Hematopoietic Stem Cell Aging: Mechanisms, Microenvironment Influences, and Rejuvenation Strategies
Abstract
1. Introduction
2. Functional Deterioration and Systemic Consequences of Aged HSCs
2.1. Self-Renewal Depletion
2.2. Myeloid Differentiation Bias
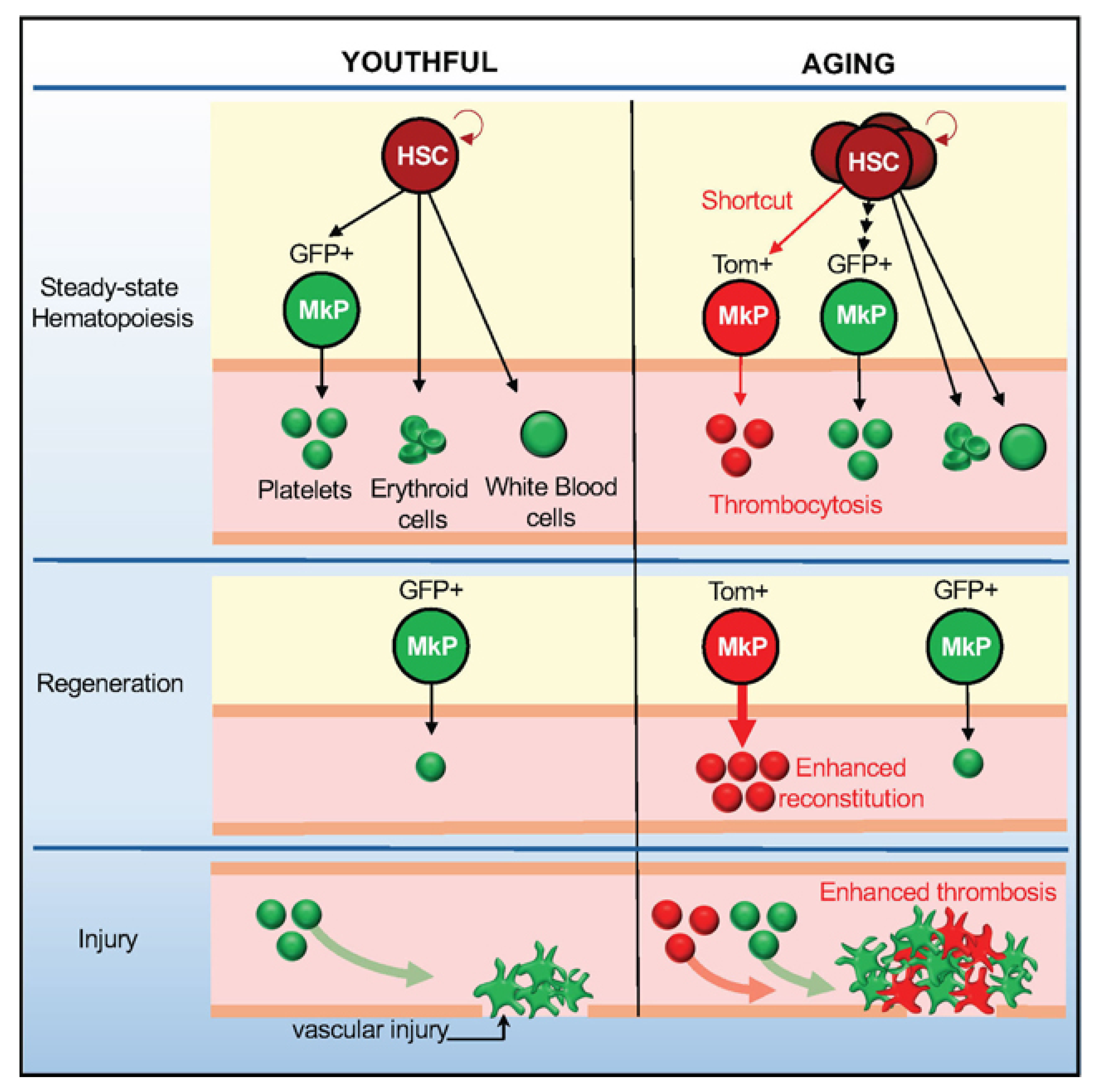
2.3. Dysfunctional Differentiation and Platelet Hyperactivity
3. Cell-Autonomous Mechanisms Driving HSC Aging
3.1. Genomic Instability
3.2. Epigenetic and Metabolic Disorders
3.3. Inflammations
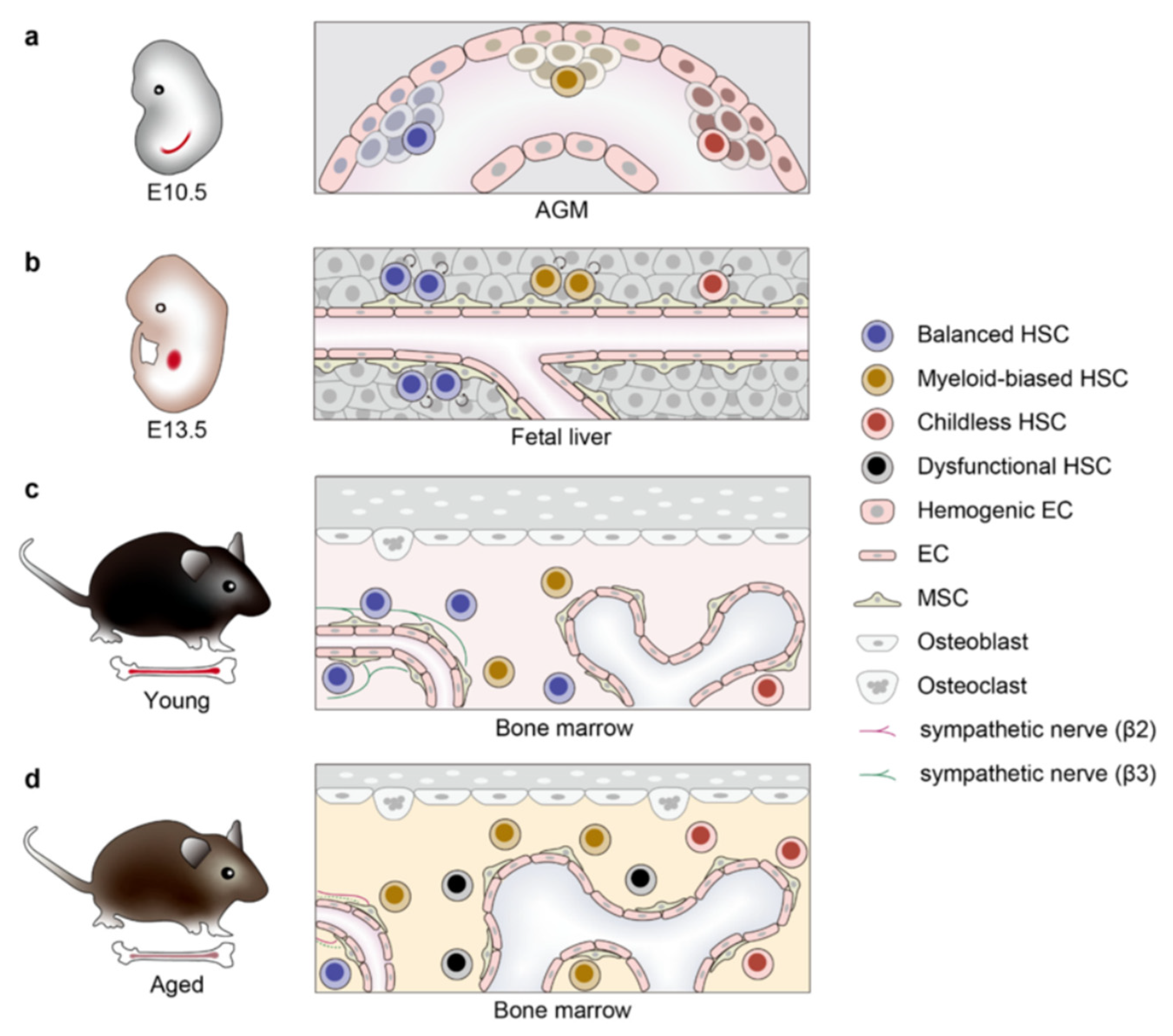
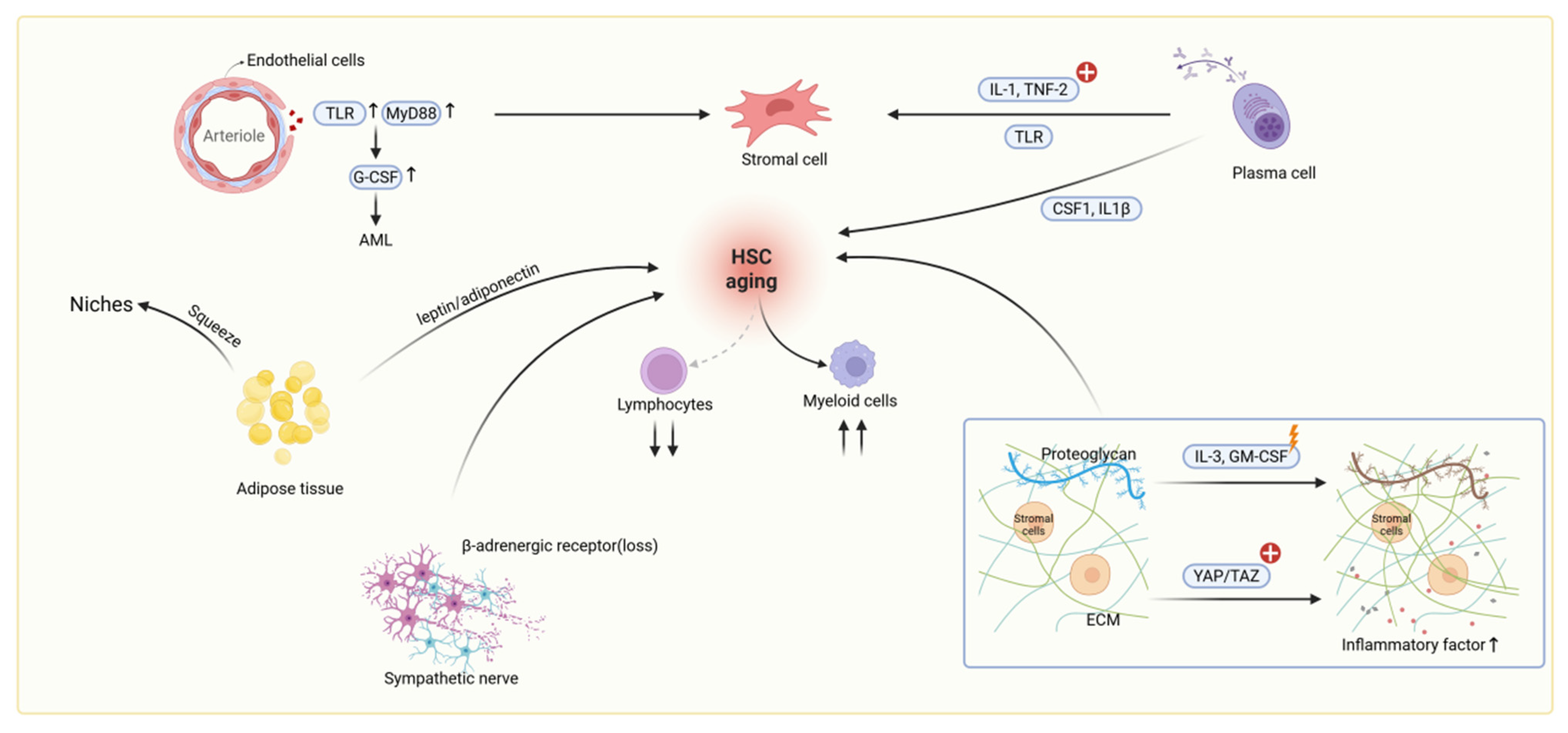
4. Microenvironment Niches of HSC Aging
4.1. Niche Cell-Mediated Inflammatory Activation in Aging
4.2. Extracellular Matrix Dysregulation in Aging
4.3. Metabolic Reprogramming and Additional Niche Alterations
5. Induced Pluripotent Stem Cell (iPSC) Differentiation Platform for HSC Aging Investigation
5.1. Modeling HSC Aging Using iPSC-Based Differentiation Platforms
5.2. Decoding HSC Aging Through Single-Cell and Multi-Omics Technologies
5.3. Pluripotent Stem Cell-Based Platforms for Anti-Aging Drug Discovery and Functional Restoration of HSCs
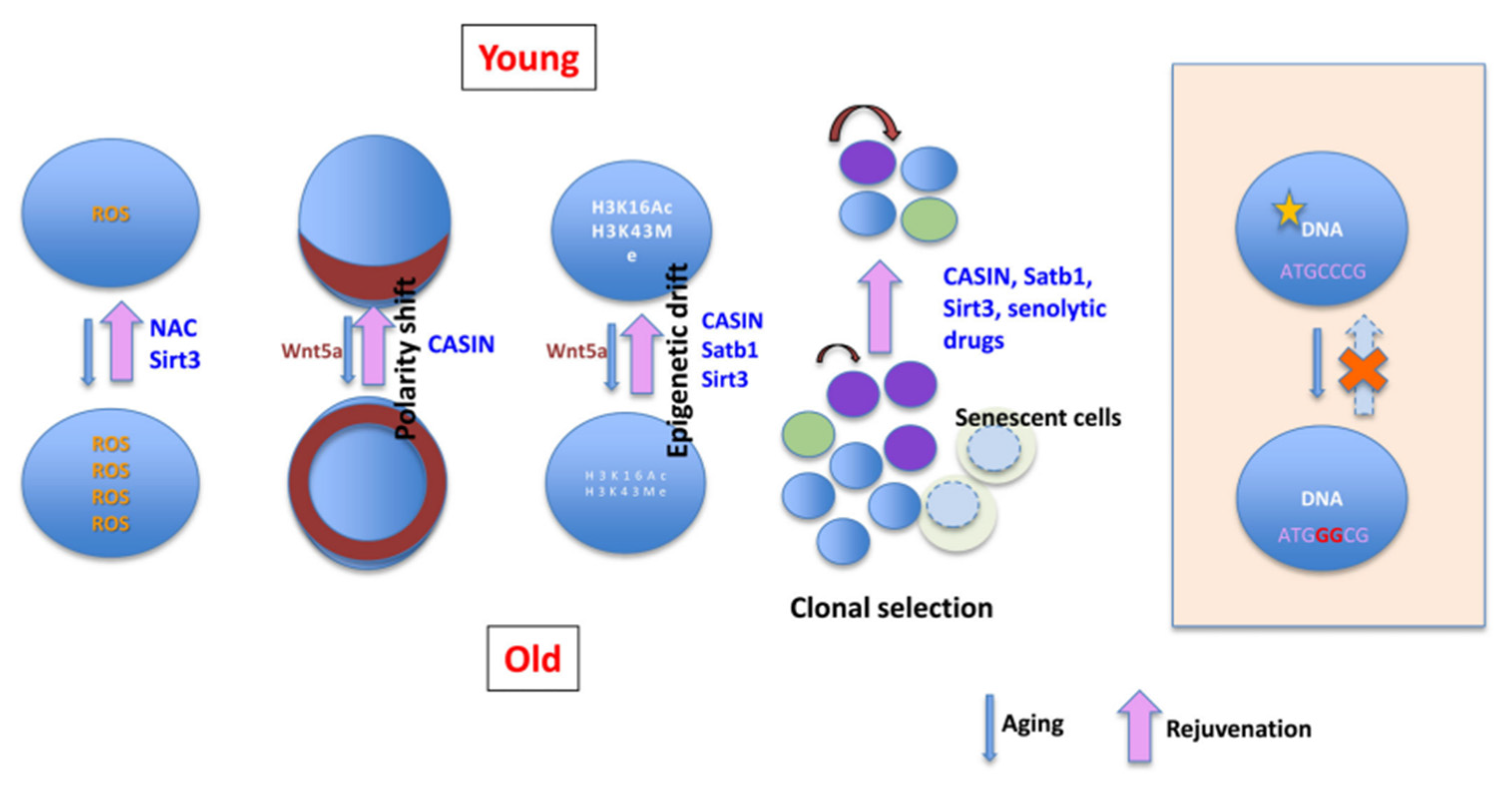
6. Rejuvenation Strategies of HSC Aging
6.1. Targeting Intrinsic Aging Mechanisms to Restore HSC Function
6.2. Engineering and Modulating the Aged Niche to Rejuvenate HSC Function
6.3. Therapeutic Strategies Targeting Inflammation and Metabolic Pathways to Rejuvenate Aged HSCs
6.4. HSC Transplantation and Combined Interventions: Emerging Strategies for Reversing Hematopoietic Aging
7. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, J.; Papatsenko, D.; Niu, X.; Schaniel, C.; Moore, K. Divisional history and hematopoietic stem cell function during homeostasis. Stem Cell Rep. 2014, 2, 473–490. [Google Scholar] [CrossRef]
- Lichtman, M.A.; Rowe, J.M. The relationship of patient age to the pathobiology of the clonal myeloid diseases. Semin. Oncol. 2004, 31, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Iwama, A. Aging and Clonal Behavior of Hematopoietic Stem Cells. Int. J. Mol. Sci. 2022, 23, 1948. [Google Scholar] [CrossRef]
- Akunuru, S.; Geiger, H. Aging, Clonality, and Rejuvenation of Hematopoietic Stem Cells. Trends Mol. Med. 2016, 22, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hirao, A.; Arai, F.; Takubo, K.; Matsuoka, S.; Miyamoto, K.; Ohmura, M.; Naka, K.; Hosokawa, K.; Ikeda, Y.; et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006, 12, 446–451. [Google Scholar] [CrossRef]
- Rimmele, P.; Bigarella, C.L.; Liang, R.; Izac, B.; Dieguez-Gonzalez, R.; Barbet, G.; Donovan, M.; Brugnara, C.; Blander, J.M.; Sinclair, D.A.; et al. Aging-like phenotype and defective lineage specification in SIRT1-deleted hematopoietic stem and progenitor cells. Stem Cell Rep. 2014, 3, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.H.; Emmrich, S.; Vannini, N. Metabolic Alterations in HSCs during Aging and Leukemogenesis. Physiology 2025, 40, 329–342. