Corneal Epithelial Tissue Engineering Strategy Based on Cell Viability Optimization: A Review and Prospects
Abstract
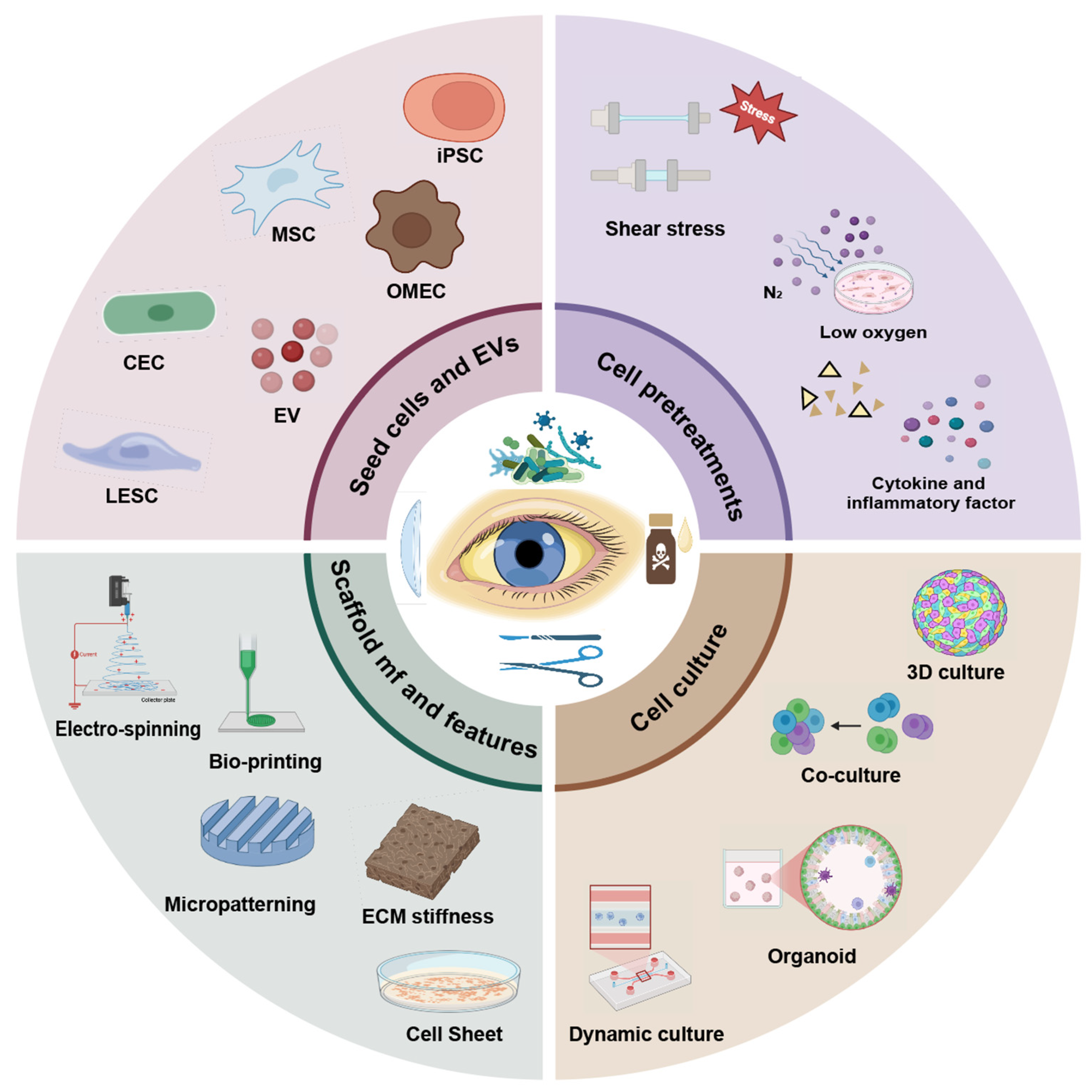
1. Introduction
2. Structure and Function of Corneal Epithelium
3. Seed Cell Resources and Cell-Free Strategies
3.1. CECs and LESCs
3.2. MSC-Induced CECs
3.3. iPSC-Induced CECs
3.4. Extracellular Vesicles and Enucleate Cells
4. Cell Pretreatment
4.1. Physical Pretreatment
4.2. Biochemical Pretreatment
5. Engineering-Oriented Culture Systems
5.1. Dynamic Culture
5.2. Co-Culture
5.3. Three-Dimensional Culture
6. Biomimetic Scaffold and Manufacture Processes
6.1. Stiffness-Regulating Mechanical Properties
6.2. Material Topography and Micropatterned Surfaces
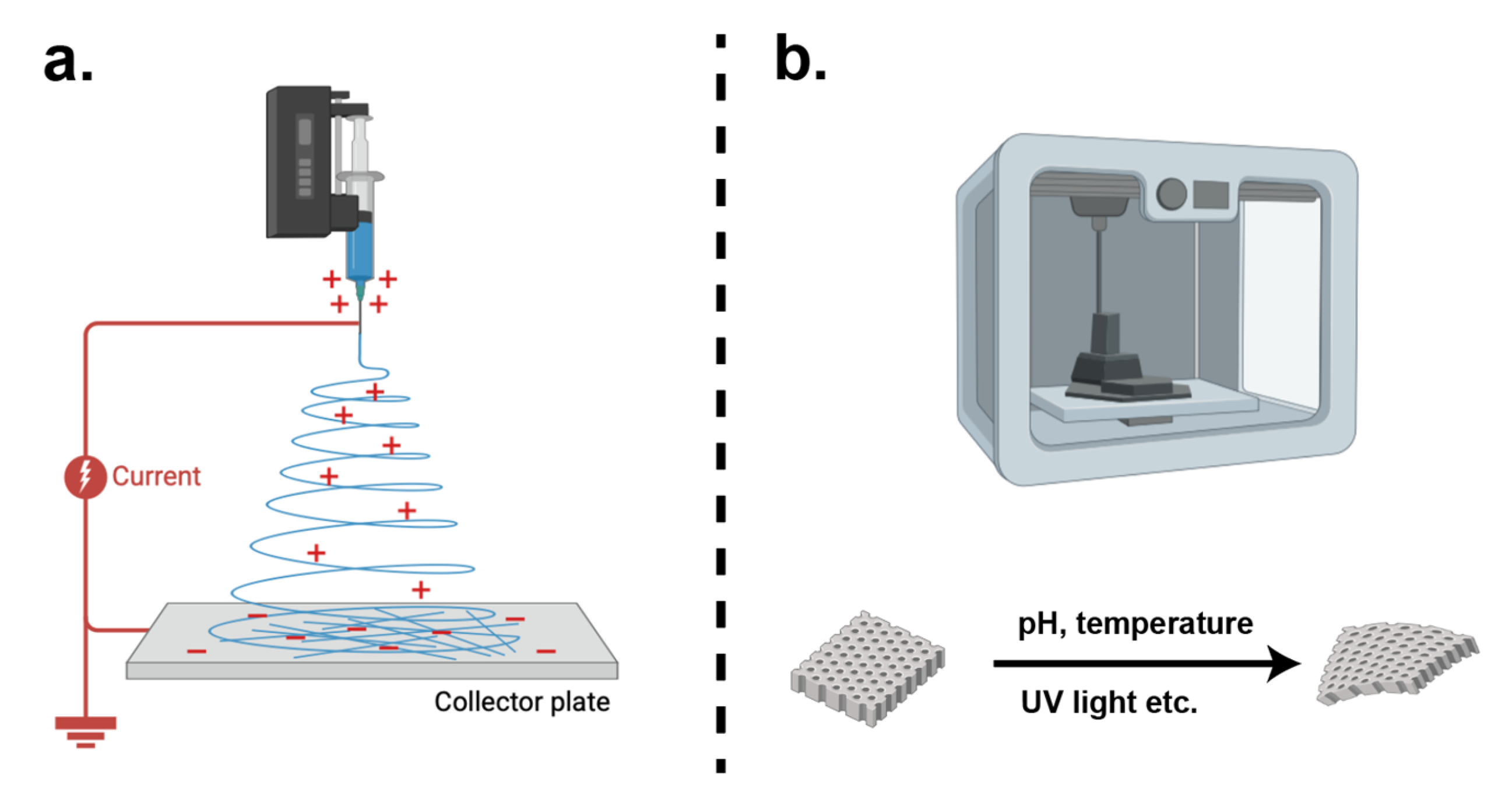
6.3. Innovative Scaffold Manufacture Processes
6.3.1. Electrospinning
6.3.2. Bioprinting
6.4. Cell Sheets
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LESC | Limbal epithelial stem cell |
| LSCD | Limbal stem cell deficiency |
| ECM | Extracellular matrix |
| CEC | Corneal epithelial cell |
| 3D | Three-dimensional |
| MSC | Mesenchymal stem cell |
| iPSC | Induced pluripotent stem cell |
| COMET | Cultured mucosal epithelial transplantation |
| CLAU | Conjunctival–limbal autograft |
| KLAL | Keratolimbal allograft |
| CLET | Cultivated limbal epithelial transplantation |
| SLET | Simple limbal epithelial transplantation |
| CALEC | Cultivated autologous limbal epithelial cell |
| hOMSC | Human oral mucosa stem cell |
| ESC | Embryonic stem cell |
| CHC | Carboxymethyl-hexanoyl chitosan |
| SEAM | Self-formed ectoderm autonomous multizone |
| EV | Extracellular vesicle |
| ALI | Air–liquid interface |
| SAW | Surface acoustic wave |
| ASC | Adipose-derived mesenchymal stem cell |
| DCM | Decellularized cornea matrix |
| LMSC | Limbal mesenchymal stem cell |
| ML | Machine learning |
References
- Barrientez, B.; Nicholas, S.E.; Whelchel, A.; Sharif, R.; Hjortdal, J.; Karamichos, D. Corneal injury: Clinical and molecular aspects. Exp. Eye Res. 2019, 186, 107709. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Kempuraj, D.; D’Souza, S.; Ghosh, A. Corneal stromal repair and regeneration. Prog. Retin. Eye Res. 2022, 91, 101090. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Kong, X.; Wolle, M.; Gasquet, N.; Ssekasanvu, J.; Mariotti, S.P.; Bourne, R.; Taylor, H.; Resnikoff, S.; West, S. Global Trends in Blindness and Vision Impairment Resulting from Corneal Opacity 1984–2020: A Meta-analysis. Ophthalmology 2023, 130, 863–871. [Google Scholar] [CrossRef]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed]
- Tran, T.M.; Duong, H.; Bonnet, C.; Kashanchi, A.; Buckshey, A.; Aldave, A.J. Corneal Blindness in Asia: A Systematic Review and Meta-Analysis to Identify Challenges and Opportunities. Cornea 2020, 39, 1196–1205. [Google Scholar] [CrossRef]
- Wu, D.H.; Lin, Y.; Wu, H.P.; Cai, J.H. Trauma-induced corneal epithelial defects may lead to persistent epithelial defects exacerbated by prolonged use of bandage lenses. Eur. J. Ophthalmol. 2025, 35, 1818–1823. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Ruiz-Lozano, R.E.; Barcelo-Canton, R.H.; Marines-Sanchez, H.M.; Homar Paez-Garza, J. The etiologic and pathogenic spectrum of exposure keratopathy: Diagnostic and therapeutic implications. Surv. Ophthalmol. 2025, 70, 882–899. [Google Scholar] [CrossRef]
- Georgoudis, P. Persistent epithelial defects in neurotrophic keratopathy. Acta Ophthalmol. 2024, 102. [Google Scholar] [CrossRef]
- Moussa, S.M.; Mahmoud, S.S.; Aly, E.M.; Talaat, M.S. Bio-spectroscopic analysis of corneal structural alterations in dry eye disease: A study of collagen, co-enzymes, lipids, and proteins with emphasis on phytotherapy intervention. Int. J. Biol. Macromol. 2024, 280 Pt 3, 136010. [Google Scholar] [CrossRef]
- Ladea, L.; Zemba, M.; Calancea, M.I.; Caltaru, M.V.; Dragosloveanu, C.D.M.; Coroleuca, R.; Catrina, E.L.; Brezean, I.; Dinu, V. Corneal Epithelial Changes in Diabetic Patients: A Review. Int. J. Mol. Sci. 2024, 25, 3471. [Google Scholar] [CrossRef]
- Anju, M.S.; Mathew, A.I.; Raj, D.K.; Vinod, D.; Kasoju, N.; Raghavan, C.; Kumar, P.R.A. Bioengineered Human Limbal Stem Cell-Derived Epithelial Sheets for Ocular Surface Reconstruction. Regen. Eng. Transl. Med. 2025, 11, 449–463. [Google Scholar]
- Lazzara, F.; Conti, F.; Maugeri, G.; D’Agata, V.; Sotera, L.; Bucolo, C. Corneal protective effects of a new ophthalmic formulation based on vitamin B12 and sodium hyaluronate. Front. Pharmacol. 2025, 16, 1548213. [Google Scholar] [CrossRef] [PubMed]
- Gelles, J.D.; Hillier, K.E.; Krisa, S.; Greenstein, S.A.; Hersh, P.S. Lipid Keratopathy Management with Therapeutic Scleral Lens Wear. Eye Contact Lens 2022, 48, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, S.M.; Hyon, J.Y.; Kim, M.K.; Oh, J.Y.; Choi, H.J. Large diameter scleral lens benefits for Asians with intractable ocular surface diseases: A prospective, single-arm clinical trial. Sci. Rep. 2021, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Dikmetas, O.; Kapucu, Y.; Cankaya, A.B.; Kocabeyoglu, S. Outcomes and success of amniotic membrane transplantation for the treatment of corneal diseases. Cutan. Ocul. Toxicol. 2024, 43, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Schuerch, K.; Baeriswyl, A.; Frueh, B.E.; Tappeiner, C. Efficacy of Amniotic Membrane Transplantation for the Treatment of Corneal Ulcers. Cornea 2020, 39, 479–483. [Google Scholar] [CrossRef]
- Tsai, R.J.; Li, L.M.; Chen, J.K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N. Engl. J. Med. 2000, 343, 86–93. [Google Scholar] [CrossRef]
- Nguyen, K.N.; Bobba, S.; Richardson, A.; Park, M.; Watson, S.L.; Wakefield, D.; Di Girolamo, N. Native and synthetic scaffolds for limbal epithelial stem cell transplantation. Acta Biomater. 2018, 65, 21–35. [Google Scholar] [CrossRef]
