Evaluating the Predictive Potential of Patient-Specific Biomechanical Models in Class III Protraction Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Registration and Ethical Approval
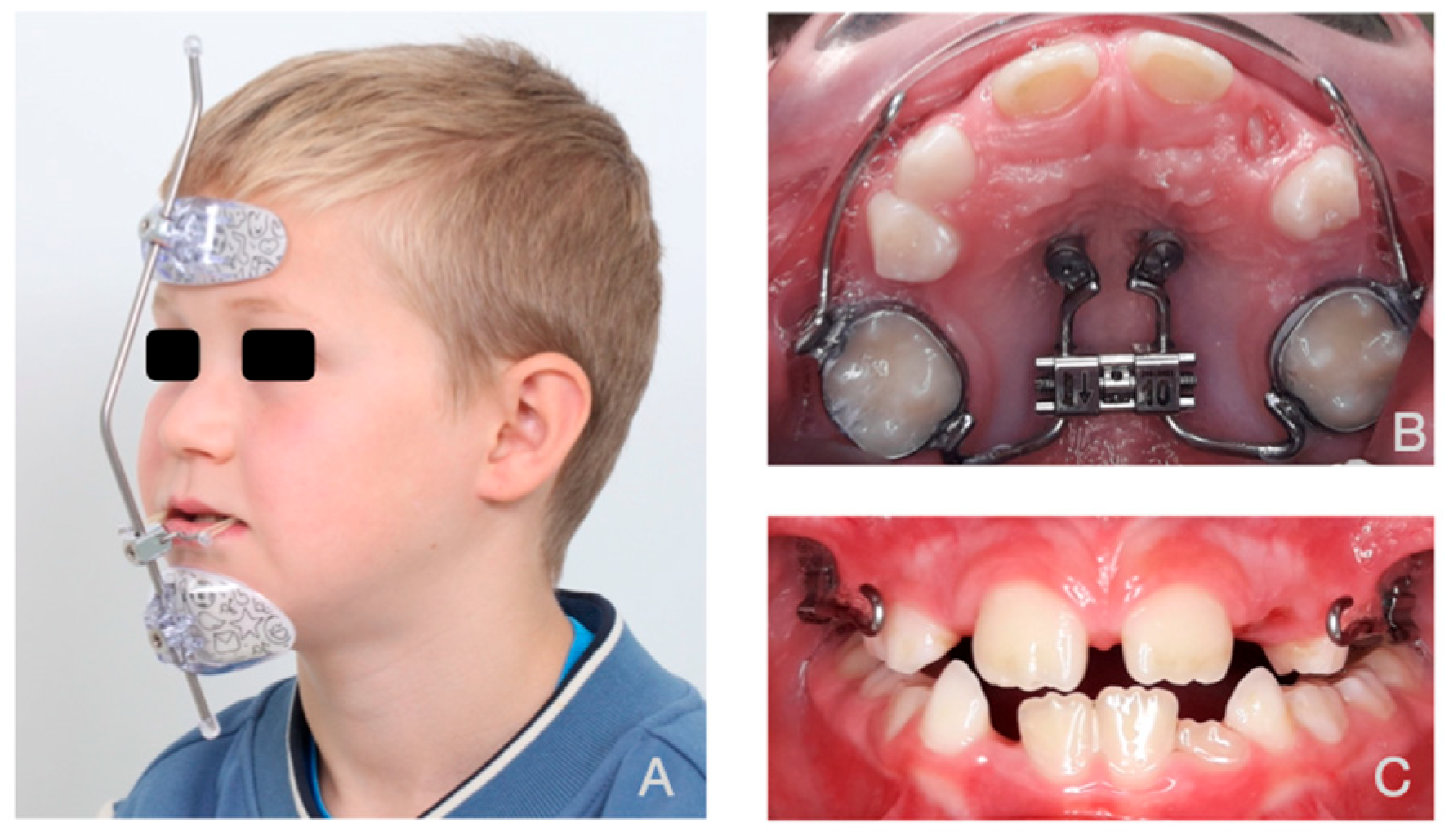
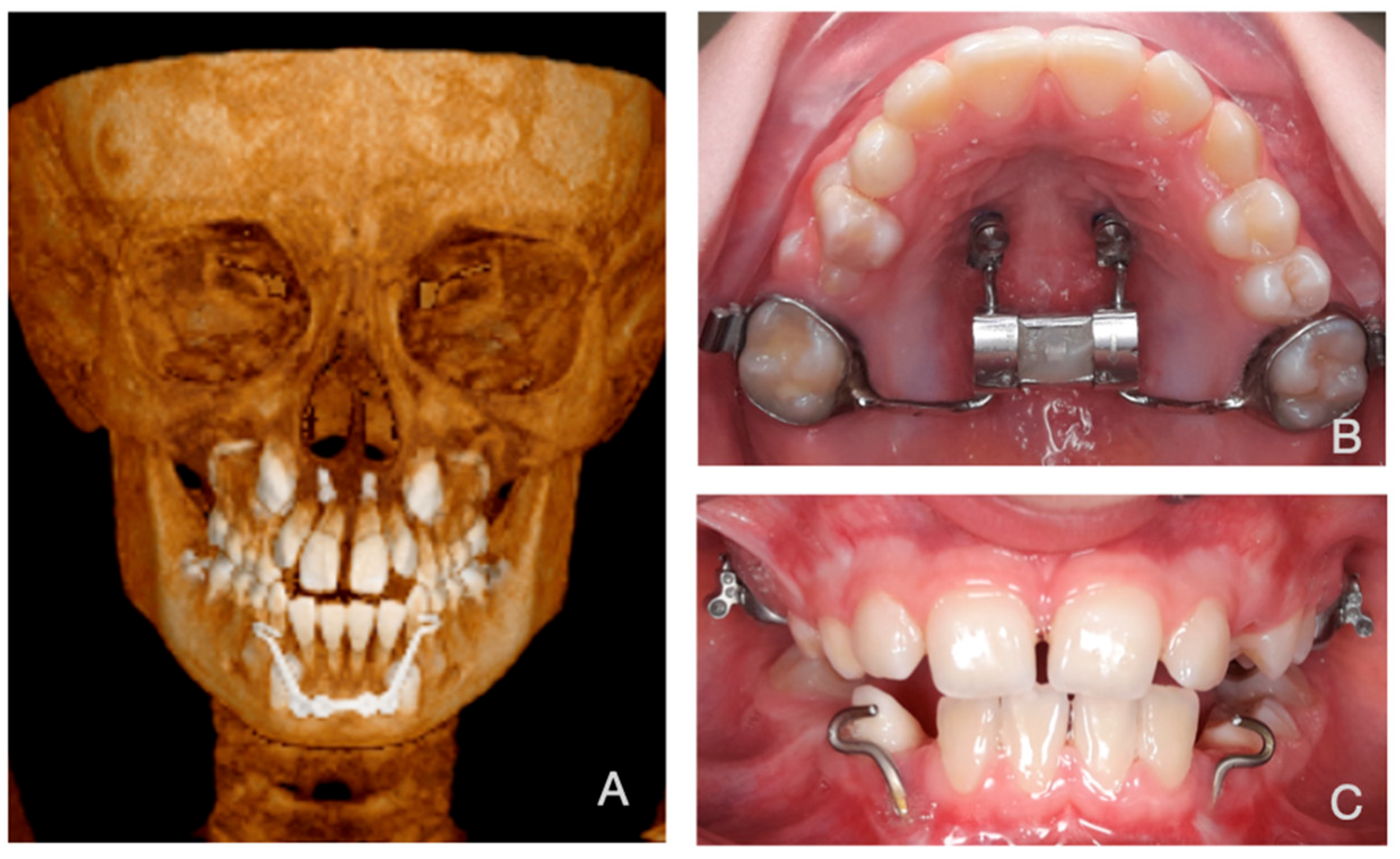
2.2. Subjects
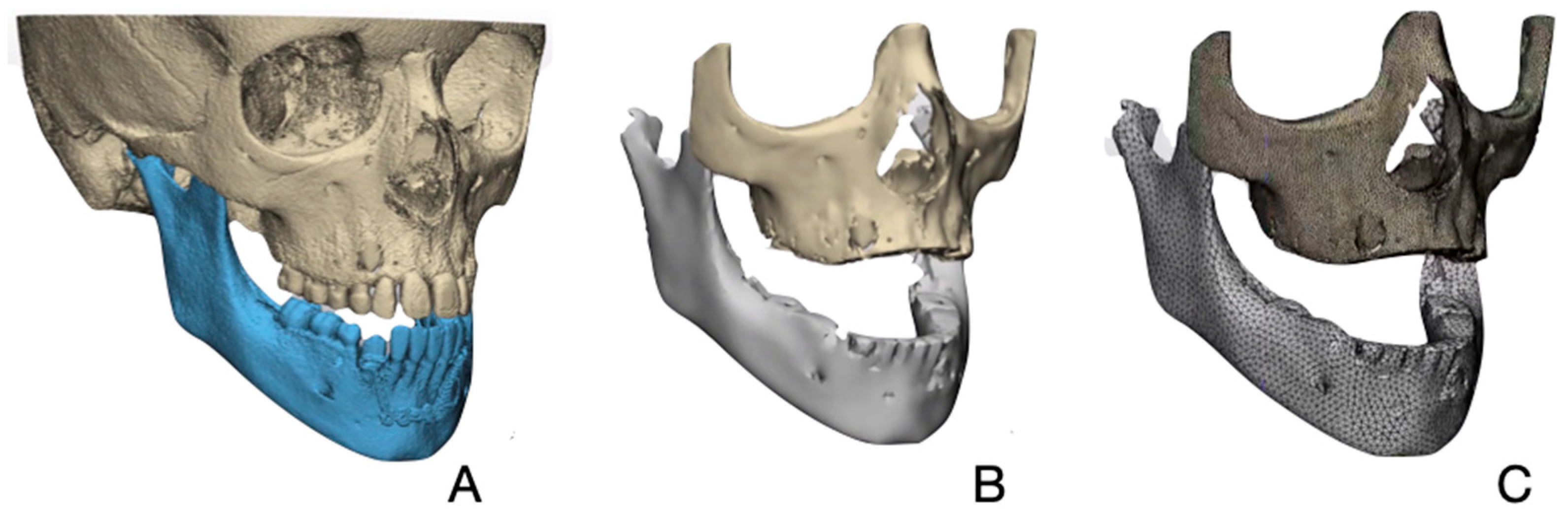
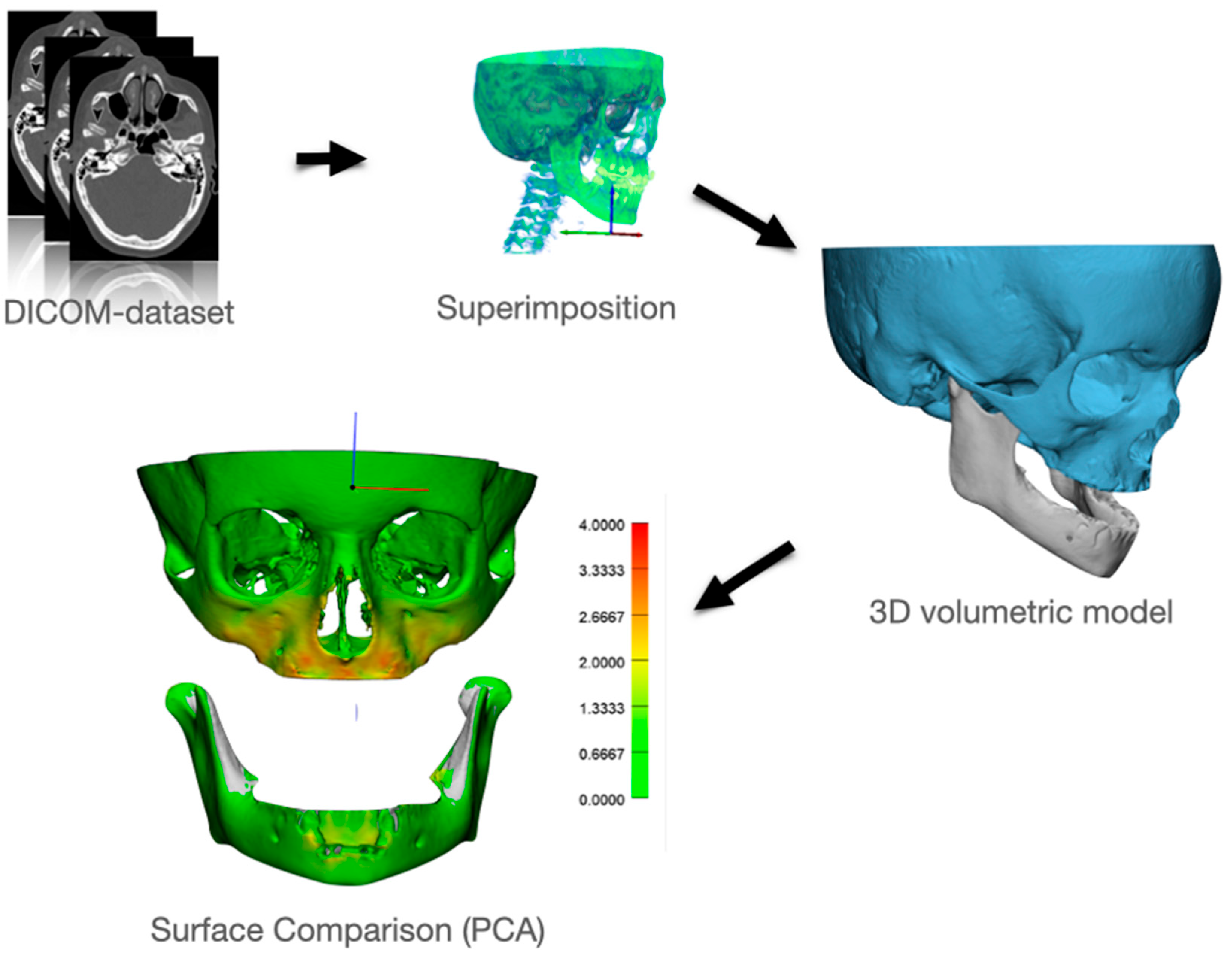
2.3. Creating a Patient Specific 3D FE Model
2.4. Finite Element Analysis (FEA)
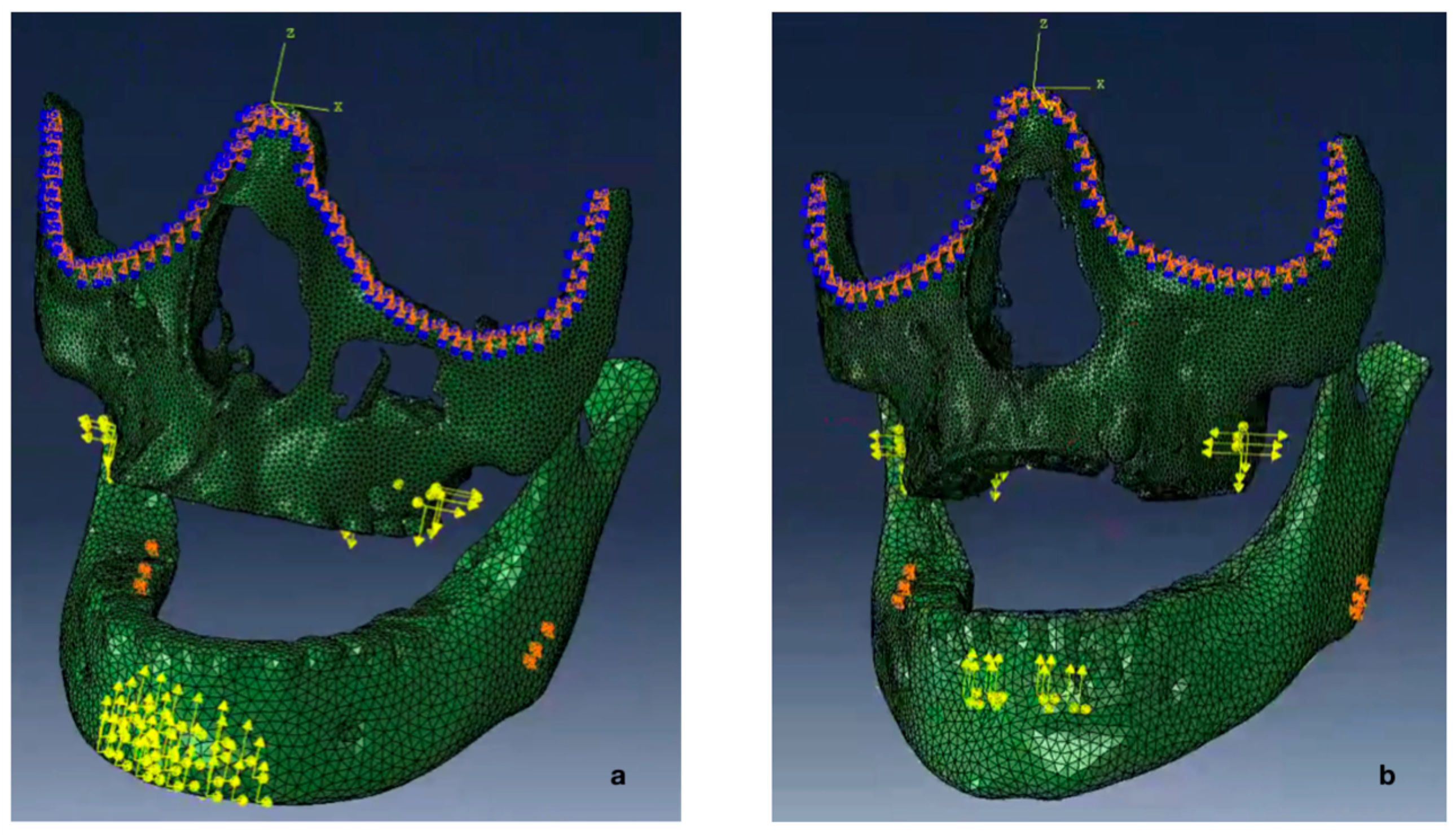
2.5. Boundary Conditions
2.6. Magnitude and Vector of the Forces
2.7. Force Simulation
2.8. Actual Treatment Effect
2.9. Comparing Modeled Deformation with Actual Deformation
3. Results
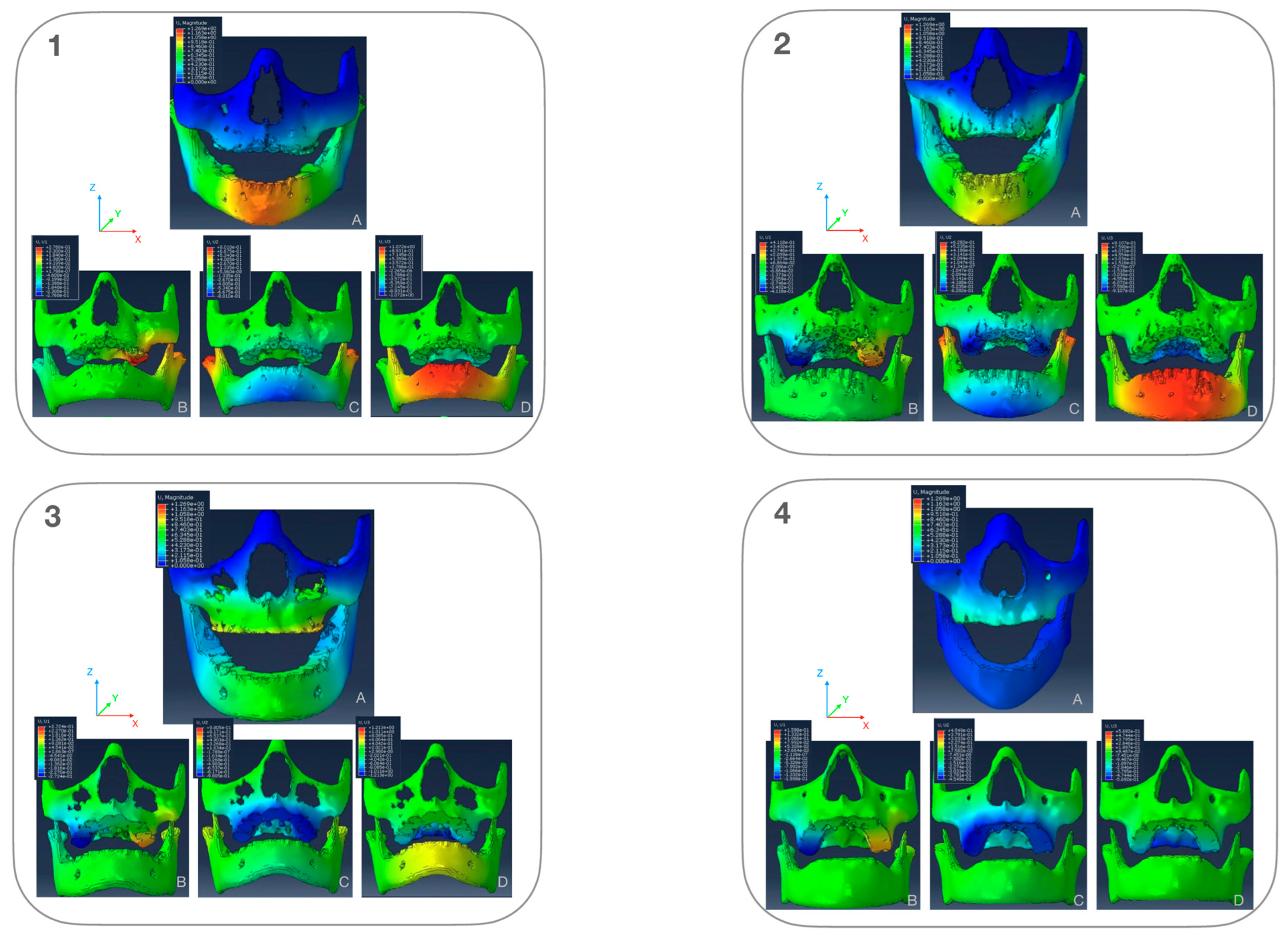
3.1. Stress-Distribution
3.1.1. Maxillary Stress Distribution (Figure 7)
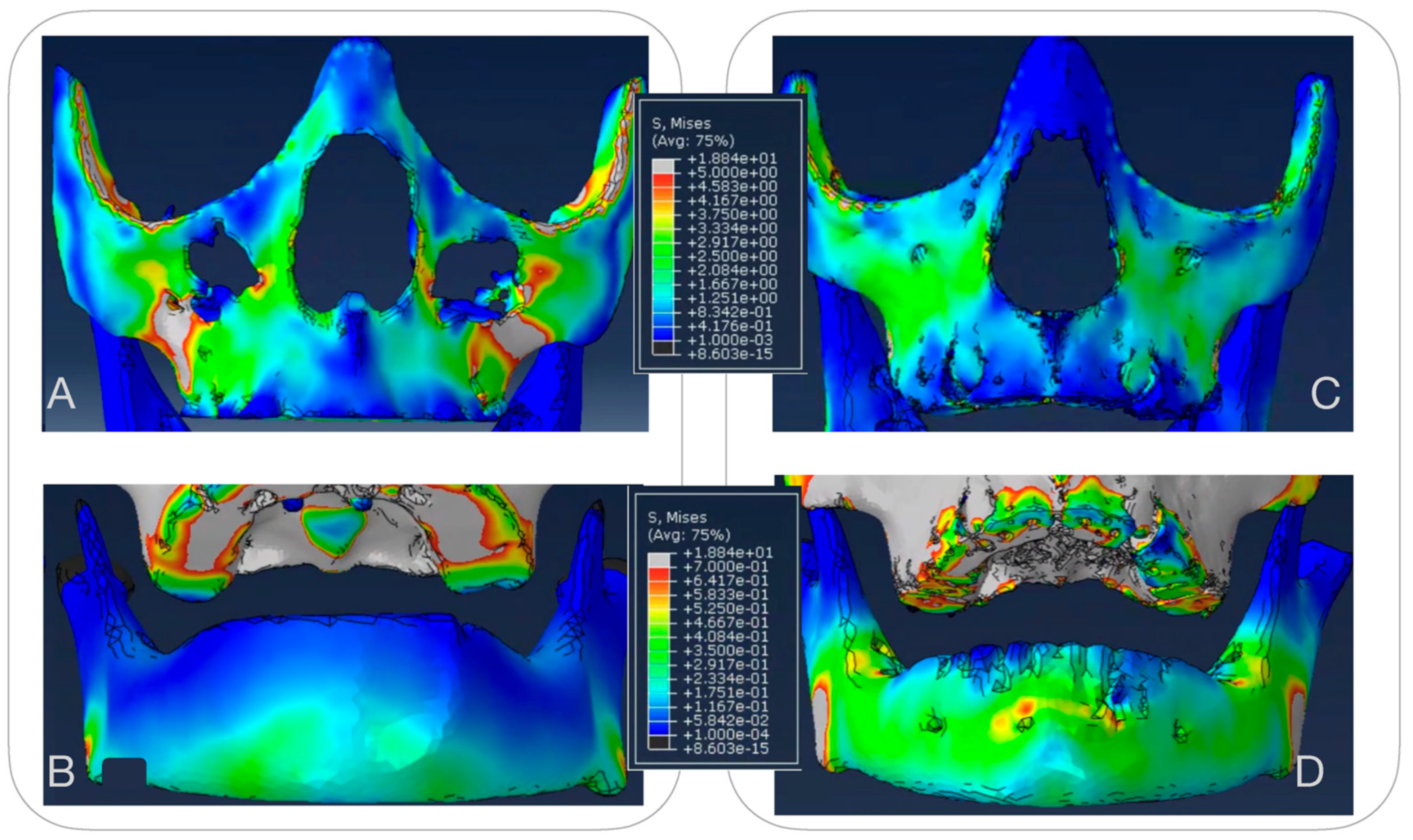
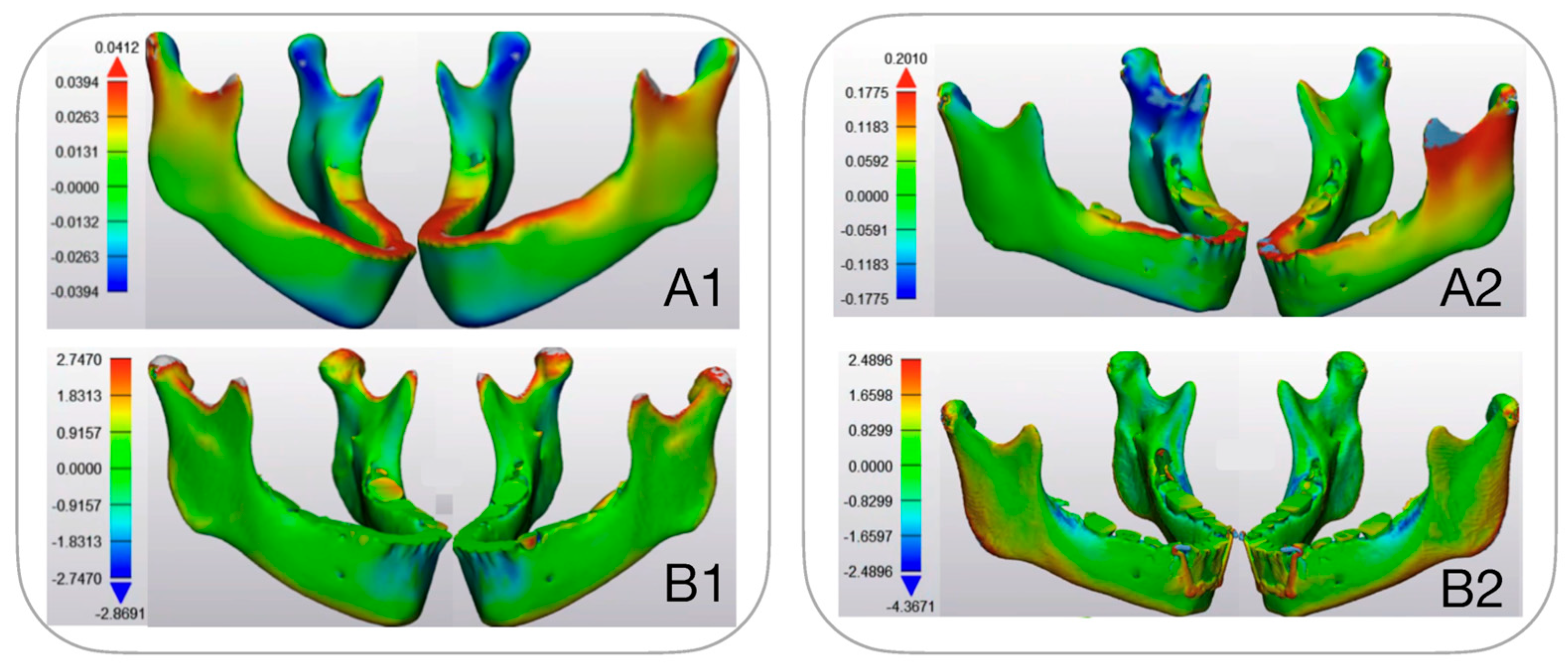
3.1.2. Mandibular Stress Distribution (Figure 7)
3.2. Modelled Deformation (PCAmD)
3.3. Comparing Modeled Deformation (PCAmD) with Actual Deformation (PCAaD)
4. Discussion
5. Conclusions
6. Clinical Relevance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| Alt-RAMEC | Alternate Rapid Maxillary Expansion and Constriction |
| BAMP | Bone Anchored Maxillary Protraction |
| CBCT | Cone-Beam Computed Tomography |
| CCW | Counterclockwise |
| CT | Computed Tomography |
| DICOM | Digital Imaging and Communications in Medicine |
| FE | Finite Element |
| FEA | Finite Element Analysis |
| FEM | Finite Element Model |
| FM | Facemask |
| HH | Hybrid Hyrax |
| MP | Mentoplate |
| PCA | Part Comparison Analysis |
| PCAmD | Part Comparison Analysis modeled Deformation |
| PCAaD | Part Comparison Analysis actual Deformation |
| RCT | Randomized Controlled Trial |
| RPE | Rapid Palatal Expansion |
| SA | Skeletal Anchorage |
| TB | Tooth-Borne |
| TMJ | Temporomandibular Joint |
| 3D | Three-Dimensional |
| 2D | Two-Dimensional |
References
- Woon, S.C.; Thiruvenkatachari, B. Early orthodontic treatment for Class III malocclusion: A systematic review and meta-analysis. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 28–52. [Google Scholar] [CrossRef]