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Ehsani, A.; Fathi, E.; Farahzadi, R. Cellular and Molecular Mechanisms Involved in Hematopoietic Stem Cell Aging as a Clinical Prospect. Oxid. Med. Cell Longev. 2022, 2022, 2713483. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, D.; Xu, L.; Xu, Y.; Gao, Z.; Pan, Y.; Jiang, M.; Wei, Y.; Wang, L.; Liao, Y.; et al. Harnessing matrix stiffness to engineer a bone marrow niche for hematopoietic stem cell rejuvenation. Cell Stem Cell 2023, 30, 378–395.e378. [Google Scholar] [CrossRef]
- Beerman, I.; Seita, J.; Inlay, M.A.; Weissman, I.L.; Rossi, D.J. Quiescent Hematopoietic Stem Cells Accumulate DNA Damage during Aging that Is Repaired upon Entry into Cell Cycle. Cell Stem Cell 2014, 15, 37–50. [Google Scholar] [CrossRef]
- Flach, J.; Bakker, S.T.; Mohrin, M.; Conroy, P.C.; Pietras, E.M.; Reynaud, D.; Alvarez, S.; Diolaiti, M.E.; Ugarte, F.; Forsberg, E.C.; et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature 2014, 512, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Su, T.Y.; Hauenstein, J.; Somuncular, E.; Dumral, Ö.; Leonard, E.; Gustafsson, C.; Tzortzis, E.; Forlani, A.; Johansson, A.S.; Qian, H.; et al. Aging is associated with functional and molecular changes in distinct hematopoietic stem cell subsets. Nat. Commun. 2024, 15, 7966. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Zhang, C.; Van, H.Q.T.; Seino, T.; Zhang, Y. Reducing functionally defective old HSCs alleviates aging-related phenotypes in old recipient mice. Cell Res. 2025, 35, 45–58. [Google Scholar] [CrossRef]
- Poscablo, D.M.; Worthington, A.K.; Smith-Berdan, S.; Forsberg, E.C. Megakaryocyte progenitor cell function is enhanced upon aging despite the functional decline of aged hematopoietic stem cells. Stem Cell Rep. 2021, 16, 1598–1613. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Li, X.; Chavakis, T. Immunometabolic control of hematopoiesis. Mol. Asp. Med. 2021, 77, 100923. [Google Scholar] [CrossRef]
- Totani, H.; Matsumura, T.; Yokomori, R.; Umemoto, T.; Takihara, Y.; Yang, C.; Chua, L.H.; Watanabe, A.; Sanda, T.; Suda, T. Mitochondria-enriched hematopoietic stem cells exhibit elevated self-renewal capabilities, thriving within the context of aged bone marrow. Nat. Aging 2025, 5, 831–847. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Zhang, X.; Li, X.; Liu, C.; Yan, D.; Deng, H.; Sun, W.; Yi, C.; Wang, J. Age-related noncanonical TRMT6-TRMT61A signaling impairs hematopoietic stem cells. Nat. Aging 2024, 4, 213–230. [Google Scholar] [CrossRef]
- Song, Z.; Park, S.H.; Mu, W.C.; Feng, Y.; Wang, C.L.; Wang, Y.; Barthez, M.; Maruichi, A.; Guo, J.; Yang, F.; et al. An NAD(+)-dependent metabolic checkpoint regulates hematopoietic stem cell activation and aging. Nat. Aging 2024, 4, 1384–1393. [Google Scholar] [CrossRef]