- Alio, J.L.; Montesel, A.; El Sayyad, F.; Barraquer, R.I.; Arnalich-Montiel, F. Corneal graft failure: An update. Br. J. Ophthalmol. 2021, 105, 1049–1058. [Google Scholar] [CrossRef]
- Kumar, R.; Sinha, N.R.; Mohan, R.R. Corneal gene therapy: Structural and mechanistic understanding. Ocul. Surf. 2023, 29, 279–297. [Google Scholar] [CrossRef]
- Li, S.; Sun, H.; Chen, L.; Fu, Y. Targeting limbal epithelial stem cells: Master conductors of corneal epithelial regeneration from the bench to multilevel theranostics. J. Transl. Med. 2024, 22, 794. [Google Scholar] [CrossRef]
- Nosrati, H.; Alizadeh, Z.; Nosrati, A.; Ashrafi-Dehkordi, K.; Banitalebi-Dehkordi, M.; Sanami, S.; Khodaei, M. Stem cell-based therapeutic strategies for corneal epithelium regeneration. Tissue Cell 2021, 68, 101470. [Google Scholar] [CrossRef]
- Bisevac, J.; Katta, K.; Petrovski, G.; Moe, M.C.; Noer, A. Wnt/β-Catenin Signaling Activation Induces Differentiation in Human Limbal Epithelial Stem Cells Cultured Ex Vivo. Biomedicines 2023, 11, 1829. [Google Scholar] [CrossRef]
- Shafiq, M.; Ali, O.; Han, S.B.; Kim, D.H. Mechanobiological Strategies to Enhance Stem Cell Functionality for Regenerative Medicine and Tissue Engineering. Front. Cell Dev. Biol. 2021, 9, 747398. [Google Scholar] [CrossRef]
- Ricciotti, L.; Apicella, A.; Perrotta, V.; Aversa, R. Geopolymer Materials for Bone Tissue Applications: Recent Advances and Future Perspectives. Polymers 2023, 15, 1087. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Q.; Liu, J.C.; Zhang, Z.Y.; Xu, C.X. A review on cell damage, viability, and functionality during 3D bioprinting. Mil. Med. Res. 2022, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, R.M.; Vajda, F.; Wibowo, J.A.; Figueiredo, F.; Connon, C.J. YAP, ΔNp63, and β-Catenin Signaling Pathways Are Involved in the Modulation of Corneal Epithelial Stem Cell Phenotype Induced by Substrate Stiffness. Cells 2019, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, Z.X.; Gao, W.D.; He, R.Y.; Li, Y.L.; Crawford, R.; Zhou, Y.H.; Xiao, L.; Xiao, Y. Current Advances in 3D Dynamic Cell Culture Systems. Gels 2022, 8, 829. [Google Scholar] [CrossRef]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar]
- Chirila, T.V.; Hicks, C.R.; Dalton, P.D.; Vijayasekaran, S.; Lou, X.; Hong, Y.; Clayton, A.B.; Ziegelaar, B.W.; Fitton, J.H.; Platten, S.; et al. Artificial cornea. Prog. Polym. Sci. 1998, 23, 447–473. [Google Scholar] [CrossRef]
- Steinhoff, G. Regenerative Medicine—From Protocol to Patient: 1. Biology of Tissue Regeneration; Springer International Publishing: Cham, Switzerland, 2016; p. 1. [Google Scholar]
- Gonzalez, G.; Sasamoto, Y.; Ksander, B.R.; Frank, M.H.; Frank, N.Y. Limbal stem cells: Identity, developmental origin, and therapeutic potential. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e303. [Google Scholar] [CrossRef]
- Hanna, C.; Bicknell, D.S.; O’Brien, J.E. Cell turnover in the adult human eye. Arch. Ophthalmol. 1961, 65, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Sartaj, R.; Zhang, C.; Wan, P.; Pasha, Z.; Guaiquil, V.; Liu, A.; Liu, J.; Luo, Y.; Fuchs, E.; Rosenblatt, M.I. Characterization of slow cycling corneal limbal epithelial cells identifies putative stem cell markers. Sci. Rep. 2017, 7, 3793. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, A.; Amitai-Lange, A.; Tarazi, N.; Dey, S.; Strinkovsky, L.; Hadad-Porat, S.; Bhattacharya, S.; Nasser, W.; Imeri, J.; Ben-David, G.; et al. Discrete limbal epithelial stem cell populations mediate corneal homeostasis and wound healing. Cell Stem Cell 2021, 28, 1248–1261.e8. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Hou, A.H.; Ali, S.M.; Png, E.; Hunziker, W.; Tong, L.I. Transglutaminase-2 is critical for corneal epithelial barrier function via positive regulation of Claudin-1. Ocul. Surf. 2023, 28, 155–164. [Google Scholar] [CrossRef]
- Giasson, C.J.; Deschambeault, A.; Carrier, P.; Germain, L. Adherens junction proteins are expressed in collagen corneal equivalents produced in vitro with human cells. Mol. Vis. 2014, 20, 386–394. [Google Scholar]
- Bush, J.; Cabe, J.I.; Conway, D.; Maruthamuthu, V. E-cadherin adhesion dynamics as revealed by an accelerated force ramp are dependent upon the presence of α-catenin. Biochem. Biophys. Res. Commun. 2023, 682, 308–315. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Y. Restoration of corneal epithelial barrier function: A possible target for corneal neovascularization. Ocul. Surf. 2024, 34, 38–49. [Google Scholar] [CrossRef]
- Ebrahim, A.S.; Carion, T.W.; Ebrahim, T.; Win, J.; Kani, H.; Wang, Y.X.; Stambersky, A.; Ibrahim, A.S.; Sosne, G.; Berger, E.A. A Novel Combination Therapy Tβ4/VIP Protects against Hyperglycemia-Induced Changes in Human Corneal Epithelial Cells. Biosensors 2023, 13, 974. [Google Scholar] [CrossRef]
- Wang, L.Y.; Xu, X.Z.; Chen, Q.K.; Wei, Y.; Wei, Z.Y.; Jin, Z.B.; Liang, Q.F. Extracellular Vesicle MicroRNAs from Corneal Stromal Stem Cell Enhance Stemness of Limbal Epithelial Stem Cells by Targeting the Notch Pathway. Investig. Ophthalmol. Vis. Sci. 2023, 64, 42. [Google Scholar] [CrossRef]
- Plamann, K. Optics and quantitative assessment of corneal transparency. Acta Ophthalmol. 2024, 102. [Google Scholar] [CrossRef]
- Maurice, D.M. The Structure and Transparency of the Cornea. J. Physiol. 1957, 136, 263. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.B.; Yan, Y.Y.; Shen, Z.Z.; Cao, Y.Y.; Duan, Q.Q.; He, M.; Zhang, Q. Photo-crosslinked hydrogels for tissue engineering of corneal epithelium. Exp. Eye Res. 2022, 218, 109027. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.J.; You, D.B.; Wang, W.; Feng, Y. Preliminary studies of constructing a tissue-engineered lamellar corneal graft by culturing mesenchymal stem cells onto decellularized corneal matrix. Int. J. Ophthalmol. 2021, 14, 10–18. [Google Scholar] [CrossRef]