- De Toffol, L.; Pavoni, C.; Baccetti, T.; Franchi, L.; Cozza, P. Orthopedic Treatment Outcomes in Class III Malocclusion. Angle Orthod. 2008, 78, 561–573. [Google Scholar] [CrossRef]
- Rongo, R.; D’Antò, V.; Bucci, R.; Polito, I.; Martina, R.; Michelotti, A. Skeletal and dental effects of Class III orthopaedic treatment: A systematic review and meta-analysis. J. Oral Rehabil. 2017, 44, 545–562. [Google Scholar] [CrossRef]
- Mandall, N.; Cousley, R.; Dibiase, A.; Dyer, F.; Littlewood, S.; Mattick, R.; Nute, S.J.; Doherty, B.; Stivaros, N.; McDowall, R.; et al. Early class III protraction facemask treatment reduces the need for orthognathic surgery: A multi-centre, two-arm parallel randomized, controlled trial. J. Orthod. 2016, 43, 164–175. [Google Scholar] [CrossRef]
- Ngan, P.W.; Hagg, U.; Yiu, C.; Wei, S.H. Treatment response and long-term dentofacial adaptations to maxillary expansion and protraction. Semin. Orthod. 1997, 3, 255–264. [Google Scholar] [CrossRef]
- Wilmes, B.; Nienkemper, M.; Ludwig, B.; Kau, C.H.; Drescher, D. Early Class III treatment with a hybrid hyrax-mentoplate combination. J. Clin. Orthod. 2011, 45, 15–21. [Google Scholar]
- Hu, S.; An, K.; Peng, Y. Comparative efficacy of the bone-anchored maxillary protraction protocols for orthopaedic treatment in skeletal Class III malocclusion: A Bayesian network meta-analysis. Orthod. Craniofac. Res. 2022, 25, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, G.; Onem Ozbilen, E.; Özdemir, F. Retrospective 3-dimensional Evaluation of Skeletal and Dental Structures Following Treatment with Hybrid Hyrax-Mentonplate with Class III Elastics in Class III Patients with Vertical Growth Pattern: A Pilot Study. Eur. J. Res. Dent. 2023, 7, 79–88. [Google Scholar] [CrossRef]
- Willmann, J.H.; Nienkemper, M.; Tarraf, N.E.; Wilmes, B.; Drescher, D. Early Class III treatment with Hybrid-Hyrax-Facemask in comparison to Hybrid-Hyrax-Mentoplate—Skeletal and dental outcomes. Prog. Orthod. 2018, 19, 42. [Google Scholar] [CrossRef]