- Bogeska, R.; Mikecin, A.M.; Kaschutnig, P.; Fawaz, M.; Büchler-Schäff, M.; Le, D.; Ganuza, M.; Vollmer, A.; Paffenholz, S.V.; Asada, N.; et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell 2022, 29, 1273–1284.e8. [Google Scholar] [CrossRef]
- Cui, X.; Dong, Y.; Zhan, Q.; Huang, Y.; Zhu, Q.; Zhang, Z.; Yang, G.; Wang, L.; Shen, S.; Zhao, J.; et al. Altered 3D genome reorganization mediates precocious myeloid differentiation of aged hematopoietic stem cells in inflammation. Sci. China. Life Sci. 2025, 68, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Telpoukhovskaia, M.A.; Hofmann, J.; Mistry, J.J.; Kokkaliaris, K.D.; Trowbridge, J.J. Variation in mesenchymal KITL/SCF and IGF1 expression in middle age underlies steady-state hematopoietic stem cell aging. Blood 2024, 144, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, P.; Poulos, M.G.; Gutkin, M.C.; Katsnelson, L.; Freire, A.G.; Lazzari, E.; Butler, J.M. Endothelial mTOR maintains hematopoiesis during aging. J. Exp. Med. 2020, 217, e20191212. [Google Scholar] [CrossRef] [PubMed]
- Vandoorne, K.; Rohde, D.; Kim, H.-Y.; Courties, G.; Wojtkiewicz, G.; Honold, L.; Hoyer, F.F.; Frodermann, V.; Nayar, R.; Herisson, F.; et al. Imaging the Vascular Bone Marrow Niche During Inflammatory Stress. Circ. Res. 2018, 123, 415–427. [Google Scholar] [CrossRef]
- Poulos, M.G.; Ramalingam, P.; Gutkin, M.C.; Llanos, P.; Gilleran, K.; Rabbany, S.Y.; Butler, J.M. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J. Clin. Investig. 2017, 127, 4163–4178. [Google Scholar] [CrossRef]
- Helbling, P.M.; Piñeiro-Yáñez, E.; Gerosa, R.; Boettcher, S.; Al-Shahrour, F.; Manz, M.G.; Nombela-Arrieta, C. Global Transcriptomic Profiling of the Bone Marrow Stromal Microenvironment during Postnatal Development, Aging, and Inflammation. Cell Rep. 2019, 29, 3313–3330.e3314. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Ho, Y.-H.; del Toro, R.; Rivera-Torres, J.; Rak, J.; Korn, C.; García-García, A.; Macías, D.; González-Gómez, C.; del Monte, A.; Wittner, M.; et al. Remodeling of Bone Marrow Hematopoietic Stem Cell Niches Promotes Myeloid Cell Expansion during Premature or Physiological Aging. Cell Stem Cell 2019, 25, 407–418.e6. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, X.; Yin, Y.; Luo, B.; Liu, Y.; Yang, C. Inflammatory Microenvironment Accelerates Bone Marrow Mesenchymal Stem Cell Aging. Front. Bioeng. Biotechnol. 2022, 10, 870324. [Google Scholar] [CrossRef]
- Wang, N.; Chen, C.; Cheng, Y.; Fu, Y.; Zhong, Z.; Yang, Y.; Lv, L.; Chen, H.; Huang, J.; Duan, Y. Hypoxia drives hematopoiesis with the enhancement of T lineage through eliciting arterial specification of hematopoietic endothelial progenitors from hESC. Stem Cell Res. Ther. 2022, 13, 282. [Google Scholar] [CrossRef]
- Ma, S.; Wang, S.; Ye, Y.; Ren, J.; Chen, R.; Li, W.; Li, J.; Zhao, L.; Zhao, Q.; Sun, G.; et al. Heterochronic parabiosis induces stem cell revitalization and systemic rejuvenation across aged tissues. Cell Stem Cell 2022, 29, 990–1005.e10. [Google Scholar] [CrossRef]