- Visalli, F.; Fava, F.; Capobianco, M.; Musa, M.; D’Esposito, F.; Russo, A.; Scollo, D.; Longo, A.; Gagliano, C.; Zeppieri, M. Innovative Bioscaffolds in Stem Cell and Regenerative Therapies for Corneal Pathologies. Bioengineering 2024, 11, 859. [Google Scholar] [CrossRef]
- Kato, D.; Hirano, K.; Tanikawa, A.; Ito, Y. Corneal surface reconstruction for the chemical injured eye by transplanting autologous cultivated limbal epithelial sheet “Nepic®”. Fujita Med. J. 2025, 11, 142–145. [Google Scholar]
- Nakamura, T.; Kinoshita, S. Ocular surface reconstruction using cultivated mucosal epithelial stem cells. Cornea 2003, 22, S75–S80. [Google Scholar] [CrossRef]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Amemiya, T.; Kanamura, N.; Kinoshita, S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1280–1284. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef]
- Toshida, H.; Kasahara, T.; Kiriyama, M.; Iwasaki, Y.; Sugita, J.; Ichikawa, K.; Ohta, T.; Miyahara, K. Early Clinical Outcomes of the First Commercialized Human Autologous Ex Vivo Cultivated Oral Mucosal Epithelial Cell Transplantation for Limbal Stem Cell Deficiency: Two Case Reports and Literature Review. Int. J. Mol. Sci. 2023, 24, 8926. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Imai, K.; Ueno, M.; Sotozono, C. Assessment of the official national insurance coverage of regenerative medical products for ophthalmic diseases in Japan following regulatory approval. Regen. Ther. 2025, 30, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.H.; Chauhan, T.; Yung, M.; Tseng, C.H.; Deng, S.X. Outcomes of Limbal Stem Cell Transplant A Meta-analysis. JAMA Ophthalmol. 2020, 138, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Daya, S.M. Conjunctival-limbal autograft. Curr. Opin. Ophthalmol. 2017, 28, 370–376. [Google Scholar] [CrossRef]
- Eslani, M.; Cheung, A.Y.; Kurji, K.; Pierson, K.; Sarnicola, E.; Holland, E.J. Long-term outcomes of conjunctival limbal autograft in patients with unilateral total limbal stem cell deficiency. Ocul. Surf. 2019, 17, 670–674. [Google Scholar] [CrossRef]
- Kenyon, K.R.; Tseng, S.C.G. Limbal Autograft Transplantation for Ocular Surface Disorders. Ophthalmology 1989, 96, 709–723. [Google Scholar] [CrossRef]
- Chan, C.C.; Biber, J.M.; Holland, E.J. The Modified Cincinnati Procedure: Combined Conjunctival Limbal Autografts and Keratolimbal Allografts for Severe Unilateral Ocular Surface Failure. Cornea 2012, 31, 1264–1272. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Eslani, M.; Kurji, K.H.; Wright, E.; Sarnicola, E.; Govil, A.; Holland, E.J. Long-term Outcomes of Living-Related Conjunctival Limbal Allograft Compared with Keratolimbal Allograft in Patients with Limbal Stem Cell Deficiency. Cornea 2020, 39, 980–985. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; DeLuca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Liu, J.B.; Lawrence, B.D.; Liu, A.H.; Schwab, I.R.; Oliveira, L.A.; Rosenblatt, M.I. Silk Fibroin as a Biomaterial Substrate for Corneal Epithelial Cell Sheet Generation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4130–4138. [Google Scholar] [CrossRef]
- Schwab, I.R.; Reyes, M.; Isseroff, R.R. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea 2000, 19, 421–426. [Google Scholar] [CrossRef]
- Ghareeb, A.E.; Lako, M.; Figueiredo, F.C. Recent Advances in Stem Cell Therapy for Limbal Stem Cell Deficiency: A Narrative Review. Ophthalmol. Ther. 2020, 9, 809–831. [Google Scholar] [CrossRef]
- Pellegrini, G.; Ardigò, D.; Milazzo, G.; Iotti, G.; Guatelli, P.; Pelosi, D.; De Luca, M. Navigating Market Authorization: The Path Holoclar Took to Become the First Stem Cell Product Approved in the European Union. Stem Cell Transl. Med. 2018, 7, 146–154. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; MacNeil, S.; Balasubramanian, D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 931–934. [Google Scholar] [CrossRef]
- Jurkunas, U.; Johns, L.; Armant, M. Cultivated Autologous Limbal Epithelial Cell Transplantation: New Frontier in the Treatment of Limbal Stem Cell Deficiency. Am. J. Ophthalmol. 2022, 239, 244–268. [Google Scholar] [CrossRef]
- Jurkunas, U.V.; Kaufman, A.R.; Yin, J.; Ayala, A.; Maguire, M.; Samarakoon, L.; Johns, L.K.; Parekh, M.; Li, S.; Gauthier, A.; et al. Cultivated autologous limbal epithelial cell (CALEC) transplantation for limbal tem cell deficiency: A phase I/II clinical trial of the first xenobiotic-free, serum-free, antibiotic-free manufacturing protocol developed in the US. Nat. Commun. 2025, 16, 1607. [Google Scholar] [CrossRef]
- Kacham, S.; Bhure, T.S.; Eswaramoorthy, S.D.; Naik, G.; Rath, S.N.; Parcha, S.R.; Basu, S.; Sangwan, V.S.; Shukla, S. Human Umbilical Cord-Derived Mesenchymal Stem Cells Promote Corneal Epithelial Repair In Vitro. Cells 2021, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.R.; Huang, C.; Zhang, L.Y.; Jiang, H.; Zhao, S.; Zhang, L.L.; Zheng, X.E.; Ou, S.K.; Gu, H. A novel tissue-engineered corneal epithelium based on ultra-thin amniotic membrane and mesenchymal stem cells. Sci. Rep. 2024, 14, 17407. [Google Scholar] [CrossRef] [PubMed]
- Kobal, N.; Marzidovsek, M.; Schollmayer, P.; Malicev, E.; Hawlina, M.; Marzidovsek, Z.L. Molecular and Cellular Mechanisms of the Therapeutic Effect of Mesenchymal Stem Cells and Extracellular Vesicles in Corneal Regeneration. Int. J. Mol. Sci. 2024, 25, 11121. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, Z.R.; Su, W.R.; Li, Y.P.; Lin, M.L.; Zhang, W.X.; Liu, Y.; Wan, Q.; Liang, D. Role of Mesenchymal Stem Cells on Cornea Wound Healing Induced by Acute Alkali Burn. PLoS ONE 2012, 7, e30842. [Google Scholar] [CrossRef]
