeiter, S.; Eberli, U.; Richards, G.; Blauth, M. Subchondral screw abutment: Does it harm the joint cartilage? An in vivo study on sheep tibiae. Int. Orthop. 2017, 41, 1607–1615. [Google Scholar] [CrossRef]
- Holme, M.N.; Schulz, G.; Deyhle, H.; Weitkamp, T.; Beckmann, F.; Lobrinus, J.A.; Rikhtegar, F.; Kurtcuoglu, V.; Zanette, I.; Saxer, T.; et al. Complementary X-ray tomography techniques for histology-validated 3D imaging of soft and hard tissues using plaque-containing blood vessels as examples. Nat. Protoc. 2014, 9, 1401–1415. [Google Scholar] [CrossRef]
- Smets, J.; Shevroja, E.; Hügle, T.; Leslie, W.D.; Hans, D. Machine Learning Solutions for Osteoporosis—A Review. J. Bone Miner. Res. 2021, 36, 833–851. [Google Scholar] [CrossRef] [PubMed]
- Pauchard, Y.; Liphardt, A.-M.; Macdonald, H.M.; Hanley, D.A.; Boyd, S.K. Quality control for bone quality parameters affected by subject motion in high-resolution peripheral quantitative computed tomography. Bone 2012, 50, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Walle, M.; Eggemann, D.; Atkins, P.R.; Kendall, J.J.; Stock, K.; Müller, R.; Collins, C.J. Motion grading of high-resolution quantitative computed tomography supported by deep convolutional neural networks. Bone 2023, 166, 116607. [Google Scholar] [CrossRef] [PubMed]
- Parr, W.C.H.; Chamoli, U.; Jones, A.; Walsh, W.R.; Wroe, S. Finite element micro-modelling of a human ankle bone reveals the importance of the trabecular network to mechanical performance: New methods for the generation and comparison of 3D models. J. Biomech. 2013, 46, 200–205. [Google Scholar] [CrossRef]
- Dailey, H.L.; Kersh, M.E.; Collins, C.J.; Troy, K.L. Mechanical Biomarkers in Bone Using Image-Based Finite Element Analysis. Curr. Osteoporos. Rep. 2023, 21, 266–277. [Google Scholar] [CrossRef]
- San Cheong, V.; Palanca, M.; Dall’Ara, E. Chapter 22—Bone strength, bone remodeling, and Biomechanics of fracture. In Digital Human Modeling and Medicine; Paul, G., Hamdy Doweidar, M., Eds.; Academic Press: Oxford, UK, 2023; pp. 515–546. [Google Scholar]
- Podshivalov, L.; Fischer, A.; Bar-Yoseph, P.Z. On the Road to Personalized Medicine: Multiscale Computational Modeling of Bone Tissue. Arch. Comput. Methods Eng. 2014, 21, 399–479. [Google Scholar] [CrossRef]
- Schileo, E.; Taddei, F.; Cristofolini, L.; Viceconti, M. Subject-specific finite element models implementing a maximum principal strain criterion are able to estimate failure risk and fracture location on human femurs tested in vitro. J. Biomech. 2008, 41, 356–367. [Google Scholar] [CrossRef]
- Engelke, K.; van Rietbergen, B.; Zysset, P. FEA to Measure Bone Strength: A Review. Clin. Rev. Bone Miner. Metab. 2016, 14, 26–37. [Google Scholar] [CrossRef]
- Lewis, G.S.; Mischler, D.; Wee, H.; Reid, J.S.; Varga, P. Finite Element Analysis of Fracture Fixation. Curr. Osteoporos. Rep. 2021, 19, 403–416. [Google Scholar] [CrossRef]
- Qasim, M.; Farinella, G.; Zhang, J.; Li, X.; Yang, L.; Eastell, R.; Viceconti, M. Patient-specific finite element estimated femur strength as a predictor of the risk of hip fracture: The effect of methodological determinants. Osteoporos. Int. 2016, 27, 2815–2822. [Google Scholar] [CrossRef]
- Liebl, H.; Garcia, E.G.; Holzner, F.; Noel, P.B.; Burgkart, R.; Rummeny, E.J.; Baum, T.; Bauer, J.S. In-Vivo Assessment of Femoral Bone Strength Using Finite Element Analysis (FEA) Based on Routine MDCT Imaging: A Preliminary Study on Patients with Vertebral Fractures. PLoS ONE 2015, 10, e0116907. [Google Scholar] [CrossRef] [PubMed]
- Varga, P.; Willie, B.M.; Stephan, C.; Kozloff, K.M.; Zysset, P.K. Finite element analysis of bone strength in osteogenesis imperfecta. Bone 2020, 133, 115250. [Google Scholar] [CrossRef] [PubMed]