- Ergul, T.; Gulec, A. Comparison of the effectiveness of skeletal and tooth-borne protraction methods with or without alternate rapid maxillary expansion and constriction protocol in patients with Class III malocclusion. Am. J. Orthod. Dentofac. Orthop. 2024, 167, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Mandall, N.; Aleid, W.; Cousley, R.; Curran, E.; Caldwell, S.; DiBiase, A.; Dyer, F.M.; Littlewood, S.J.; Nute, S.; Campbell, S.J.; et al. The effectiveness of bone anchored maxillary protraction (BAMP) in the management of class III skeletal malocclusion in children aged 11–14 years compared with an untreated control group: A multicentre two-arm parallel randomised controlled trial. J. Orthod. 2024, 51, 228–239. [Google Scholar] [CrossRef]
- Cornelis, M.A.; Tepedino, M.; Riis, N.D.V.; Niu, X.; Cattaneo, P.M. Treatment effect of bone-Anchored maxillary protraction in growing patients compared to controls: A systematic review with meta-Analysis. Eur. J. Orthod. 2021, 43, 51–68. [Google Scholar] [CrossRef]
- Meyns, J.; Brasil, D.M.; Mazzi-Chaves, J.F.; Politis, C.; Jacobs, R. The clinical outcome of skeletal anchorage in interceptive treatment (in growing patients) for class III malocclusion. Int. J. Oral. Maxillofac. Surg. 2018, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Kinzinger, G.S.M.; Hourfar, J.; Sommer, J.N.; Lisson, J.A. Age-dependent effects of Delaire facemask therapy for class III malocclusion: Impact on maxillary sutures and palatal morphology. J. Orofac. Orthop. 2024, 86, 197–215. [Google Scholar] [CrossRef]
- Fudalej, P.; Dragan, M.; Wedrychowska-Szulc, B. Prediction of the outcome of orthodontic treatment of Class III malocclusions-a systematic review. Eur. J. Orthod. 2011, 33, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Le, V.N.T.; Kim, J.G.; Yang, Y.M.; Lee, D.W. Prediction Model for Future Success of Early Orthopedic Treatment of Class III Malocclusion. Children 2023, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Chang, J.E.; Chung, C.J.; Tahk, J.H.; Kim, K.H. Prediction of long-term success of orthopedic treatment in skeletal Class III malocclusions. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 193–203. [Google Scholar] [CrossRef]