- Fu, Y.O.; Chen, P.R.; Yeh, C.C.; Pan, J.Y.; Kuo, W.C.; Tseng, K.W. Human Umbilical Mesenchymal Stem Cell Xenografts Repair UV-Induced Photokeratitis in a Rat Model. Biomedicines 2022, 10, 1125. [Google Scholar] [CrossRef]
- Venkatakrishnan, J.; Saeed, Y.; Kao, W.W.Y. Trends in using mesenchymal stromal/stem cells (MSCs) in treating corneal diseases. Ocul. Surf. 2022, 26, 255–267. [Google Scholar] [CrossRef]
- Marynka-Kalmani, K.; Treves, S.; Yafee, M.; Rachima, H.; Gafni, Y.; Cohen, M.A.; Pitaru, S. The Lamina Propria of Adult Human Oral Mucosa Harbors a Novel Stem Cell Population. Stem Cells 2010, 28, 984–995. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Hoz, L.; Tenorio, E.P.; Buentello, B.; Magaña, F.S.; Wintergerst, A.; Navas, A.; Garfias, Y.; Arzate, H. Can Human Oral Mucosa Stem Cells Differentiate to Corneal Epithelia? Int. J. Mol. Sci. 2021, 22, 5976. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Liao, Y.W.; Liu, D.M.; Lin, H.L.; Chen, S.J.; Chen, H.L.; Peng, C.H.; Liang, C.M.; Mou, C.Y.; Chiou, S.H. Corneal repair by human corneal keratocyte-reprogrammed iPSCs and amphiphatic carboxymethyl-hexanoyl chitosan hydrogel. Biomaterials 2012, 33, 8003–8016. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Chen, M.F.; Sun, X.R.; Ge, J. Differentiation of mouse induced pluripotent stem cells into corneal epithelial-like cells. Cell Biol. Int. 2013, 37, 87–94. [Google Scholar] [CrossRef]
- Lee, S.; Han, J.; Yang, J.; Lyu, J.; Park, H.; Bang, J.; Kim, Y.; Chang, H.; Park, T. Exosomes from Human iPSC-Derived Retinal Organoids Enhance Corneal Epithelial Wound Healing. Int. J. Mol. Sci. 2024, 25, 8925. [Google Scholar] [CrossRef]
- Hayashi, R.; Ishikawa, Y.; Sasamoto, Y.; Katori, R.; Nomura, N.; Ichikawa, T.; Araki, S.; Soma, T.; Kawasaki, S.; Sekiguchi, K.; et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 2016, 531, 376–380. [Google Scholar] [CrossRef]
- Soma, T.; Oie, Y.; Takayanagi, H.; Matsubara, S.; Yamada, T.; Nomura, M.; Yoshinaga, Y.; Maruyama, K.; Watanabe, A.; Takashima, K.; et al. Induced pluripotent stem-cell-derived corneal epithelium for transplant surgery: A single-arm, open-label, first-in-human interventional study in Japan. Lancet 2024, 404, 1929–1939. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.R.; Wang, Z.; Li, J.J.; Shahzad, K.; Zheng, J.L. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.M.; Lu, B.; He, J.; Chen, X.; Fu, Q.L.; Han, H.J.; Luo, C.Q.; Yin, H.F.; Qin, Z.W.; Lyu, D.N.; et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Song, W.J.; Teng, L.J.; Huang, Y.R.; Liu, J.; Peng, Y.H.; Lu, X.T.; Yuan, J.; Zhao, X.; Zhao, Q.; et al. MiRNA 24-3p-rich exosomes functionalized DEGMA-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioact. Mater. 2023, 25, 640–656. [Google Scholar] [CrossRef]
- Xu, Y.H.; Wei, C.; Ma, L.; Zhao, L.; Li, D.F.; Lin, Y.L.; Zhou, Q.J.; Xie, L.X. 3D mesenchymal stem cell exosome-functionalized hydrogels for corneal wound healing. J. Control. Release 2025, 380, 630–646. [Google Scholar] [CrossRef]
- Wang, H.; Alarcon, C.N.; Liu, B.; Watson, F.; Searles, S.; Lee, C.K.; Keys, J.; Pi, W.; Allen, D.; Lammerding, J.; et al. Genetically engineered and enucleated human mesenchymal stromal cells for the targeted delivery of therapeutics to diseased tissue. Nat. Biomed. Eng. 2022, 6, 882–897. [Google Scholar] [CrossRef]
- Hampel, U.; Garreis, F.; Burgemeister, F.; Essel, N.; Paulsen, F. Effect of intermittent shear stress on corneal epithelial cells using an flow culture model. Ocul. Surf. 2018, 16, 341–351. [Google Scholar] [CrossRef]
- Molladavoodi, S.; Robichaud, M.; Wulff, D.; Gorbet, M. Corneal epithelial cells exposed to shear stress show altered cytoskeleton and migratory behaviour. PLoS ONE 2017, 12, e0178981. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Ishibazawa, A.; Yoshioka, T.; Song, Y.S.; Yoshida, K. Assessing effects of mechanical stimulation of fluid shear stress on inducing matrix Metalloproteinase-9 in cultured corneal epithelial cells. Exp. Eye Res. 2023, 234, 109571. [Google Scholar] [CrossRef]
- Wu, M.F.; Peng, X.; Zhao, J.L.; Zhang, M.C.; Xie, H.T. Mitophagy and mitochondrion-related expression profiles in response to physiological and pathological hypoxia in the corneal epithelium. Genomics 2023, 115, 110739. [Google Scholar] [CrossRef]
- Ma, X.L.; Liu, H.Q. Effect of hypoxia on the proliferation of murine cornea limbal epithelial progenitor cells. Int. J. Ophthalmol. 2011, 4, 147–149. [Google Scholar]
- Fehrer, C.; Brunauer, R.; Laschober, G.; Unterluggauer, H.; Reitinger, S.; Kloss, F.; Gülly, C.; Gassner, R.; Lepperdinger, G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell 2007, 6, 745–757. [Google Scholar] [CrossRef]
- Han, K.H.; Kim, A.K.; Kim, M.H.; Kim, D.H.; Go, H.N.; Kim, D.I. Enhancement of angiogenic effects by hypoxia-preconditioned human umbilical cord-derived mesenchymal stem cells in a mouse model of hindlimb ischemia. Cell Biol. Int. 2016, 40, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kaitsuka, T.; Hakim, F. Response of Pluripotent Stem Cells to Environmental Stress and Its Application for Directed Differentiation. Biology 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Yu, L.; Liu, Y.; Xiao, B.; Ye, X.J.; Zhao, H.; Xi, Y.H.; Shi, Z.C.; Wang, W.H. Hypoxia regulates adipose mesenchymal stem cells proliferation, migration, and nucleus pulposus-like differentiation by regulating endoplasmic reticulum stress via the HIF-1α pathway. J. Orthop. Surg. Res. 2023, 18, 339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Xu, L.L.; Zhao, J.P.; Liang, J.C.; Zhang, Z.X.; Li, Q.; Zhang, J.H.; Wan, P.X.; Wu, Z. Reconstructing auto tissue engineering lamellar cornea with aspartic acid modified acellular porcine corneal stroma and preconditioned limbal stem cell for corneal regeneration. Biomaterials 2022, 289, 121745. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, T.H.; Kim, H.S. Current Strategies to Enhance Adipose Stem Cell Function: An Update. Int. J. Mol. Sci. 2019, 20, 3827. [Google Scholar] [CrossRef]