- Arai, F.; Stumpf, P.S.; Ikushima, Y.M.; Hosokawa, K.; Roch, A.; Lutolf, M.P.; Suda, T.; MacArthur, B.D. Machine Learning of Hematopoietic Stem Cell Divisions from Paired Daughter Cell Expression Profiles Reveals Effects of Aging on Self-Renewal. Cell Syst. 2020, 11, 640–652.e5. [Google Scholar] [CrossRef]
- Du, L.; Freitas-Cortez, M.A.; Zhang, J.; Xue, Y.; Veettil, R.T.; Zhao, Z.; Morrison, S.J. Periarteriolar niches become inflamed in aging bone marrow, remodeling the stromal microenvironment and depleting lymphoid progenitors. Proc. Natl. Acad. Sci. USA 2025, 122, e2412317122. [Google Scholar] [CrossRef]
- Pei, W.; Shang, F.; Wang, X.; Fanti, A.K.; Greco, A.; Busch, K.; Klapproth, K.; Zhang, Q.; Quedenau, C.; Sauer, S.; et al. Resolving Fates and Single-Cell Transcriptomes of Hematopoietic Stem Cell Clones by PolyloxExpress Barcoding. Cell Stem Cell 2020, 27, 383–395 e388. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; DeLaughter, D.M.; Trembley, M.A.; Saifee, S.; Xiao, F.; Chen, J.; Zhou, P.; Seidman, C.E.; Seidman, J.G.; et al. Molecular and Spatial Signatures of Mouse Embryonic Endothelial Cells at Single-Cell Resolution. Circ. Res. 2024, 134, 529–546. [Google Scholar] [CrossRef]
- Gao, D.; Yi, W.W.; Liu, B.; Zhang, C.E.; Yang, C.C.; Zeng, L.; Li, L.; Luo, G.; Zhang, L.; Ju, Z.Y.; et al. Tetrahydroxy stilbene glucoside rejuvenates aging hematopoietic stem cells with predilection for lymphoid differentiation via AMPK and Tet2. J. Adv. Res. 2025, 70, 515–529. [Google Scholar] [CrossRef]
- Li, M.; Guo, H.; Carey, M.; Huang, C. Transcriptional and epigenetic dysregulation impairs generation of proliferative neural stem and progenitor cells during brain aging. Nat. Aging 2024, 4, 62–79. [Google Scholar] [CrossRef]
- Aoyama, Y.; Yamazaki, H.; Nishimura, K.; Nomura, M.; Shigehiro, T.; Suzuki, T.; Zang, W.; Tatara, Y.; Ito, H.; Hayashi, Y.; et al. Selenoprotein-mediated redox regulation shapes the cell fate of HSCs and mature lineages. Blood 2025, 145, 1149–1163. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Hughes-Davies, A.; Clayfield, L.; Menendez-Gonzalez, J.B.; Almotiri, A.; Alotaibi, B.; Tonks, A.; Rodrigues, N.P. Gata2 haploinsufficiency promotes proliferation and functional decline of hematopoietic stem cells with myeloid bias during aging. Blood Adv. 2021, 5, 4285–4290. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, H.S.; Yon, S.J.; Matsumoto, T.; Lee, S.K.; Lee, K.Y. In vitro culture of hematopoietic stem cell niche using angiopoietin-1-coupled alginate hydrogel. Int. J. Biol. Macromol. 2022, 209 Pt B, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Aprile, A.; Raggi, L.; Bolamperti, S.; Villa, I.; Storto, M.; Morello, G.; Marktel, S.; Tripodo, C.; Cappellini, M.D.; Motta, I.; et al. Inhibition of FGF23 is a therapeutic strategy to target hematopoietic stem cell niche defects in β-thalassemia. Sci. Transl. Med. 2023, 15, eabq3679. [Google Scholar] [CrossRef] [PubMed]