- Ngan, P.; Wei, S.H.-Y. Early Treatment of Class III Patients To Improve Facial Aesthetics and Predict Future Growth. Hong Kong Dent. J. 2004, 1, 24–30. [Google Scholar]
- Miki, M. An experimental research on the directional control of the nasomaxillary complex by means of external force--two dimensional analysis on the sagittal plane of the craniofacial skeleton (author’s transl). Shikwa Gakuho 1979, 79, 1563–1597. [Google Scholar]
- Hirato, R. An experimental study on the center of resistance of the nasomaxillary complex. 2-dimensional analysis of the coronal plane in the dry skull. Shikwa Gakuho 1984, 84, 1225–1262. [Google Scholar]
- Lee, K.G.; Ryu, Y.K.; Park, Y.C.; Rudolph, D.J. A study of holographic interferometry on the initial reaction of maxillofacial complex during protraction. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 623–632. [Google Scholar] [CrossRef]
- Nanda, R. Protraction of maxilla in rhesus monkeys by controlled extraoral forces. Am. J. Orthod. 1978, 74, 121–141. [Google Scholar] [CrossRef]
- Billiet, T.; De Pauw, G.; Dermaut, L. Location of the centre of resistance of the upper dentition and the nasomaxillary complex. An experimental study. Eur. J. Orthod. 2001, 23, 263–273. [Google Scholar] [CrossRef]
- Itoh, T.; Chaconas, S.J.; Caputo, A.A.; Matyas, J. Photoelastic effects of maxillary protraction on the craniofacial complex. Am. J. Orthod. 1985, 88, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.A.; Chaconas, S.J.; Hayashi, R.K. Photoelastic visualization of orthodontic forces during canine retraction. Am. J. Orthod. 1974, 65, 250–259. [Google Scholar] [CrossRef]
- Suresh, S.; Sundareswaran, S.; Sathyanadhan, S. Effect of microimplant assisted rapid palatal expansion on bone-anchored maxillary protraction: A finite element analysis. Am. J. Orthod. Dentofac. Orthop. 2021, 160, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Bayome, M.; Park, J.H.; Kim, K.B.; Mo, S.S.; Kook, Y.A. Displacement and stress distribution of the maxillofacial complex during maxillary protraction with buccal versus palatal plates: Finite element analysis. Eur. J. Orthod. 2015, 37, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; He, W.; Lin, T.; Liu, J.; Bai, X.; Yan, G.; Lu, L. Three-dimensional finite element analysis of the craniomaxillary complex during maxillary protraction with bone anchorage vs conventional dental anchorage. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 197–205. [Google Scholar] [CrossRef]