- Takeuchi, S.; Tsuchiya, A.; Iwasawa, T.; Nojiri, S.; Watanabe, T.; Ogawa, M.; Yoshida, T.; Fujiki, K.; Koui, Y.; Kido, T.; et al. Small extracellular vesicles derived from interferon-γ pre-conditioned mesenchymal stromal cells effectively treat liver fibrosis. npj Regen. Med. 2021, 6, 19. [Google Scholar] [CrossRef]
- Nakao, Y.; Fukuda, T.; Zhang, Q.Z.; Sanui, T.; Shinjo, T.; Kou, X.X.; Chen, C.; Liu, D.W.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021, 122, 306–324. [Google Scholar] [CrossRef]
- Heo, S.C.; Jeon, E.S.; Lee, I.H.; Kim, H.S.; Kim, M.B.; Kim, J.H. Tumor Necrosis Factor-α-Activated Human Adipose Tissue-Derived Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing through Paracrine Mechanisms. J. Investig. Dermatol. 2011, 131, 1559–1567. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-based 3D cell culture models in cancer research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef]
- Monostori, T.; Szucs, D.; Lovászi, B.; Kemény, L.; Veréb, Z. Advances in tissue engineering and 3D bioprinting for corneal regeneration. Int. J. Bioprint. 2024, 10, 107–130. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, F.; Han, D.; Zhang, S.Y.; Dong, Y.; Li, X.; Ling, L.; Deng, Z.; Cao, X.; Tian, J.; et al. 3D bioprinting of corneal decellularized extracellular matrix: GelMA composite hydrogel for corneal stroma engineering. Int. J. Bioprint. 2023, 9, 774. [Google Scholar] [CrossRef]
- Lekkala, V.K.R.; Kang, S.Y.; Liu, J.; Shrestha, S.; Acharya, P.; Joshi, P.; Zolfaghar, M.; Lee, M.; Vanga, M.G.; Jamdagneya, P.; et al. A Pillar/Perfusion Plate Enhances Cell Growth, Reproducibility, Throughput, and User Friendliness in Dynamic 3D Cell Culture. ACS Biomater. Sci. Eng. 2024, 10, 3478–3488. [Google Scholar] [CrossRef] [PubMed]
- Abdalkader, R.; Kamei, K.I. Multi-corneal barrier-on-a-chip to recapitulate eye blinking shear stress forces. Lab Chip 2020, 20, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Bennet, D.; Estlack, Z.; Reid, T.; Kim, J. A microengineered human corneal epithelium-on-a-chip for eye drops mass transport evaluation. Lab Chip 2018, 18, 1539–1551. [Google Scholar] [CrossRef]
- Lee, R.; Kim, H.; Kim, H.; Lee, J.; Cho, K.J.; Kim, J. High throughput microfluidic drug screening system for corneal epithelial wound healing. J. Micromech. Microeng. 2023, 33, 125007. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Q.; Duan, H.Y.; Wang, X.R.; Xiao, J.H.; Duan, H.C.; Li, N.Y.; Li, C.Y.; Wan, P.X.; Liu, Y.; et al. Reconstruction of Auto-Tissue-Engineered Lamellar Cornea by Dynamic Culture for Transplantation: A Rabbit Model. PLoS ONE 2014, 9, e93012. [Google Scholar]
- Greco, G.; Agostini, M.; Tonazzin, I.; Sallemi, D.; Barone, S.; Cecchini, M. Surface-Acoustic-Wave (SAW)-Driven Device for Dynamic Cell Cultures. Anal. Chem. 2018, 90, 7450–7457. [Google Scholar] [CrossRef]
- Gao, Y.; Fajrial, A.K.; Yang, T.; Ding, X.Y. Emerging on-chip surface acoustic wave technology for small biomaterials manipulation and characterization. Biomater. Sci. 2021, 9, 1574–1582. [Google Scholar] [CrossRef]
- Gosselin, E.A.; Torregrosa, T.; Ghezzi, C.E.; Mendelsohn, A.C.; Gomes, R.; Funderburgh, J.L.; Kaplan, D.L. Multi-layered silk film coculture system for human corneal epithelial and stromal stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 285–295. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Anwar, K.; Ashraf, M.; Lee, H.; Jung, R.B.C.; Koganti, R.; Ghassemi, M.; Djalilian, A.R. Wound-Healing Effects of Mesenchymal Stromal Cell Secretome in the Cornea and the Role of Exosomes. Pharmaceutics 2023, 15, 1486. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Shen, J.; Zheng, Q.Q.; Li, Q.S.; Zhao, H.L.; Cui, L.; Hong, C.Y. Cell viability and extracellular matrix synthesis in a co-culture system of corneal stromal cells and adipose-derived mesenchymal stem cells. Int. J. Ophthalmol. 2017, 10, 670–678. [Google Scholar] [PubMed]
- Soleimanifar, F.; Mortazavi, Y.; Nadri, S.; Islami, M.; Vakilian, S. Coculture of conjunctiva derived mesenchymal stem cells (CJMSCs) and corneal epithelial cells to reconstruct the corneal epithelium. Biologicals 2018, 54, 39–43. [Google Scholar] [CrossRef]
- Moreno, I.Y.; Parsaie, A.; Verma, S.; Gesteira, T.F.; Coulson-Thomas, V.J. Characterization of the Limbal Epithelial Stem Cell Niche. Investig. Ophthalmol. Vis. Sci. 2023, 64, 48. [Google Scholar] [CrossRef]
- Ahmadiankia, N.; Ebrahimi, M.; Hosseini, A.; Baharvand, H. Effects of different extracellular matrices and co-cultures on human limbal stem cell expansion in vitro. Cell Biol. Int. 2009, 33, 978–987. [Google Scholar] [CrossRef]
- Nam, S.M.; Maeng, Y.S.; Kim, E.K.; Seo, K.Y.; Lew, H. Ex Vivo Expansion of Human Limbal Epithelial Cells Using Human Placenta-Derived and Umbilical Cord-Derived Mesenchymal Stem Cells. Stem Cells Int. 2017, 2017, 4206187. [Google Scholar] [CrossRef]
- Dziasko, M.A.; Tuft, S.J.; Daniels, J.T. Limbal melanocytes support limbal epithelial stem cells in 2D and 3D microenvironments. Exp. Eye Res. 2015, 138, 70–79. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chen, H.; Zhou, Q.; Wu, J.; Zhang, X.L.; Wang, L.; Qin, X.Y. Tissue engineering of lamellar cornea using human amniotic epithelial cells and rabbit cornea stroma. Int. J. Ophthalmol. 2013, 6, 425–429. [Google Scholar]
- Ajjarapu, S.M.; Tiwari, A.; Kumar, S. Applications and Utility of Three-Dimensional In Vitro Cell Culture for Therapeutics. Future Pharmacol. 2023, 3, 213–228. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Carter, K.; Lee, H.J.; Na, K.S.; Fernandes-Cunha, G.M.; Blanco, I.J.; Djalilian, A.; Myung, D. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019, 99, 247–257. [Google Scholar] [CrossRef]