- Cooney, J. Expansion of Cord Blood Stem Cells and Enhancing Their Mobilization and Homing Potential Using Mesenchymal Stromal Cells. Blood 2018, 132 (Suppl. S1), 3344. [Google Scholar] [CrossRef]
- Lin, W.; Chen, S.; Wang, Y.; Wang, M.; Lee, W.Y.; Jiang, X.; Li, G. Dynamic regulation of mitochondrial-endoplasmic reticulum crosstalk during stem cell homeostasis and aging. Cell Death Dis. 2021, 12, 794. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Zhu, Y.; Liu, Z.; Huang, L.; Zhao, H.; Zhou, Z.; Wu, Q. Anti-aging mechanism of different age donor-matched adipose-derived stem cells. Stem Cell Res. Ther. 2023, 14, 192. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, K.A.; Hoban, M.D.; Proctor, J.L.; Adams, H.L.; Hyzy, S.L.; Boitano, A.E.; Cooke, M.P. Phenotype Does Not Always Equal Function: HDAC Inhibitors and UM171, but Not SR1, Lead to Rapid Upregulation of CD90 on Non-Engrafting CD34+CD90-Negative Human Cells. Blood 2017, 130, 659. [Google Scholar] [CrossRef]
- Sanders, M.A.; Zeilemaker, A.; al Hinai, A.; Hoogenboezem, R.; Kavelaars, F.G.; Rijken, M.; Bindels, E.M.J.; Löwenberg, B.; Valk, P.J.M. DNMT3A Mutations Enhance CpG Mutagenesis through Deregulation of the Active DNA Demethylation Pathway. Blood 2016, 128, 1076. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, S.; Wang, S.; Wang, Y.; Li, W.; Huang, Y.; Zhao, H.; Wu, X.; An, C.; Guo, X.; et al. TET2 deficiency leads to stem cell factor-dependent clonal expansion of dysfunctional erythroid progenitors. Blood 2018, 132, 2406–2417. [Google Scholar] [CrossRef]
- Guderyon, M.J.; Chen, C.; Bhattacharjee, A.; Ge, G.; Fernandez, R.A.; Gelfond, J.A.L.; Gorena, K.M.; Cheng, C.J.; Li, Y.; Nelson, J.F.; et al. Mobilization-based transplantation of young-donor hematopoietic stem cells extends lifespan in mice. Aging Cell 2020, 19, e13110. [Google Scholar] [CrossRef]
- He, H.; Xu, P.; Zhang, X.; Liao, M.; Dong, Q.; Cong, T.; Tang, B.; Yang, X.; Ye, M.; Chang, Y.; et al. Aging-induced IL27Ra signaling impairs hematopoietic stem cells. Blood 2020, 136, 183–198. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Tang, B.; Dong, Q.; Wu, C.; Sun, W.; Wang, J. Aging-induced MCPH1 translocation activates necroptosis and impairs hematopoietic stem cell function. Nat. Aging 2024, 4, 510–526. [Google Scholar] [CrossRef]
- Tang, B.; Wang, X.; He, H.; Chen, R.; Qiao, G.; Yang, Y.; Xu, Z.; Wang, L.; Dong, Q.; Yu, J.; et al. Aging-disturbed FUS phase transition impairs hematopoietic stem cells by altering chromatin structure. Blood 2024, 143, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.D.; San Antonio, J.D.; Jacenko, O. Heparan sulfate proteoglycans: A GAGgle of skeletal-hematopoietic regulators. Dev. Dyn. 2008, 237, 2622–2642. [Google Scholar] [CrossRef]