- Shyagali, T.R.; Patidar, R.; Gupta, A.; Kapoor, S.; Tiwari, A. Evaluation of stresses and displacement in the craniofacial region as a reaction to bone-anchored maxillary protraction in conjugation with posterior bite plane and rapid maxillary expansion in patients with Class III malocclusion: A finite element analys. Am. J. Orthod. Dentofac. Orthop. 2023, 164, 253–264. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Sengottuvel, N.; Altaf, S.K.; Bhandari, P.K.; Kumaragurubaran, P.; Antony, M. Three-Dimensional Finite Element Analysis of Maxillary Protraction Using Diverse Modes of Rapid Palatal Expansion. Cureus 2023, 15, e36328. [Google Scholar] [CrossRef]
- Meyns, J.; Meewis, J.; Dons, F.; Schreurs, A.; Aerts, J.; Shujaat, S.; Politis, C.; Jacobs, R. Long-term comparison of maxillary protraction with hybrid hyrax-facemask vs. hybrid hyrax-mentoplate protocols using Alt-RAMEC: A 5-year randomized controlled trial. Eur. J. Orthod. 2025, 47, cjaf011. [Google Scholar] [CrossRef] [PubMed]
- Meyns, J.; Thanatchaporn, J.; Shujaat, S.; Politis, C.; Jacobs, R. Long-term three-dimensional skeletal effects of hybrid hyrax with facemask versus mentoplate in growing Class III patients: A randomized controlled trial. Prog. Orthod. 2025, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Liou, E.J.W.; Tsai, W.C. A new protocol for maxillary protraction in cleft patients: Repetitive weekly protocol of alternate rapid maxillary expansions and constrictions. Cleft Palate-Craniofacial J. 2005, 42, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Strait, D.S.; Dumont, E.R.; Ross, C.F.; Grosse, I.R. The effects of modeling simplifications on craniofacial finite element models: The alveoli (tooth sockets) and periodontal ligaments. J. Biomech. 2011, 44, 1831–1838. [Google Scholar] [CrossRef]