- Sumbalova Koledova, Z. 3D Cell Culture: Techniques For and Beyond Organoid Applications. In Methods in Molecular Biology; Humana: New York, NY, USA, 2024; Volume 2764, pp. 1–12. [Google Scholar]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, Y.; Lei, H.; Zeng, Y.; Cui, Z.; Zeng, Q.; Zhu, D.; Lian, R.; Zhang, J.; Chen, Z.; et al. In vitro biomimetic platforms featuring a perfusion system and 3D spheroid culture promote the construction of tissue-engineered corneal endothelial layers. Sci. Rep. 2017, 7, 777. [Google Scholar] [CrossRef] [PubMed]
- Higa, K.; Higuchi, J.; Kimoto, R.; Miyashita, H.; Shimazaki, J.; Tsubota, K.; Shimmura, S. Human corneal limbal organoids maintaining limbal stem cell niche function. Stem Cell Res. 2020, 49, 102012. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; Torricelli, A.A.M.; Marino, G.K. Corneal epithelial basement membrane: Structure, function and regeneration. Exp. Eye Res. 2020, 194, 108002. [Google Scholar] [CrossRef]
- Smita, S.S.; Pramanik, K. Fabrication and characterization of an electrospun silk fibroin/gelatin transparent graft material for corneal epithelial regeneration. Int. J. Polym. Mater. Polym. Biomater. 2025, 74, 1182–1198. [Google Scholar] [CrossRef]
- Last, J.A.; Liliensiek, S.J.; Nealey, P.F.; Murphy, C.J. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. J. Struct. Biol. 2009, 167, 19–24. [Google Scholar] [CrossRef]
- Salimbeigi, G.; Vrana, N.E.; Ghaemmaghami, A.M.; Huri, P.Y.; McGuinness, G.B. Basement membrane properties and their recapitulation in organ-on-chip applications. Mater. Today Bio 2022, 15, 100301. [Google Scholar] [CrossRef]
- Sun, M.G.; Luo, Y.C.; Teng, T.; Guaiquil, V.; Zhou, Q.; McGinn, L.; Nazzal, O.; Walsh, M.; Lee, J.; Rosenblatt, M.I. Silk Film Stiffness Modulates Corneal Epithelial Cell Mechanosignaling. Macromol. Chem. Phys. 2021, 222, 2100013. [Google Scholar] [CrossRef]
- Lei, H.Y.; Yi, T.; Fan, H.Y.; Pei, X.; Wu, L.N.; Xing, F.; Li, M.X.; Liu, L.; Zhou, C.C.; Fan, Y.J.; et al. Customized additive manufacturing of porous Ti6Al4V scaffold with micro-topological structures to regulate cell behavior in bone tissue engineering. Mater. Sci. Eng. C 2021, 120, 111789. [Google Scholar] [CrossRef]
- Hu, X.; Bao, M. Advances in micropatterning technology for mechanotransduction research. Mechanobiol. Med. 2024, 2, 100066. [Google Scholar] [CrossRef] [PubMed]
- Aboal-Castro, L.; Radziunas-Salinas, Y.; Pita-Vilar, M.; Carnero, B.; Mikos, A.G.; Alvarez-Lorenzo, C.; Flores-Arias, M.T.; Diaz-Gomez, L. Laser-Assisted Micropatterned 3D Printed Scaffolds with Customizable Surface Topography and Porosity for Modulation of Cell Function. Adv. Healthc. Mater. 2025, 14, e2403992. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.D.; Pan, Z.; Liu, A.; Kaplan, D.L.; Rosenblatt, M.I. Human corneal limbal epithelial cell response to varying silk film geometric topography in vitro. Acta Biomater. 2012, 8, 3732–3743. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.B.; Lawrence, B.D.; Gao, X.R.; Guaiquil, V.H.; Liu, A.; Rosenblatt, M.I. The Effect of Micro- and Nanoscale Surface Topographies on Silk on Human Corneal Limbal Epithelial Cell Differentiation. Sci. Rep. 2019, 9, 1507. [Google Scholar] [CrossRef]
- Öncel, M.Ö.Ö.; Erkoc-Biradli, F.Z.; Rasier, R.; Marcali, M.; Elbuken, C.; Garipcan, B. Rose petal topography mimicked poly(dimethylsiloxane) substrates for enhanced corneal endothelial cell behavior. Mat. Sci. Eng. C 2021, 126, 112147. [Google Scholar]
- Wang, Q.S.; Liu, Q.S.; Gao, J.M.; He, J.H.; Zhang, H.J.; Ding, J.D. Stereo Coverage and Overall Stiffness of Biomaterial Arrays Underly Parts of Topography Effects on Cell Adhesion. ACS Appl. Mater. Inter. 2023, 15, 6142–6155. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.L.; Backman, L.J.; Malm, A.D.; Danielson, P. Surface Topography and Mechanical Strain Promote Keratocyte Phenotype and Extracellular Matrix Formation in a Biomimetic 3D Corneal Model. Adv. Healthc. Mater. 2017, 6, 1601238. [Google Scholar] [CrossRef]
- Song, E.; Chen, K.M.; Margolis, M.S.; Wungcharoen, T.; Koh, W.G.; Myung, D. Electrospun Nanofiber Membrane for Cultured Corneal Endothelial Cell Transplantation. Bioengineering 2024, 11, 54. [Google Scholar] [CrossRef]
- Dong, Y.H.; Jaleh, B.; Ashrafi, G.; Kashfi, M.; Rhee, K.Y. Mechanical properties of the hybrids of natural (alginate, collagen, chitin, cellulose, gelatin, chitosan, silk, and keratin) and synthetic electrospun nanofibers: A review. Int. J. Biol. Macromol. 2025, 312, 143742. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, J.Y.; Park, C.H. Fabrication of transparent hemispherical 3D nanofibrous scaffolds with radially aligned patterns via a novel electrospinning method. Sci. Rep. 2018, 8, 3424. [Google Scholar] [PubMed]
- Arabpour, Z.; Baradaran-Rafii, A.; Bakhshaiesh, N.L.; Ai, J.; Ebrahimi-Barough, S.; Esmaeili Malekabadi, H.; Nazeri, N.; Vaez, A.; Salehi, M.; Sefat, F.; et al. Design and characterization of biodegradable multi layered electrospun nanofibers for corneal tissue engineering applications. J. Biomed. Mater. Res. Part A 2019, 107, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Sun, W.; Chen, G.S.; Tang, S.; Li, M.; Shao, Z.W.; Mi, S.L. Tissue-engineered cornea constructed with compressed collagen and laser-perforated electrospun mat. Sci. Rep. 2017, 7, 970. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Sefat, E.; Roberts, D.; Gilger, B.C.; Gluck, J.M. Application of Noggin-Coated Electrospun Scaffold in Corneal Wound Healing. Transl. Vis. Sci. Technol. 2023, 12, 15. [Google Scholar] [CrossRef]