- Fujino, T.; Asada, S.; Goyama, S.; Kitamura, T. Mechanisms involved in hematopoietic stem cell aging. Cell Mol. Life Sci. 2022, 79, 473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hansen, M.; Di Summa, F.; Von Lindern, M.; Gillemans, N.; Van, I.W.F.J.; Svendsen, A.F.; Philipsen, S.; Van der Reijden, B.; Varga, E.; et al. LSD1/KDM1A and GFI1B repress endothelial fate and induce hematopoietic fate in induced pluripotent stem cell-derived hemogenic endothelium. Haematologica 2024, 109, 3975–3988. [Google Scholar] [CrossRef]
- Shen, J.; Xu, Y.; Zhang, S.; Lyu, S.; Huo, Y.; Zhu, Y.; Tang, K.; Mou, J.; Li, X.; Hoyle, D.L.; et al. Single-cell transcriptome of early hematopoiesis guides arterial endothelial-enhanced functional T cell generation from human PSCs. Sci. Adv. 2021, 7, eabi9787. [Google Scholar] [CrossRef] [PubMed]
- Desterke, C.; Bennaceur-Griscelli, A.; Turhan, A.G. EGR1 dysregulation defines an inflammatory and leukemic program in cell trajectory of human-aged hematopoietic stem cells (HSC). Stem Cell Res. Ther. 2021, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tristan, C.A.; Chen, L.; Jovanovic, V.M.; Malley, C.; Chu, P.H.; Ryu, S.; Deng, T.; Ormanoglu, P.; Tao, D.; et al. A versatile polypharmacology platform promotes cytoprotection and viability of human pluripotent and differentiated cells. Nat. Methods 2021, 18, 528–541. [Google Scholar] [CrossRef]
- Guan, J.; Wang, G.; Wang, J.; Zhang, Z.; Fu, Y.; Cheng, L.; Meng, G.; Lyu, Y.; Zhu, J.; Li, Y.; et al. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature 2022, 605, 325–331. [Google Scholar] [CrossRef]
- Ren, S.; Wu, D.; Shen, X.; Wu, Q.; Li, C.; Xiong, H.; Xiong, Z.; Gong, R.; Liu, Z.; Wang, W.; et al. Deciphering the role of extrachromosomal circular DNA in adipose stem cells from old and young donors. Stem Cell Res. Ther. 2023, 14, 341. [Google Scholar] [CrossRef]
- Hua, P.; Hester, J.; Adigbli, G.; Li, R.; Psaila, B.; Roy, A.; Bataille, C.J.; Wynne, G.M.; Jackson, T.; Milne, T.A.; et al. The BET inhibitor CPI203 promotes ex vivo expansion of cord blood long-term repopulating HSCs and megakaryocytes. Blood 2020, 136, 2410–2415. [Google Scholar] [CrossRef]
- Yuan, N.; Wei, W.; Ji, L.; Qian, J.; Jin, Z.; Liu, H.; Xu, L.; Li, L.; Zhao, C.; Gao, X.; et al. Young donor hematopoietic stem cells revitalize aged or damaged bone marrow niche by transdifferentiating into functional niche cells. Aging Cell 2023, 22, e13889. [Google Scholar] [CrossRef]
- Ramalingam, P.; Gutkin, M.C.; Poulos, M.G.; Winiarski, A.; Smith, A.; Carter, C.; Doughty, C.; Tillery, T.; Redmond, D.; Freire, A.G.; et al. Suppression of thrombospondin-1-mediated inflammaging prolongs hematopoietic health span. Sci. Immunol. 2025, 10, eads1556. [Google Scholar] [CrossRef] [PubMed]
- Lee-Thedieck, C.; Schertl, P.; Klein, G. The extracellular matrix of hematopoietic stem cell niches. Adv. Drug Deliv. Rev. 2022, 181, 114069. [Google Scholar] [CrossRef]