- Lee, B.; Flores-Mir, C.; Lagravère, M.O. Normal orbit skeletal changes in adolescents as determined through cone-beam computed tomography. Head Face Med. 2016, 12, 10–15. [Google Scholar] [CrossRef]
- Nguyen, T.; Cevidanes, L.; Franchi, L.; Ruellas, A.; Jackson, T. Three-dimensional mandibular regional superimposition in growing patients. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 747–754. [Google Scholar] [CrossRef]
- LeCornu, M.; Cevidanes, L.H.S.; Hongtu, Z.; Wu, C.-D.; Larson, B.; Nguyen, T.T. Three-dimensional treatment outcomes in class II patientes treated with Herbst appliance: A pilot study. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 818–830. [Google Scholar] [CrossRef]
- Ngan, P.; Wilmes, B.; Drescher, D.; Martin, C.; Weaver, B.; Gunel, E. Comparison of two maxillary protraction protocols: Tooth-borne versus bone-anchored protraction facemask treatment. Prog. Orthod. 2015, 16, 26. [Google Scholar] [CrossRef]
- Kim, J.H.; Viana, M.A.; Graber, T.M.; Omerza, F.F.; BeGole, E.A. The effectiveness of protraction face mask therapy: A meta-analysis. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 675–685. [Google Scholar] [CrossRef]
- Gazzani, F.; Pavoni, C.; Giancotti, A.; Cozza, P.; Lione, R. Facemask performance during maxillary protraction: A finite element analysis (FEA) evaluation of load and stress distribution on Delaire facemask. Prog. Orthod. 2018, 19, 21. [Google Scholar] [CrossRef]
- Anirudh Agarwal, R.M. Maxillary Expansion. Int. J. Clin. Pediatr. Dent. 2010, 3, 139–146. [Google Scholar] [CrossRef]
- Goeckner, K.; Pepakayala, V.; Nervina, J.; Gianchandani, Y.; Kapila, S. Three-dimensional force measurements during rapid palatal expansion in Sus scrofa. Micromachines 2016, 7, 64. [Google Scholar] [CrossRef]
- Isaacson, R.J.; Wood, J.L.; Ingram, A.H. Forces produced by rapid maxillary expansion: II. Forces present during treatment. Angle Orthod. 1964, 34, 261–278. [Google Scholar]
- Pluim, J.P.W.; Maintz, J.B.A.A.; Viergever, M.A. Mutual-information-based registration of medical images: A survey. IEEE Trans. Med. Imaging 2003, 22, 986–1004. [Google Scholar] [CrossRef] [PubMed]