- Jafar, H.; Ahmed, K.; Rayyan, R.; Sotari, S.; Buqain, R.; Ali, D.; Al Bdour, M.; Awidi, A. Plasma-Treated Electrospun PLGA Nanofiber Scaffold Supports Limbal Stem Cells. Polymers 2023, 15, 4244. [Google Scholar] [CrossRef]
- Abdullah, K.K.; Molnár, K. Current Trends and Future Prospects of Integrating Electrospinning With 3D Printing Techniques for Mimicking Bone Extracellular Matrix Scaffolds. J. Polym. Sci. 2025, 63, 1481–1504. [Google Scholar] [CrossRef]
- Zhang, Y.N.; O’Mahony, A.; He, Y.; Barber, T. Hydrodynamic shear stress’ impact on mammalian cell properties and its applications in 3D bioprinting. Biofabrication 2024, 16, 022003. [Google Scholar] [CrossRef]
- Malekpour, A.; Chen, X.B. Printability and Cell Viability in Extrusion-Based Bioprinting from Experimental, Computational, and Machine Learning Views. J. Funct. Biomater. 2022, 13, 40. [Google Scholar] [CrossRef]
- Wang, P.J.; Sun, Y.Z.; Diao, L.W.; Liu, H.T. Considering cell viability in 3D printing of structured inks: A comparative and equivalent analysis of fluid forces. Int. J. Bioprint. 2024, 10, 238–262. [Google Scholar] [CrossRef]
- Wang, Q.S.; Liao, Y.H.; Ho, Y.H.; Wang, K.; Jin, W.Z.; Guan, Y.M.; Fu, W.X. A study on cell viability based on thermal inkjet three-dimensional bioprinting. Phys. Fluids 2023, 35, 082007. [Google Scholar] [CrossRef]
- Ouyang, L.L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Boularaoui, S.; Shanti, A.; Khan, K.A.; Iacoponi, S.; Christoforou, N.; Stefanini, C. Harnessing shear stress preconditioning to improve cell viability in 3D post-printed biostructures using extrusion bioprinting. Bioprinting 2022, 25, e00184. [Google Scholar] [CrossRef]
- Basoz, D.; Akalinli, A.; Buyuksungur, S.; Celebi, A.R.C.; Yucel, D.; Hasirci, N.; Hasirci, V. Preclinical Testing of 3D Printed, Cell Loaded Hydrogel Based Corneal Substitutes on Rabbit Model. Macromol. Biosci. 2025, 25, e2400595. [Google Scholar] [CrossRef] [PubMed]
- He, B.B.; Wang, J.; Xie, M.T.; Xu, M.Y.; Zhang, Y.H.; Hao, H.J.; Xing, X.L.; Lu, W.; Han, Q.H.; Liu, W.G. 3D printed biomimetic epithelium/stroma bilayer hydrogel implant for corneal regeneration. Bioact. Mater. 2022, 17, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.N.; Liu, W.F.; Zhao, Q.; Feng, X.Y.; Li, Z.B.; Huang, Y.R.; Liu, J.; Peng, Y.H.; Song, W.J.; Ren, L. 3D bioprinted GelMA/collagen hydrogel for corneal stroma regeneration. Biofabrication 2025, 17, 045002. [Google Scholar] [CrossRef]
- Ghosh, A.; Bera, A.K.; Ghosh, S.; Singh, V.; Basu, S.; Pati, F. Bioprinting a resilient and transparent cornea stroma equivalent: Harnessing dual crosslinking strategy with decellularized cornea matrix and silk fibroin hybrid. Biofabrication 2024, 17, 015028. [Google Scholar] [CrossRef]
- Pietryga, K.; Jesse, K.; Drzyzga, R.; Konka, A.; Zembala-John, J.; Kowalik, A.; Kielbowicz, Z.; Cwirko, M.; Buldak, R.J.; Dobrowolski, D.; et al. Bio-printing method as a novel approach to obtain a fibrin scaffold settled by limbal epithelial cells for corneal regeneration. Sci. Rep. 2024, 14, 23352. [Google Scholar] [CrossRef]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef]
- Bektas, C.K.; Hasirci, V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater. Sci. 2020, 8, 438–449. [Google Scholar] [CrossRef]
- Moro, A.; Samanta, S.; Honkamaki, L.; Rangasami, V.K.; Puistola, P.; Kauppila, M.; Narkilahti, S.; Miettinen, S.; Oommen, O.; Skottman, H. Hyaluronic acid based next generation bioink for 3D bioprinting of human stem cell derived corneal stromal model with innervation. Biofabrication 2022, 15, 015020. [Google Scholar] [CrossRef]
- Gronroos, P.; Moro, A.; Puistola, P.; Hopia, K.; Huuskonen, M.; Viheriala, T.; Ilmarinen, T.; Skottman, H. Bioprinting of human pluripotent stem cell derived corneal endothelial cells with hydrazone crosslinked hyaluronic acid bioink. Stem Cell Res. Ther. 2024, 15, 81. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Sun, L.; Zhang, X.; Guo, M.; Liu, J.; Wang, W.; Zhao, N. 4D printing of biological macromolecules employing handheld bioprinters for in situ wound healing applications. Int. J. Biol. Macromol. 2024, 280 Pt 3, 135999. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Malviya, R.; Sridhar, S.B.; Wadhwa, T.; Talath, S.; Shareef, J. Trends in 4D Printed Shape Memory Biomaterials for Tissue Engineering Applications. Curr. Pharm. Des. 2025, 31, 3285–3302. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulou, M.; Díaz-Payno, P.J.; Mirzaali, M.J.; van Osch, G.J.V.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A. 4D printed shape-shifting biomaterials for tissue engineering and regenerative medicine applications. Biofabrication 2024, 16, 022002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Jiang, C.Q.; Fan, Y.Q.; Hao, X.D.; Dong, Y.H.; He, X.J.; Gao, J.N.; Zhang, Y.C.; Li, M.; Wang, M.Y.; et al. The application of a 4D-printed chitosan-based stem cell carrier for the repair of corneal alkali burns. Stem Cell Res. Ther. 2024, 15, 41. [Google Scholar] [CrossRef]
- Song, W.L.; Huang, W.H.; Qu, J.Z.; Xiao, C.J.; Yin, H.N.; Liu, X.Z.; Xu, W.K. The application prospects of 4D printing tissue engineering materials in oral bone regeneration. Int. J. Bioprint. 2025, 11, 139–191. [Google Scholar] [CrossRef]
- Stepanovska, J.; Otahal, M.; Hanzalek, K.; Supova, M.; Matejka, R. pH Modification of High-Concentrated Collagen Bioinks as a Factor Affecting Cell Viability, Mechanical Properties, and Printability. Gels 2021, 7, 252. [Google Scholar] [CrossRef]
- Lameirinhas, N.S.; Teixeira, M.C.; Carvalho, J.P.F.; Valente, B.F.A.; Luís, J.L.; Duarte, I.F.; Pinto, R.J.B.; Oliveira, H.; Oliveira, J.M.; Silvestre, A.J.D.; et al. Biofabrication of HepG2 Cells-Laden 3D Structures Using Nanocellulose-Reinforced Gelatin-Based Hydrogel Bioinks: Materials Characterization, Cell Viability Assessment, and Metabolomic Analysis. Acs Biomater. Sci. Eng. 2025, 11, 3043–3057. [Google Scholar] [CrossRef]