- Kluess, D.; Souffrant, R.; Mittelmeier, W.; Wree, A.; Schmitz, K.-P.; Bader, R. A convenient approach for finite-element-analyses of orthopaedic implants in bone contact: Modeling and experimental validation. Comput. Methods Programs Biomed. 2009, 95, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Limbert, G.; van Lierde, C.; Muraru, O.L.; Walboomers, X.F.; Frank, M.; Hansson, S.; Middleton, J.; Jaecques, S. Trabecular bone strains around a dental implant and associated micromotions—A micro-CT-based three-dimensional finite element study. J. Biomech. 2010, 43, 1251–1261. [Google Scholar] [CrossRef]
- Stadelmann, V.A.; Conway, C.M.; Boyd, S.K. In vivo monitoring of bone–implant bond strength by microCT and finite element modelling. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 993–1001. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.J.; Jang, I.G. Patient-Specific Phantomless Estimation of Bone Mineral Density and Its Effects on Finite Element Analysis Results: A Feasibility Study. Comput. Math. Methods Med. 2019, 2019, 4102410. [Google Scholar] [CrossRef]
- Zysset, P.; Qin, L.; Lang, T.; Khosla, S.; Leslie, W.D.; Shepherd, J.A.; Schousboe, J.T.; Engelke, K. Clinical Use of Quantitative Computed Tomography–Based Finite Element Analysis of the Hip and Spine in the Management of Osteoporosis in Adults: The 2015 ISCD Official Positions—Part II. J. Clin. Densitom. 2015, 18, 359–392. [Google Scholar] [CrossRef]
- Viceconti, M.; Olsen, S.; Nolte, L.P.; Burton, K. Extracting clinically relevant data from finite element simulations. Clin. Biomech. 2005, 20, 451–454. [Google Scholar] [CrossRef]
- Keaveny, T.M.; Morgan, E.F.; Niebur, G.L.; Yeh, O.C. Biomechanics of trabecular bone. Annu. Rev. Biomed. Eng. 2001, 3, 307–333. [Google Scholar] [CrossRef]
- Chevalier, Y.; Pahr, D.; Allmer, H.; Charlebois, M.; Zysset, P. Validation of a voxel-based FE method for prediction of the uniaxial apparent modulus of human trabecular bone using macroscopic mechanical tests and nanoindentation. J. Biomech. 2007, 40, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Bini, F.; Pica, A.; Novelli, S.; Pecci, R.; Bedini, R.; Marinozzi, A.; Marinozzi, F. 3D FEM model to simulate Brownian motion inside trabecular tissue from human femoral head. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2022, 10, 500–507. [Google Scholar] [CrossRef]
- Benedikt, S.; Zelger, P.; Horling, L.; Stock, K.; Pallua, J.; Schirmer, M.; Degenhart, G.; Ruzicka, A.; Arora, R. Deep Convolutional Neural Networks Provide Motion Grading for High-Resolution Peripheral Quantitative Computed Tomography of the Scaphoid. Diagnostics 2024, 14, 568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hann, E.; Werys, K.; Wu, C.; Popescu, I.; Lukaschuk, E.; Barutcu, A.; Ferreira, V.M.; Piechnik, S.K. Deep learning with attention supervision for automated motion artefact detection in quality control of cardiac T1-mapping. Artif. Intell. Med. 2020, 110, 101955. [Google Scholar] [CrossRef]
- Fink, H.A.; Langsetmo, L.; Vo, T.N.; Orwoll, E.S.; Schousboe, J.T.; Ensrud, K.E. Association of High-resolution Peripheral Quantitative Computed Tomography (HR-pQCT) bone microarchitectural parameters with previous clinical fracture in older men: The Osteoporotic Fractures in Men (MrOS) study. Bone 2018, 113, 49–56. [Google Scholar] [CrossRef]
- Su, B.; Wen, Y.; Liu, Y.; Liao, S.; Fu, J.; Quan, G.; Li, Z. A deep learning method for eliminating head motion artifacts in computed tomography. Med. Phys. 2022, 49, 411–419. [Google Scholar] [CrossRef]
- Neeteson, N.J.; Besler, B.A.; Whittier, D.E.; Boyd, S.K. Automatic segmentation of trabecular and cortical compartments in HR-pQCT images using an embedding-predicting U-Net and morphological post-processing. Sci. Rep. 2023, 13, 252. [Google Scholar] [CrossRef]
- Lee, Y.; Bandara, W.R.; Park, S.; Lee, M.; Seo, C.; Yang, S.; Lim, K.J.; Moe, S.M.; Warden, S.J.; Surowiec, R.K. Integrating deep learning and machine learning for improved CKD-related cortical bone assessment in HRpQCT images: A pilot study. Bone Rep. 2025, 24, 101821. [Google Scholar] [CrossRef]