- Maes, F.; Collignon, A.; Vandermeulen, D.; Marchal, G.; Suetens, P. Multi-modality image registration by maximization of mutual information. In Proceedings of the Workship on Mathematical Methods in Biomedical Image Analysis, San Francisco, CA, USA, 21–22 June 1996; Volume 16, pp. 14–22. [Google Scholar] [CrossRef]
- Owens, D.; Watkinson, S.; Harrison, J.E.; Turner, S.; Worthington, H.V. Orthodontic treatment for prominent lower front teeth (Class III malocclusion) in children (Review). Cochrane Database Syst. Rev. 2024, 2024, CD003451. [Google Scholar] [CrossRef]
- Lin, Y.; Guo, R.; Hou, L.; Fu, Z.; Li, W. Stability of maxillary protraction therapy in children with Class III malocclusion: A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2639–2652. [Google Scholar] [CrossRef]
- Knop, L.; Gandini, L.G.; Shintcovsk, R.L.; Gandini, M.R.E.A.S. Scientific use of the finite element method in orthodontics. Dent. Press J. Orthod. 2015, 20, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Likitmongkolsakul, U.; Smithmaitrie, P.; Samruajbenjakun, B.; Aksornmuang, J. Development and Validation of 3D Finite Element Models for Prediction of Orthodontic Tooth Movement. Int. J. Dent. 2018, 2018, 4927503. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, L.; Zhang, Y.; Ma, X.; Guo, Y.; Liu, Y. Establishment, FEM analysis and experimental validation of tooth movement prediction model of orthodontic archwire T-loop. BMC Oral Health 2022, 22, 406. [Google Scholar] [CrossRef]
- Rai, P.; Garg, D.; Tripathi, T.; Kanase, A.; Ganesh, G. Biomechanical effects of Skeletally anchored Class III elastics on the maxillofacial complex: A 3D finite element analysis. Prog. Orthod. 2021, 22, 36. [Google Scholar] [CrossRef]
- Peterson, J.; Wang, Q.; Dechow, P.C. Material properties of the dentate maxilla. Anat. Rec.—Part A Discov. Mol. Cell. Evol. Biol. 2006, 288, 962–972. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, H.; Nguyen, T.; de Paula, L.K.; Cevidanes, L. Three-dimensional assessment of mandibular and glenoid fossa changes after bone-anchored Class III intermaxillary traction. Am. J. Orthod. Dentofac. Orthop. 2012, 142, 25–31. [Google Scholar] [CrossRef] [PubMed]