- Limon, S.M.; Sarah, R.; Habib, A. Integrating Decision Trees and Clustering for Efficient Optimization of Bioink Rheology and 3D Bioprinted Construct Microenvironments. J. Manuf. Sci. Eng. 2025, 147, 091003. [Google Scholar] [CrossRef]
- Zhang, C.L.; Elvitigala, K.C.M.L.; Mubarok, W.; Okano, Y.; Sakai, S. Machine learning-based prediction and optimisation framework for as-extruded cell viability in extrusion-based 3D bioprinting. Virtual Phys. Prototyp. 2024, 19, e2400330. [Google Scholar] [CrossRef]
- Shao, L.; Gao, Q.; Xie, C.Q.; Fu, J.Z.; Xiang, M.X.; He, Y. Synchronous 3D Bioprinting of Large-Scale Cell-Laden Constructs with Nutrient Networks. Adv. Healthc. Mater. 2020, 9, e1901142. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xie, C.N.; Zhang, Z.M.; Liu, H.N.; Xu, H.X.; Peng, Z.Y.; Liu, C.; Li, J.J.; Wang, C.Q.; Xu, T.; et al. 3D Printed Integrated Bionic Oxygenated Scaffold for Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 29506–29520. [Google Scholar] [CrossRef]
- Wang, X.C.; Yang, C.Y.; Yu, Y.R.; Zhao, Y.J. In Situ 3D Bioprinting Living Photosynthetic Scaffolds for Autotrophic Wound Healing. Research 2022, 2022, 9794745. [Google Scholar] [CrossRef]
- Guo, B.J.; Duan, Y.C.; Li, Z.W.; Tian, Y.; Cheng, X.D.; Liang, C.X.; Liu, W.J.; An, B.; Wei, W.M.; Gao, T.T.; et al. High-Strength Cell Sheets and Vigorous Hydrogels from Mesenchymal Stem Cells Derived from Human Embryonic Stem Cells. ACS Appl. Mater. Interfaces 2023, 15, 27586–27599. [Google Scholar] [CrossRef]

| Therapy | Cell Resource | Description | Advantages | Disadvantages | Translation |
|---|---|---|---|---|---|
| CLAU | Autologous conjunctiva and corneal limbal tissue | Autologous transplantation of a large limbal tissue biopsy containing stem cells from the healthy contralateral eye to the affected eye | Simple to operate, high success rate | Suitable for unilateral LSCD, may induce iatrogenic LSCD in the donor eye, corneal conjunctivalization | Clinically conventional application |
| CLET | Autologous corneal limbal tissue | Harvesting a small amount of limbal tissue and expanding ex vivo on scaffolds to form epithelial cell sheets | Suitable for bilateral LSCD, less traumatic | Complicated technique, high cost | Holoclar |
| SLET | Autologous/allogeneic corneal limbal tissue | Harvesting a small amount of limbal tissue from a healthy contralateral eye, which is divided into micrografts and directly adhered to an amniotic membrane carrier | Less traumatic, no need for ex vivo culture, simple to operate, high success rate | Suitable for unilateral LSCD, need for long-term immunosuppression | Clinically conventional application clinically |
| CALEC | Autologous corneal limbal tissue | A standardized CLET technique utilizing a xeno-free culture system | Xeno-free, production reproducibility | Complicated technique, the highest cost | Phase II completes |
| COMET | Autologous oral mucosal epithelium | Harvesting a small amount of oral mucosal epithelium tissue and expanding ex vivo on scaffolds to form epithelial cell sheets | Suitable for bilateral LSCD, convenient material selection, adequate sources | Morphological and functional mismatch with corneal epithelium, poor visual outcome | Ocural®, Sakracy® |
| MSC therapy | Allogeneic MSC | Allogeneic MSC-induced CEC with bioscaffolds | Suitable for bilateral LSCD, adequate sources | Complicated technique, immunosuppression | Preclinical study |
| iPSC therapy | Autologous somatic cell | Reprogrammed autologous somatic cell to CEC with bioscaffolds | Adequate source, free of immunosuppression | Extremely complicated technique, high cost, tumorigenicity | Preclinical study |
| Cell sheet | Autologous/Allogeneic CEC | Formed primarily through self-assembled cell-secreted ECM | Closest to a natural structure, free of external materials | Complicated technique, poor mechanical strength | Nepic® |
| Bioink | Nozzle Diameters | Printing Temperature | Fixation | Cell Viability | Reference |
|---|---|---|---|---|---|
| GelMA, keratocyte | Not mentioned | 20 °C | UV | ≈80% | [155] |
| PEGDA + GelMA, CEC + ASC | Not mentioned | 37 °C | Blue light | 90% | [156] |
| EPTAC-Col + GelMA, keratocyte | 0.34 mm | 20 °C | UV | 95% | [157] |
| DCM + SF, LMSC | 0.33 mm | 15 °C | Green light | 92% | [158] |
| Fibrin, LESC | 0.2 mm | Not mentioned | CaCl2 | 91.1% | [159] |
| Sodium alginate +ColMA, keratocytes | 0.2 mm | 37 °C | CaCl2 | 83% | [160] |
| GelMA, keratocytes | 0.26 mm | Not mentioned | UV | 98% | [161] |
| HA-CDH + HA-Ald, ASC + keratocyte | 0.1 mm | 20 °C | Hydrazone | 95% | [162] |
| HA-CDH + HA-Ald, iPSC-CEnC | 0.1 mm | Room temperature | Hydrazone | 92.5% | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, G.; Chi, M.; Zhai, Y.; Peng, R.; Hong, J. Corneal Epithelial Tissue Engineering Strategy Based on Cell Viability Optimization: A Review and Prospects. Bioengineering 2025, 12, 1175. https://doi.org/10.3390/bioengineering12111175
Tang G, Chi M, Zhai Y, Peng R, Hong J. Corneal Epithelial Tissue Engineering Strategy Based on Cell Viability Optimization: A Review and Prospects. Bioengineering. 2025; 12(11):1175. https://doi.org/10.3390/bioengineering12111175
Chicago/Turabian StyleTang, Guoguo, Miaomiao Chi, Yang Zhai, Rongmei Peng, and Jing Hong. 2025. "Corneal Epithelial Tissue Engineering Strategy Based on Cell Viability Optimization: A Review and Prospects" Bioengineering 12, no. 11: 1175. https://doi.org/10.3390/bioengineering12111175
APA StyleTang, G., Chi, M., Zhai, Y., Peng, R., & Hong, J. (2025). Corneal Epithelial Tissue Engineering Strategy Based on Cell Viability Optimization: A Review and Prospects. Bioengineering, 12(11), 1175. https://doi.org/10.3390/bioengineering12111175