- Surowiec, R.K.; Swallow, E.A.; Warden, S.J.; Allen, M.R. Tracking changes of individual cortical pores over 1 year via HR-pQCT in a small cohort of 60-year-old females. Bone Rep. 2022, 17, 101633. [Google Scholar] [CrossRef]
- Lu, S.; Fuggle, N.R.; Westbury, L.D.; Breasail, M.Ó.; Bevilacqua, G.; Ward, K.A.; Dennison, E.M.; Mahmoodi, S.; Niranjan, M.; Cooper, C. Machine learning applied to HR-pQCT images improves fracture discrimination provided by DXA and clinical risk factors. Bone 2023, 168, 116653. [Google Scholar] [CrossRef]
- Atkinson, E.J.; Therneau, T.M.; Melton, L.J., 3rd; Camp, J.J.; Achenbach, S.J.; Amin, S.; Khosta, S. Assessing fracture risk using gradient boosting machine (GBM) models. J. Bone Miner. Res. 2012, 27, 1397–1404. [Google Scholar] [CrossRef]
- Gazzotti, S.; Aparisi Gómez, M.P.; Schileo, E.; Taddei, F.; Sangiorgi, L.; Fusaro, M.; Miceli, M.; Guglielmi, G.; Bazzocchi, A. High-resolution peripheral quantitative computed tomography: Research or clinical practice? Br. J. Radiol. 2023, 96, 20221016. [Google Scholar] [CrossRef]
- Whittier, D.E.; Samelson, E.J.; Hannan, M.T.; Burt, L.A.; Hanley, D.A.; Biver, E.; Szulc, P.; Sornay-Rendu, E.; Merle, B.; Chapurlat, R.; et al. A Fracture Risk Assessment Tool for High Resolution Peripheral Quantitative Computed Tomography. J. Bone Miner. Res. 2023, 38, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Lagzouli, A.; Pivonka, P.; Cooper, D.M.L.; Sansalone, V.; Othmani, A. A robust deep learning approach for segmenting cortical and trabecular bone from 3D high resolution µCT scans of mouse bone. Sci. Rep. 2025, 15, 8656. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Moore, J.M.; Covarrubias, B.V.; Lynch, L.M. Segmentation of cortical bone, trabecular bone, and medullary pores from micro-CT images using 2D and 3D deep learning models. Anat. Rec. 2025. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Goldstein, S.A.; Parfitt, M.A.; Jesion, G.; Kleerekoper, M. The direct examination of three-dimensional bone architecture in vitro by computed tomography. J. Bone Miner. Res. 1989, 4, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Petrik, V.; Apok, V.; Britton, J.A.; Bell, B.A.; Papadopoulos, M.C. Godfrey Hounsfield and the Dawn of Computed Tomography. Neurosurgery 2006, 58, 780–787. [Google Scholar] [CrossRef]
- Rüegsegger, P.; Koller, B.; Müller, R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif. Tissue Int. 1996, 58, 24–29. [Google Scholar] [CrossRef]
- Jaecques, S.V.N.; Van Oosterwyck, H.; Muraru, L.; Van Cleynenbreugel, T.; De Smet, E.; Wevers, M.; Naert, I.; Vander Sloten, J. Individualised, micro CT-based finite element modelling as a tool for biomechanical analysis related to tissue engineering of bone. Biomaterials 2004, 25, 1683–1696. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Q.; Huang, J.; Zhang, Z.; Wang, X.; Han, Q.; Shi, Y.; Ji, R.; Li, Y. Design and manufacturing of biomimetic scaffolds for bone repair inspired by bone trabeculae. Comput. Biol. Med. 2023, 165, 107369. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Schulte, F.A.; Lambers, F.M.; Kuhn, G.; Müller, R. In vivo micro-computed tomography allows direct three-dimensional quantification of both bone formation and bone resorption parameters using time-lapsed imaging. Bone 2011, 48, 433–442. [Google Scholar] [CrossRef]



| Modality | Typical Spatial Resolution | Contrast Mechanism | Ionising Radiation | Soft-Tissue Contrast | 3D Bone Microarchitecture (Trabeculae/Cortex) | In Vivo Suitability | Typical Bone Uses | Key Limitations |
|---|---|---|---|---|---|---|---|---|
| Micro-CT (lab, ex vivo) | ~1–10 µm voxels (sub-µm with nano-CT) | X-ray attenuation (calibratable to mineral density) | Yes (specimen only) | Limited without contrast agents | Excellent (quantitative trabecular/cortical metrics; FE models) | Ex vivo; small-animal in vivo variants exist | Morphometry, porosity, BMD, implants/scaffolds, digital histology, FE | Dose/scan time; FoV and specimen-size constraints; beam hardening |