- Kruta, M.; Sunshine, M.J.; Chua, B.A.; Fu, Y.; Chawla, A.; Dillingham, C.H.; Hidalgo San Jose, L.; De Jong, B.; Zhou, F.J.; Signer, R.A.J. Hsf1 promotes hematopoietic stem cell fitness and proteostasis in response to ex vivo culture stress and aging. Cell Stem Cell 2021, 28, 1950–1965 e1956. [Google Scholar] [CrossRef]
- Matteini, F.; Mulaw, M.A.; Florian, M.C. Aging of the Hematopoietic Stem Cell Niche: New Tools to Answer an Old Question. Front. Immunol. 2021, 12, 738204. [Google Scholar] [CrossRef] [PubMed]
- Tobin, S.W.; Alibhai, F.J.; Weisel, R.D.; Li, R.K. Considering Cause and Effect of Immune Cell Aging on Cardiac Repair after Myocardial Infarction. Cells 2020, 9, 1894. [Google Scholar] [CrossRef]
- Ho, T.T.; Warr, M.R.; Adelman, E.R.; Lansinger, O.M.; Flach, J.; Verovskaya, E.V.; Figueroa, M.E.; Passegué, E. Autophagy maintains the metabolism and function of young and old stem cells. Nature 2017, 543, 205–210. [Google Scholar] [CrossRef]
- Chandra, A.; Law, S.F.; Pignolo, R.J. Changing landscape of hematopoietic and mesenchymal cells and their interactions during aging and in age-related skeletal pathologies. Mech. Ageing Dev. 2025, 225, 112059. [Google Scholar] [CrossRef]
- Gong, Y.; Zhan, H.; Wei, N.; Liu, M.; Liu, Y.; Guan, P.; Xie, Y.; Deng, Y.; Pu, Q.; Lou, X.; et al. Acetylation profiling by Iseq-Kac reveals insights into HSC aging and lineage decision. Nat. Chem. Biol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Poscablo, D.M.; Worthington, A.K.; Smith-Berdan, S.; Rommel, M.G.E.; Manso, B.A.; Adili, R.; Mok, L.; Reggiardo, R.E.; Cool, T.; Mogharrab, R.; et al. An age-progressive platelet differentiation path from hematopoietic stem cells causes exacerbated thrombosis. Cell 2024, 187, 3090–3107.e3021. [Google Scholar] [CrossRef]
- Pioli, P.D.; Casero, D.; Montecino-Rodriguez, E.; Morrison, S.L.; Dorshkind, K. Plasma Cells Are Obligate Effectors of Enhanced Myelopoiesis in Aging Bone Marrow. Immunity 2019, 51, 351–366.e356. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Eudy, E.; Bell, R.; Loberg, M.A.; Stearns, T.; Sharma, D.; Velten, L.; Haas, S.; Filippi, M.-D.; Trowbridge, J.J. Decline in IGF1 in the bone marrow microenvironment initiates hematopoietic stem cell aging. Cell Stem Cell 2021, 28, 1473–1482.e1477. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Pan, C.; Zheng, X.; Hu, B.; Xie, R.; Hu, J.; Shang, X.; Yang, H. Single-cell RNA sequencing to track novel perspectives in HSC heterogeneity. Stem Cell Res. Ther. 2022, 13, 39. [Google Scholar] [CrossRef]
- Meng, Y.; Nerlov, C. Epigenetic regulation of hematopoietic stem cell fate. Trends Cell Biol. 2025, 35, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Yasui, R.; Sekine, K.; Taniguchi, H. Clever Experimental Designs: Shortcuts for Better iPSC Differentiation. Cells 2021, 10, 3540. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, H.; Zhao, X.; Liu, S.; Kong, S.K.; Ho, A.; Chen, T.; Feng, H.; He, H. Stem cell differentiation with consistent lineage commitment induced by a flash of ultrafast-laser activation in vitro and in vivo. Cell Rep. 2022, 38, 110486. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Caserta, S.; Mirabile, G.; Gangemi, S. Aging and Age-Related Epigenetic Drift in the Pathogenesis of Leukemia and Lymphomas: New Therapeutic Targets. Cells 2023, 12, 2392. [Google Scholar] [CrossRef]