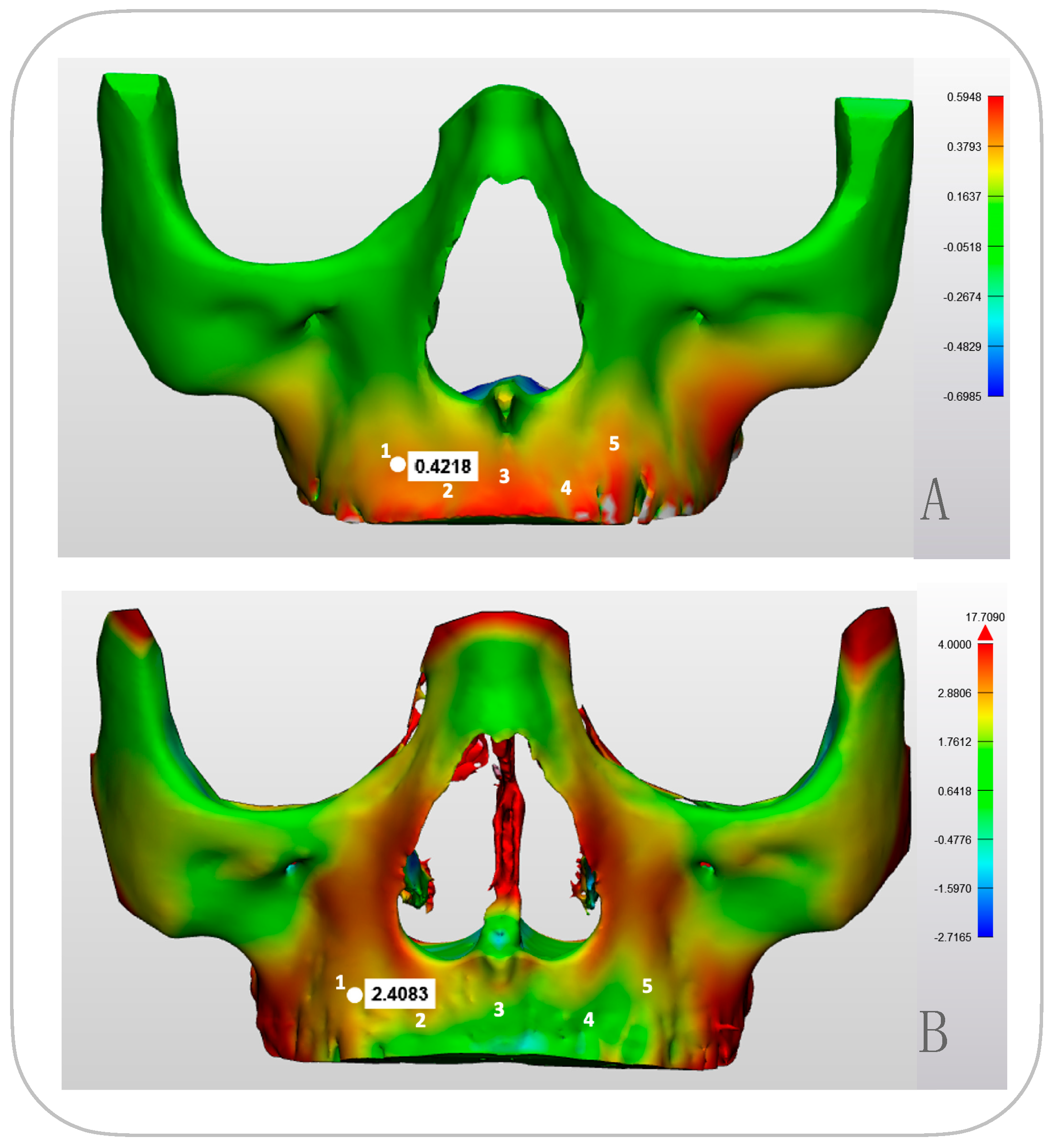

| Point 1 | Point 2 | Point 3 | Point 4 | Point 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCAmD | PCAaD | PCAmD | PCAaD | PCAmD | PCAaD | PCAmD | PCAaD | PCAmD | PCAaD | |
| MP 1 | 0.22 | 1.75 | 0.33 | 1.50 | 0.35 | 1.57 | 0.51 | 1.03 | 0.49 | 1.90 |
| MP 2 | 0.22 | 0.43 | 0.30 | 1.42 | 0.38 | 1.16 | 0.46 | 0.71 | 0.34 | 0.59 |
| MP 3 | 0.11 | 3.12 | 0.15 | 2.42 | 0.15 | 2.73 | 0.16 | 2.08 | 0.18 | 2.84 |
| FM 1 | 0.42 | 2.41 | 0.43 | 2.33 | 0.48 | 0.75 | 0.43 | 0.69 | 0.41 | 1.78 |
| FM2 | 0.39 | 0.90 | 0.49 | 1.66 | 0.46 | 1.79 | 0.48 | 1.55 | 0.38 | 1.28 |
| FM 3 | 0.30 | 2.21 | 0.35 | 1.85 | 0.43 | 1.10 | 0.34 | 1.48 | 0.43 | 1.37 |
| mean | 0.28 | 1.80 | 0.34 | 1.86 | 0.37 | 1.52 | 0.40 | 1.25 | 0.37 | 1.63 |
| SD | 0.12 | 1.00 | 0.12 | 0.42 | 0.12 | 0.70 | 0.13 | 0.54 | 0.11 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyns, J.; Vertenten, W.; Shujaat, S.; Van Cauter, S.; Politis, C.; Vander Sloten, J.; Jacobs, R. Evaluating the Predictive Potential of Patient-Specific Biomechanical Models in Class III Protraction Therapy. Bioengineering 2025, 12, 1173. https://doi.org/10.3390/bioengineering12111173
Meyns J, Vertenten W, Shujaat S, Van Cauter S, Politis C, Vander Sloten J, Jacobs R. Evaluating the Predictive Potential of Patient-Specific Biomechanical Models in Class III Protraction Therapy. Bioengineering. 2025; 12(11):1173. https://doi.org/10.3390/bioengineering12111173
Chicago/Turabian StyleMeyns, Joeri, Wout Vertenten, Sohaib Shujaat, Sofie Van Cauter, Constantinus Politis, Jos Vander Sloten, and Reinhilde Jacobs. 2025. "Evaluating the Predictive Potential of Patient-Specific Biomechanical Models in Class III Protraction Therapy" Bioengineering 12, no. 11: 1173. https://doi.org/10.3390/bioengineering12111173
APA StyleMeyns, J., Vertenten, W., Shujaat, S., Van Cauter, S., Politis, C., Vander Sloten, J., & Jacobs, R. (2025). Evaluating the Predictive Potential of Patient-Specific Biomechanical Models in Class III Protraction Therapy. Bioengineering, 12(11), 1173. https://doi.org/10.3390/bioengineering12111173