| SR micro-CT (ex vivo) | Sub-µm | Monochromatic X-ray attenuation | Yes (beamline) | Limited without contrast | Excellent (ultra-high res) | Ex vivo | Lacuno-canalicular network, micro-damage, mineral mapping | Facility access; sample size limits |
| HR-pQCT (clinical in vivo) | 61–82 µm | X-ray attenuation | Very low effective dose | Limited soft tissue | Good (coarser than micro-CT; clinical in vivo) | Yes (peripheral sites) | Osteoporosis assessment, longitudinal monitoring, FE strength | Peripheral only; motion; partial-volume at trabecular scale |
| MRI (clinical) | ~0.2–1.0 mm (sequence-dependent) | Proton density and relaxation; excellent soft tissue | No | Excellent (marrow, cartilage, synovium) | Limited (UTE/ZTE capture cortex signal; not trabecular morphometry at clinical voxel sizes) | Yes | Marrow composition, oedema, cartilage, soft tissue around bone | Lower mineral sensitivity; long scans; artefacts with metal |
| PET-CT | PET: ~4–6 mm; CT: 0.5–1 mm | Molecular radiotracer uptake + CT anatomy | Yes (radiotracer + CT) | PET indirect; CT limited | Limited (microarchitecture below PET resolution) | Yes | Turnover, infection/inflammation, oncology; CT for localization | Radiation; cost; limited spatial detail of trabeculae |
| Ultrasound (MSK) | ~0.1–0.3 mm (high-freq probes) | Acoustic impedance | No | Good for superficial soft tissue | No intraosseous (cortex blocks beam) | Yes | Tendons/ligaments, cortical surface, guidance | Operator-dependent; cannot image through cortex |
| Metric Measures | Abbreviation | Description | Standard Unit | Recommended Variable for |
|---|---|---|---|---|
| Bone volume ratio | BV/TV | Ratio of bone volume to total volume in the ROI | % | trabecular bone |
| Cortical bone area | Ct.Ar | Cortical bone area | mm2 | cortical bone |
| Total cross-sectional area | Tt.Ar | Area inside the periosteal envelope | mm2 | cortical bone |
| Cortical area fraction | Ct.Ar/Tt.Ar | Ratio of cortical bone area to total cross-sectional area | % | cortical bone |
| Cortical thickness | Ct.Th | Average cortical thickness | mm | cortical bone |
| Cortical porosity | Ct.Po | Relative voxel-based measure of the volume of the intracortical pore space normalised by the sum of the pore and cortical bone volume | % | cortical bone |
| Trabecular separation | Tb.Sp | Mean distance between trabeculae | mm | trabecular bone |
| Trabecular thickness | Tb.Th | Mean thickness of trabeculae | mm | trabecular bone |
| Trabecular number | Tb.N | Mean number of trabeculae per unit length | mm−1 | trabecular bone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindtner, R.; Kampik, L.; Putzer, D.; Klosterhuber, M.; Pallua, A.K.; Streif, W.; Schirmer, M.; Degenhart, G.; Arora, R.; Pallua, J.D. Advancements in High-Resolution Computed Tomography: Revolutionising Bone Health Micro-Research. Bioengineering 2025, 12, 1189. https://doi.org/10.3390/bioengineering12111189
Lindtner R, Kampik L, Putzer D, Klosterhuber M, Pallua AK, Streif W, Schirmer M, Degenhart G, Arora R, Pallua JD. Advancements in High-Resolution Computed Tomography: Revolutionising Bone Health Micro-Research. Bioengineering. 2025; 12(11):1189. https://doi.org/10.3390/bioengineering12111189
Chicago/Turabian StyleLindtner, Richard, Lukas Kampik, David Putzer, Miranda Klosterhuber, Anton Kasper Pallua, Werner Streif, Michael Schirmer, Gerald Degenhart, Rohit Arora, and Johannes Dominikus Pallua. 2025. "Advancements in High-Resolution Computed Tomography: Revolutionising Bone Health Micro-Research" Bioengineering 12, no. 11: 1189. https://doi.org/10.3390/bioengineering12111189
APA StyleLindtner, R., Kampik, L., Putzer, D., Klosterhuber, M., Pallua, A. K., Streif, W., Schirmer, M., Degenhart, G., Arora, R., & Pallua, J. D. (2025). Advancements in High-Resolution Computed Tomography: Revolutionising Bone Health Micro-Research. Bioengineering, 12(11), 1189. https://doi.org/10.3390/bioengineering12111189