- Uemura, S.; Yamashita, M.; Aihara, A.; Iwawaki, T.; Koide, S.; Nakajima-Takagi, Y.; Oshima, M.; Masuko, M.; Takizawa, J.; Sone, H.; et al. YAP/TAZ Promote Hematopoietic Regeneration Via Accelerating the Recovery of Bone Marrow Niche. Blood 2021, 138, 199. [Google Scholar] [CrossRef]
- Hettler, F.; Schreck, C.; Marquez, S.R.; Engleitner, T.; Vilne, B.; Landspersky, T.; Weidner, H.; Hausinger, R.; Mishra, R.; Oellinger, R.; et al. Osteoprogenitor SFRP1 prevents exhaustion of hematopoietic stem cells via PP2A-PR72/130-mediated regulation of p300. Haematologica 2023, 108, 490–501. [Google Scholar] [CrossRef]
- Grants, J.M.; Wegrzyn, J.; Hui, T.; O’Neill, K.; Shadbolt, M.; Knapp, D.; Parker, J.; Deng, Y.; Gopal, A.; Docking, T.R.; et al. Altered microRNA expression links IL6 and TNF-induced inflammaging with myeloid malignancy in humans and mice. Blood 2020, 135, 2235–2251. [Google Scholar] [CrossRef]
- Li, L.; Xu, D.; Huang, X. SERCA-mediated endoplasmic reticulum stress facilitates hematopoietic stem cell mobilization. Stem Cell Res. Ther. 2025, 16, 208. [Google Scholar] [CrossRef]
- Zeytin, I.C.; Alkan, B.; Ozdemir, C.; Cetinkaya, D.U.; Okur, F.V. Alterations in Hematopoietic and Mesenchymal Stromal Cell Components of the Osteopetrotic Bone Marrow Niche. Stem Cells Transl. Med. 2022, 11, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Wang, Y.; Wu, J.; Zeng, T.; Cui, H.; Tao, Z.; Lei, L.; Yu, L.; Liu, A.; Wang, H.; et al. Long-term mid-onset dietary restriction rejuvenates hematopoietic stem cells and improves regeneration capacity of total bone marrow from aged mice. Aging Cell 2020, 19, e13241. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Li, X.; Liu, B.; Dong, C.; Chang, Y. Hematopoietic Stem Cell Aging: Mechanisms, Microenvironment Influences, and Rejuvenation Strategies. Bioengineering 2025, 12, 1166. https://doi.org/10.3390/bioengineering12111166
Cui J, Li X, Liu B, Dong C, Chang Y. Hematopoietic Stem Cell Aging: Mechanisms, Microenvironment Influences, and Rejuvenation Strategies. Bioengineering. 2025; 12(11):1166. https://doi.org/10.3390/bioengineering12111166
Chicago/Turabian StyleCui, Jiaqi, Xincan Li, Bin Liu, Cheng Dong, and Yun Chang. 2025. "Hematopoietic Stem Cell Aging: Mechanisms, Microenvironment Influences, and Rejuvenation Strategies" Bioengineering 12, no. 11: 1166. https://doi.org/10.3390/bioengineering12111166
APA StyleCui, J., Li, X., Liu, B., Dong, C., & Chang, Y. (2025). Hematopoietic Stem Cell Aging: Mechanisms, Microenvironment Influences, and Rejuvenation Strategies. Bioengineering, 12(11), 1166. https://doi.org/10.3390/bioengineering12111166








